Use ofδ18O,δ13C and NO3-to identify hydrogeochemical processes related to contamination in an aquifer located in central Mexico
2022-07-11JoseIvaMoralesArredondoMarAuroraArmientaHernandezFatimaJuarezAparicioJorgeFedericoLandaArreguItzamnaZakniteFloresOcampo
Jose´Iva´n Morales-Arredondo ·Marı´a Aurora Armienta Herna´ndez·Fa´tima Jua´rez-Aparicio·Jorge Federico Landa-Arreguı´n·Itzamna Zaknite Flores-Ocampo
Abstract In this work,an isotopic analysis ofδ18O,δ13C,and NO3-concentrations was carried out to identify the origin and the processes related to the contamination of an aquifer located in the state of Guanajuato,Mexico.The research identified the possible sources ofδ13C in groundwater.During groundwater flow,CO2 participates in different hydrogeochemical reactions in which the dissolution of carbonates or biochemical processes related to biodegradation stand out.Isotopic data ofδ13C,δ18O,and the hydrogeochemical behavior of NO3-and HCO3-in water,in addition to isotopic data and the chemical composition of limestones in the study area,were determined to establish the isotopic signature and the processes undergone by the rocks.The isotopic signature of rock and water samples indicated that metamorphic limestones contributed with carbon dioxide to deep groundwater,while in the upper aquifer,bacterial metabolic reactions during nitrification-denitrification could modify the isotopic signature ofδ13C in some wells,although atmospheric contribution also plays a role.The modification of the carbon isotopic component is related to the precipitation of calcite in specific regions of the study area,input of atmospheric CO2,and soil(e.g.the possible participation of C4-type plants in the assimilation-release of carbon).This process is not confirmed or completely ruled out in this study since agriculture is excessively developed throughout the region.The joint interpretation of isotopic values and the hydrogeochemical behavior of major and conservative elements help in identifying possible pollution processes in which different carbon sources are related.
Keywords 18O and 13C isotopes·Groundwater pollution·Nitrate·Limestone
1 Introduction
Stable isotopes are useful tracers to identify the origin of groundwater contamination and processes related to the release of contaminants,to define trajectories of carbonate in groundwater,or to evaluate if alkalinity in an aquifer has its origin in the hydrolysis of carbonate or silicate minerals by CO2(e.g.,Kalin 2000;Walker 2005;Han&Plummer 2016;Bottrell et al.2017).In the same way,stable isotopes(e.g.,2H,18O,and13C)are applied in water quality studies,serve to complement geochemical(and biochemical)studies in a groundwater system,since during the hydrogeological cycle show a specific behavior that is influenced by hydrogeochemical(and biochemical)reactions in both closed and open systems(Akbar et al.2020).A specific isotopic signature of13C allows tracing the trajectory of carbonate and origin from the source of CO2(Bottrell et al.2017;Sabagh et al.2021).This peculiarity,together with hydrogeochemical behavior of major and minor ions,trace elements,and isotopic information of2H,18O,helps to determine different pollution processes,geochemical evolution,recharge procedures,water-rock interactions,and salinity sources(Clark and Fitz 1997;Clark 2015).
Tracking the dynamics of pollutants in a natural hydrological environment(for example,source and destination of nitrates)requires knowledge of the18O isotopic composition of the water and contaminant(Peters et al.2015).Variation in the stable isotope ratios(δ18O andδ2H)of surface and groundwater bodies has been shown to reflect evaporation conditions at the precipitation source,mainly in arid and semi-arid regions,and have also been correlated with evaporation and transpiration processes at the land surface(Rozanski et al.1992;Katz et al.1997;Clark and Fritz 1997;Winter et al.1998;Skrzypek et al.2015).Stable isotopes with contaminant indicators are used to identify the possible sources of pollution(Minet et al.2017),and their possible changes in rainy and dry seasons and to elucidate the transport mechanisms of water and some contaminants(Skrzypek et al.2015;Pu et al.2020),for instances water in the wet months shows a depletion in δ18O as opposed to the dry months in which there is enrichment.This process reflects the effect of evaporation resulting from lower precipitation and higher solar energy in the dry season,leading to the enrichment in heavy isotopes compared to the wet season (Clark 2015).Stable isotopes of carbon(δ13C andδ12C)help to define the evolution of water throughout the hydrological cycle(whether as rainwater,surface water,or groundwater),provide information during the CO2(aq)incorporation in an aqueous medium,as the water infiltrates and recharges the groundwater or while the water is hosted in a geological medium and dissolution proceeds,mainly in carbonate rocks(Gonfiantini 1981),as they preserve the effect on water mineralization(e.g.,carbonate dissolution and precipitation in different environments)(Akbar et al.2020),which can be identified through enrichment or depletion of their isotopes either in a closed or an open system(Han and Plummer 2016).Stable isotopes of carbon area tracer of CO2emissions derived from degradation of organic carbon by microorganisms and other chemical reactions(geogenic processes)or anthropogenic activities(Hounslow 1999;Walker 2005;Mehmood et al.2020).Moreover,in shallow aquifers,the high CO2contents present in limits between soil and vadose zone,that generated by biomass degradation produces a much more negative isotopic signature of δ13C,and closer to the vegetation type C3 and C4(due to the outward diffusion from high pCO2in soils),as12C is more diffusive than13C(Khon 2010;Clark 2015;Sabagh et al.2021).In addition,the photosynthetic cycle of C-3 or C-4 plants may produceδ13C isotopic signatures related to the C-3 or C-4 carbon cycle,which may be reflected in shallow water mineralization,mainly when the plants are subjected to water stress,due to insufficient supply through the roots,or in arid conditions,which causes a limited circulation of CO2through them(Schwarcz and Schoeninger,2011),or even when water table decline and release unexpectedly large and relatively steady of CO2(Vogel 1993;Tokunaga et al.2016).Likewise,during the oxidation reactions of organic matter in the soil or shallow groundwater,CO2is released due to the participation of microorganisms(Han and Plummer 2016)or even when there is a deep contribution of CO2by respiration rates at greater depths or due to geological causes(Berner 2004;Macpherson et al.2008;Tokunaga et al.2016).The pCO2of rivers and springs is regularly more than an order of magnitude above atmospheric pCO2,resulting in rapid degassing,which affects theδ12C isotopic signature and leads toδ13C enrichment in the DIC(Clark 2015).
In Mexico,few comprehensive studies that include an isotopic interpretation ofδ13C in groundwater have been developed,mainly from the north and center of the country(Edmunds et al.2002;Mahlknecht et al.2004,2008;Horst et al.2008;Armienta et al.2014;).However,recently,the use of stable isotope tools has started to increase rapidly to complement hydrogeological and hydrogeochemical evaluations with different approaches;for example,in Mexico City,an isotopic study was conducted to estimate the ages of flows,geochemical evolution of groundwater,natural baseline conditions and determining water origin(Edmunds et al.2002;Montiel 2020).Likewise,in the Mexican Bajı´o area,the application ofδ13C isotopes allowed us to deduce the possible participation of carbon dioxide in different hydrogeochemical reactions related to silicate weathering or carbonate dissolution in groundwater,CO2incorporation from an unsaturated zone or surface water(Mahlknecht et al.2004,2006;Horst et al.2008;Morales-Arredondo and Armienta 2020).
This work presents the isotopic data ofδ13C in dissolved inorganic carbon,δ18O andδ2H in water molecules and carbonate rocks,and concentrations of major elements in groundwater to define water origin,characterize geochemical evolution of groundwater in a complex geological environment comprising volcano-sedimentary material interacting with a carbonate basement,and where there is a contribution of CO2from the soil(where biochemical processes related to biodegradation occurs).The purpose of this study was to identify the possible sources ofδ13C in the hydrogeological environment during groundwater migration.
In addition,characterizing the chemical behavior of major anions in groundwater will serve as a reference in the region to differentiate natural from anthropogenic actions in whichδ13C is involved,as has been done in previous studies in other aquifers of the world(e.g.,Zhang et al.2015;Vandekerckhove et al.2019;Hartland et al.2011).To meet this objective,isotope data ofδ13C,δ18O from rock and water and the chemical composition of carbonate rocks from the Mesozoic that emerge north of the study area will be used to confirm or rule out the chemical and isotopic contribution of these rocks in the various hydrogeochemical reactions occurring at depth.
2 Background
2.1 Study area
Santa Cruz de Juventino Rosas and Villagra´n are two municipalities that belong to Bajı´o Guanajuatense,and they cover an area of 554.04 km2and are in the centralsouthern part of the state of Guanajuato,Mexico.This area is known for its intense agricultural,industrial,and livestock activity,which generally uses chemical fertilizers and irrigation with wastewaters and soil nitrification are practiced(CONAGUA 2015;Valverde and Castillo 2002),as shown in Fig.1,both municipalities belong to the Celaya Valley Aquifer(AVC)located between coordinates-101°5′29′′,+100°28′52′′longitude and 20°20′12′′,20°51′57′′latitude,which in turn is part of the Lerma-Santiago hydrological region No.12 in the Medio Lerma subregion(CONAGUA 2015;CEAG 2018).

Fig.1 Location of the wells in the study area,profile AA’corresponds to the profile where the geological composition is observed at depth.Square B shows the area where a wastewater treatment plant and land where the sewage sludge is placed.Square C shows the area where two fault systems intersect and agriculture and intense fertilization are practiced
2.2 Geology
The basement complex is composed of carbonate rocks of marine origin from Mesozoic;some of them show slight metamorphism(Cerca 1998)(Kss).These rocks are correlated with the rocks of the Esperanza Formation(Baez 2012;Echegoye´n et al.1970;Cerca 1998).The age of this formation is estimated from the Lower Cretaceous according to stratigraphic correlation,but Corona(1988)indicated that the age of the formation corresponds to the Titonian-Valanginian,The basement outcrops in only some places.The approximate structural thickness of the formation was 2800 m(Mengelle et al.2013);it outcrops to the northeast of the study area,has a high silica content,and shows a microcrystalline texture and Fe oxyhydroxide laminations(Jua´rez-Aparicio 2019).Microscopic results suggest that these rocks are limestones formed by micrite with laminations of carbonaceous matter,presenting polycrystalline aggregates of calcite and quartz,in addition to detrital quartz grains;they also contain significant amounts of calcite veins of tectonic origin(Mengelle et al.2013).
The Cenozoic cover is structured by sedimentary material and volcanic rocks from different ages,the clastic sediments were deposited in tectonic basins(a red conglomerate of Guanajuato)from the Eocene(del Rı´o-Varela et al.2020);in the Oligocene,volcanic rocks were deposited (Tvr),mainly rhyolites (e.g.,Chichı´ndaro rhyolite);In addition,there is a great number of ignimbrites throughout the entire area(Tom).These structures are composed of porphyric and fluid lava,which often have domic structures(with an approximate age of 32 Mybp)(Nieto-Samaniego et al.1996).Volcanic rocks are interbedded with sediments of fluvio-lacustrine origin(Cerca et al.2000).
The Miocene rocks are represented by andesitic composition lava flows that form plateaus and stratovolcanoes(Tmo),and are related to the early stages of the formation of the Trans-Mexican Volcanic Belt(INEGI 2017;del Rı´o-Varela et al.2020).The Andesite Allende unit(Tsa)belongs to these structures(with an approximate age of 11.1±0.4 Mybp)(Pe´rez-Venzor et al.1996).Overlying these units,the Late Miocene Basalt unit(Tmb and Tpl),outcrops north of the area and whose main characteristic is the presence of a certain hydrothermal alteration in some areas,in addition to the fact that structures show the filling of their vesicles,mainly by silica(CEAG 2018;Landa-Arreguı´n et al.2021).
The dominant Pliocene rocks have an andesitic composition(Cerca 1998).The Pleistocene is represented by extrusive rocks from the monogenetic volcanoes that are present in the area,in addition to the alluvial deposits from the Pleistocene to the recent era(Qal).The grain size of the deposits ranges from clay to gravel,and the approximate thickness is 100 m(CEAG 2018).
2.3 Hydrogeology of the celaya valley aquifer
To the north of the study area,the aquifer is composed of rhyolites and ignimbrites and basaltic sequences to the south;both areas are covered by alluvial and lake deposits of varying thickness(CEAG 2018).Basalts form large shield-type monogenetic volcanoes;these structures extend around the valleys,acting as recharge zones(CONAGUA 2015).The aquifer is divided according to its depth and Physico-chemical properties.The shallow non-confined aquifer(50-150 m)is hosted in recent alluvial material interbedded with rhyolites-ignimbrites to the north and in fractured basalts to the south.
The intermediate aquifer is hosted in rhyolitic rocks,is located between 200 and 350 m deep,and shows lowtemperature geothermal characteristics,which range between 29 and 50°C(Morales et al.2018;Landa-Arreguı´n et al.2021).This aquifer has a high concentration of arsenic and fluoride(Valenzuela et al.2006;Morales-Arredondo et al.2020),whose possible origin is related to deep groundwater that migrates through faults and fractures and that causes the hydrochemical mixture of groundwater from two systems(Morales et al.2016;Moran-Ramı´rez et al.2020).According to previous hydrogeological and hydrogeochemical studies,the vertical migration of local and intermediate flows occurs through the main aquifer(Morales-Arredondo et al.2017;Moran-Ramı´rez 2020).The study area includes three dominant water types:bicarbonate-calcium,bicarbonate-calcium sodium,and bicarbonate-sodium.The latter is quite common in the central part of wells located in the basin,mainly in the municipalities of Santa Cruz de Juventino Rosas and Villagra´n(Morales-Arredondo and Armienta et al.2020).Previously,both aquifers were hydraulically connected,and the water table was shallow(Cuellar 2010).Currently,the groundwater level of the first aquifer shows a considerably piezometric level decrease,which has led to the exploitation of the second thermal aquifer as the main water supply source.The intensive extraction of the groundwater has led to the modification of the water table,acceleration of subsidence,and the formation of faults and fractures in the area;since the population needs to extract deeper water with poor quality for human consumption due to high concentrations of As and F-(Morales-Arredondo et al.2018,2020).In the last 30 years,the static level has shown a strong decrease,mainly in the center of the basin,due to the excessive use of groundwater for agriculture(Fig.2a).According to data from the Guanajuato State Water Commission(CEAG),the annual piezometric level depletion ranges from 1.0 to 1.8 m(Romero et al.2017),which has caused the static level to reach a depth of more than 150 m,compared to 50 m in the 1980s.
Excessive groundwater extraction is linked to agricultural activity,and some processes related to the natural geothermal of the region may be affecting the isotopic signature of the stable isotopes of groundwater(e.g.δ13C,δ18O,andδ2H),both shallow and deep in the study area.This work focuses on evaluating the isotopic signature of groundwater and some rocks in the region,to define a natural background value,which will help to determine possible natural or anthropogenic processes that modify the behavior of stable isotopes.
3 Methodology
3.1 Sampling and chemical analysis of groundwater samples
One carbonate rock sampling campaign was conducted north of the study area in 2017(Fig.2b),and two groundwater sampling campaigns were carried out in the municipalities of Villagran(Vill)and Juventino Rosas(JR),Guanajuato,in 2017(32 wells in the rainy season[September])and in 2019(32 wells in the dry season[April]),following the standard methods of APHAAWWA(2005)and Mexican standards(NOM-127-SSA1 and NOM-230-SSA1).Urban and agricultural wells were included in the samplings.Temperature,pH,and electrical conductivity were measured in the field.The equipment was calibrated at the water temperature at each site,and a PC18 conductometer,a pH/EC/TDS/Temp tester Model HI 98,130 m,and a Hanna Model HI 9829 multiparameter equipment were used.
Chemical analyses of anions and cations were performed at the Analytical Chemistry Laboratory of the Institute of Geophysics,UNAM,Mexico.The laboratory participates in international calibration exercises(Verma et al.2012,2015;Stewart et al.2020).Major ions were analyzed following standard methods(APHA-AWWA 2005).Bicarbonate was determined using volumetry(HCl titration)(VC=6%),and Ca2+and Mg2+were determined by volumetry(tritiation with EDTA)(VC=1%);Cl-was determined by potentiometry with selective electrodes(VC=1%)(4500-Cl-)(APHA-AWWA 2005);Na+and K+were determined by atomic emission spectrophotometry(VC=1.1%and 0.7%,respectively)(3500-Na+and K+;APHA-AWWA 2005);and SO42-was determined by turbidimetry(VC=1%)(method 4500-SO42-).The ionic balance was less than 10%in all samples except one,which had an extremely high I.B.value(Ptol06)due to its high NO3-content;however,this sample was included in the analysis to evaluate its isotopic behavior,considering that this well is contaminated.
Nitrate determination was performed by UV spectrophotometry with Thermo Evolution 300(VC=3%,detection limit=0.9 mg/L).For this analysis,H2SO4was added to the samples as a preservative during the sampling process.In the case of samples where the highest nitrate values were obtained,the results were corroborated using the methodology proposed by EPA 353.2 for the determination of nitrate-nitrite and nitrogen using automated colorimetry.The method can be applied from 0.05 to 10.0 mg/L nitrate-nitrite nitrogen,which can be scaled up with sample dilution(USEPA 1993).
Samples were collected directly from the discharge of the operating wells.Isotopes ofδ13C,δ18O,andδ2H were determined at the Stable Isotope Laboratory(SIL)of the Institute of Geology,UNAM,using a Gas Bench II coupled to a ThermoFinnigan MAT 253 stable isotope mass spectrometer,following the procedure of Epstein and Mayeda(1953),which was updated for measurement by continuous flow(Gehre et al.2004).Theδ18O andδ2H results were normalized to the Vienna Standard Mean Ocean Water(VSMOW)and Standard Light Antarctic Precipitation(SLAP)scales,respectively(Coplen 1988).Theδ13C values were normalized using NBS-19,NBS-18,and LSVEC(reference materials)to the VPDB scale(PDB=sample taken from an internal calcite structure from a fossilBelemnitella americanafrom the Cretaceous Pee Dee Formation in South Carolina).Once the standard was used up,it was replaced by VPDB,which is assumed to be identical to PDB but standardized to Vienna according to the corrections described by Coplen(1988)and Werner and Brand(2001).
The results of stable water isotopes(δ2H,δ18O)and concentrations of anions(Cl-,HCO3-and SO42-)were used to identify possible sources of nitrate and to evaluate hydrogeochemical reactions that affect the behavior of the shallow and deep aquifers.In addition,hydrogeochemical characterization was performed and spatial and temporal variations of all chemical and isotopic analyses were determined.
3.2 Sampling and chemical analysis of rocks
3.2.1 18O and 13C isotopes
Isotopic analyzes ofδ13C andδ18O were performed on limestone samples from the study area.The analyses were performed at the University Laboratory of Isotope Geochemistry of the Institute of Geophysics,UNAM.Carbon and oxygen isotopes in the carbonate fraction of the matrix(δ13Ccarb andδ18Ocarb,respectively)were determined in 60 samples extracted from the micritic matrix of limestone with a dental drill(using a tungsten carbide tip).Orthophosphoric acid was added to approximately 0.9 mg of each sample at 25°C and allowed to react for 54 h under vacuum following the guidelines of McCrea(1950).The CO2released was analyzed with a Thermo Finnigan MAT 253 mass spectrometer coupled to Gas Bench II.Carbon and oxygen isotope values are given in permil relative to the Vienna Pee Dee Belemnite(VPDB)standard(Re´ve´sz and Landwehr 2002).The reproducibility of repeated analysis of samples was generally better than a standard deviation of 0.2‰for carbon and oxygen isotopes ratios,following the procedure described by Coplen(1988),Coplen et al.(2006),and Werner and Brand(2001).Results were reported asδ13CVPDB andδ18OVPDB and were normalized using NBS-19 and LSVEC,and theδ18OVPDB values were converted to VSMOW using the formula of Faure (1977):δ18OVSMOW=1.030901*δ18OVPDB+30.91.
3.2.2 Chemical composition of carbonate rocks
Rock samples were analyzed by X-Ray Fluorescence(RIGAKU ZSX Primus II Spectrometer)by quantitative analysis for major elements at the National Laboratory of Geochemistry and Mineralogy(LANGEM)of the Institute of Geology,UNAM.Major elements were determined by the fusion of the powdered sample with 50%Li2B4O7-50%LiBO2,and the loss on ignition(LOI)was determined by heating at 950°C.Results of the major elements were reported as oxides.
4 Results and discussion
4.1 Isotopic evaluation of sampled rocks
4.1.1 Behavior of stable isotopes ofδ18O andδ13C in rocks
Theδ13C andδ18O isotopic behavior of the Mesozoic carbonate rocks(RC1701,DD1705,and DD1706)of the study area was used to interpret the primary variation of their isotopic signature by comparing their behavior with other studies on carbonate rocks(Vahrenkamp 1996)(Table 1).Currently,there are no detailed geological or mineralogical studies on these rocks,so they are considered as part of the Soyatal Formation(limestones with more than 75%CaCO3,interbedded with shales that are overlaid in angular unconformity with volcanic rocks and stressed by dynamo-metamorphism during their folding)(Miranda-Gasca 1978;Labastida et al.2017),However,there are doubts about belonging of these rocks to the Esperanza Formation(siliceous limestones with iron oxyhydroxide laminations with less than 69%of CaCO3and a maximum of 36%of SiO2,which show alterating submarine volcanic rocks and black slate)(Cerca 1998;Jua´rez-Aparicio 2019).
Rock samples DD1705 and DD1706 showed a higher content of silica,aluminum,and iron compared to sample RC1701.Jua´rez-Aparicio(2019)related this chemical behavior to the presence of alteration minerals,such as clays and iron hydroxides,which could be due to hydrothermal processes.The concentration range of δ13CV-PDB varied from-2.56‰to-3.72‰,while the δ18OV-PDB range varied from-6.27‰to-11.18‰.These ranges evidenced a depletion process in the isotopic behavior ofδ13CV-PDB andδ18OV-PDB.To determine this action,we applied the diagrams used by Nelson and Smith(1996),Baumgartner and Valley(2001),and Hoefs(2009),as depicted in Fig.3a and b.
Theδ13C V-PDB isotopic signature of rock RC1701 was quite close to 0‰on the PDB scale.Authors such as Clark(2015),Ayyildiz et al.(2004),and Hudson(1977)suggest that similar signatures show a relationship with carbonates of marine origin.This behavior is observed in Fig.3a,whereδ13C V-PDB andδ18O VPDB values are compared.Sample RC1701 lies at the border between Marine Limestone and Meteoric Cement(Fig.3a).
According to Nagendra et al.(2010),in general,the isotopic signature of freshwater carbonates shows negative δ13CV-PDB values;in the case of the study area,the three rocks showed negativeδ13CV-PDB values.Considering these specifications it is suggested that the studied carbonate rocks have a shallow marine carbonate origin,which becomes prone to alteration of their isotopic signatures during diagenesis(they are close to the atmosphere).These types of carbonate and isotopic signatures have been observed in other carbonate regions,in Central Mexico(Villanueva-Olea et al.2019),near the Gulf of Mexico(Muller and Mayo 1986;Saller and Moore 1991)and in other carbonates precipitated at shallow depths worldwide(Buonocunto et al.2002;Nagendra et al.2010).In the study area,some fossils found in limestone clasts are shelf shallow water fauna(Omana et al.2015).These features could be observed in the negative values of theδ13C and δ18O isotopic composition of rock RC1701.Malone et al.(2001)suggested that in environments where sea-level regression has occurred,organic matter oxidizes and causes relatively high amounts of12C and16O.These light carbon and oxygen isotopes are incorporated into calcite by reprecipitating in the diagenetic stage,causing a negative isotopic value ofδ13C andδ18O(Fisher et al.2005).Geological events have been widely reported in stratigraphic deposits that include carbonates from shallow lagoonal environments throughout Mexico(Gonza´lez-Sa´nchez et al.2008),including the study area(Omana et al.2015).
In general,when the sea-level changes in a nearshore area,the organic matter could oxidize(due to its environment being exposed to meteoric water),causingδ13C and δ18O values to decrease(Marshall 1992).Similar events have been reported in other areas of Mexico because of regional geological evolution(Sierra-Rojas et al.2016),in the state of Guanajuato they may be related to the existence of a shallow-water platform in the Arpeos basin(Omana et al.2015).All these phenomena could indicate the reasons why the isotopic component of rock RC1701 presented this behavior during its diagenetic processes.
On the other hand,the rocks DD1705 and DD1706 showed slight metamorphism(Cerca 1998;Jua´rez-Aparicio 2019).Considering this characteristic,an evaluation of the isotopic signatures ofδ13C andδ18O was carried out.Both rocks showed depletion in isotopic values forδ13C and δ18O(common in metamorphosed limestones),considering as a reference the rock without metamorphism(RC1701)(Fig.3b).According to Hoefs(2009),when a volatilization reaction occurs during the rock fluid interaction(congruent solution),it regularly generates a distinctive isotopic modification to that produced when there is a fluid-rock interaction,which generates the mineral-fluid reaction(e.g.,by metasomatic processes).This process causes a change in the signature ofδ13C between 5‰and 10‰,as metamorphic volatilization reactions involve CO2generation and H2O loss with respect to the parent rock.Furthermore,depletion depends on the fractionation carried out and the temperature at which the reaction is conducted.In most cases of volatilization,the change inδ18O isotopic behavior is about 1‰,as the amount of oxygen released is minuscule compared to the oxygen remaining in the rock during isotopic fractionation.Extremely high temperatures would be required to generate a higher loss(Baumgartner and Valley 2001).
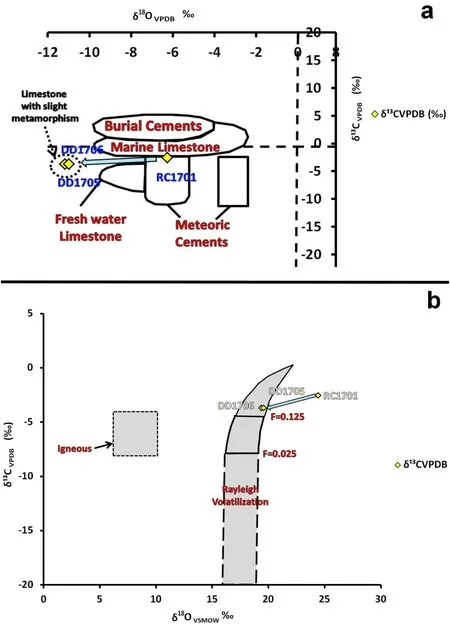
Fig.3 aδ18O vsδ13C diagram(after Nelson and Smith 1996)showing isotope fields for a selection of carbonate materials and limestones from the study area RC1701,DD1706 y DD1705(yellow diamonds)collected at Juventino Rosas and Villagra´n municipalities,b behavior ofδ13C andδ18O and the tendency of the isotopic composition of carbonate rocks with increasing degree of metamorphism in the study area,including the igneous rocks field,the Raleigh volatilization field,and the various fractionation factors F,considering the isotopic variation of 13C and 18O in rocks presenting contact metamorphism(Baumgartner and Valley;2001;Hoefs,2009)
The isotopic change helps to demonstrate that the volatilization reactions can occur during fluid-rock interaction when a metamorphic event takes place.Valley(1986)indicated that the release of coupled O-C pairs was evident in different metamorphic systems with the presence of carbonates.According to Hoefs(1992),during metamorphism in carbonate rocks,CO2is released.This process is more intense during the formation of other high-temperature metamorphic minerals(e.g.,wollastonite)(decarbonation).Taylor and O’Neil(1977)observed that metamorphic calcites have a depletion range of 3‰inδ18O and close to 5‰inδ13C(e.g.,during decarbonation).Hoefs(1992)suggested thatδ13C values close to-3‰and-4‰could be observed in geothermal environments.The isotopic behavior of the metamorphic carbonate rocks in the study area(DD1705 and DD1706)evidenced aδ13C isotopic depletion of nearly-3‰and-4‰,which is quite like that reported by the authors.In the limestone rocks that outcrop in the study area,there is sufficient evidence to explain the contact metamorphism of these rocks,a product of the hydrothermal activity associated with the various volcanic events that formed the Sierra de Codornices(Cerca 1998;Cerca et al.2020;CEAG 2018;del Rio-Varela,2020).

Table1IsotopeandX-RayFluorescencedataoftheanalyzedcarbonaterocks.InternalqualitycontrolsofthelaboratoryoftheInstituteofGeology,UNAM,(*)=totalpercentageofmajor(%)∑PxC(%)X-RF P2O5(%)X-RF K2O(%)X-RF Na2O(%)X-RF MnO(%)X-RF TiO2(%)X-RF Al2O3(%)X-RF MgO(%)X-RF Fe2O3(%)X-RF SiO2(%)X-RF CaO(%)X-RF δ13CVPDB(‰)δ18OVSMOW(‰)elementsofKT-1(Labastidaetal.2013)δ18OVPDB(‰)Description Sample number 100.05 100.02 99.96 99.96(96.54*)25 31.18 35.48 40.06(35.54*)0.158 0.105 0.078 0.053(0.05*)0.918 1.15 0.263 0.292(0.53*)0.043 0.051 0.044 0.168(0.11*)0.133 0.072 0.05 0.015(0.02*)0.208 0.174 0.094 0.091(0.1*)4.693 4.269 1.751 1.672(1.96*)0.793 0.728 0.449 0.473(0.41)2.515 1.779 0.882 0.65(1.13*)35.392 22.009 16.147 7.814(7.79*)30.192 38.502 44.721 48.671(49.31*)-3.71-3.72-2.56--19.38 19.58 24.44---11.18-10.99-6.27--Rockwithaprobable lowdegreeof metamorphism Alteredrockand possibleslight metamorphism Unalteredrock Internalreference material DD1706 DD1705 RC1701 Kit-1
Finally,in the case of the three carbonate samples rocks,it is evident that a negative slope is generated from the rock without metamorphism(RC1701)toward the rocks showing a degree of metamorphism(DD1705 and DD1706).This is remarkably similar qualitatively to the effects of devolatilization with the fluid exchange of lightδ18O and δ13C isotopic composition,where the compositional change is due to fluid-rock interaction(parent rock to metamorphic rock),causing more negativeδ13C andδ18O values(Fig.3b).
It is also evident that during the metamorphism in rocks DD1705 and DD1706,a slight depletion of theδ18O values and a more intense depletion ofδ13C were generated(Fig.3b).This can be explained by the loss in CO2content,probably due to reactions that generated carbonate dissolution,a process explained in the Rayleigh volatilization.This process indicates that when the fluid is produced,it is immediately isolated from the rock,and metamorphic volatilization reactions reduce theδ18O value in CO2generation and H2O loss,as mentioned above(Hoerfs 2009).This process may be more evident since the results of XRF show that rocks DD1705 and DD1706 have a higher SiO2,Al2O3,and Fe2O3content with respect to rock RC1701,while they present a lower CaO content(Table 1).The variation in SiO2and CaCO3concentrations could help to understand the metamorphism occurring in these rocks or could even show a possible degree of mineralogical alteration that increases iron and aluminum content,mainly in rock DD1706,reported by Jua´rez-Aparicio(2019).Additionally,as reported in Jua´rez-Aparicio (2019),RC1701 showed a no or low alteration,while DD1705 presented an intermediate alteration and DD1706 rock a greater alteration.The isotopic signature obtained from the limestones of the area serves as a background value for these rocks.
4.1.2 Hydrogeochemical evaluation and isotopic characterization of water samples
According to Piper′s diagram(Fig.4a and b,Tables 2 and 3),the water types can be divided into five groups:Watertype V(Ca-Na-HCO3-Cl):The local aquifer in this area is hosted in alluvial deposits,composed of volcanic material resulting from basaltic eruptions.The concentration of calcium,chloride and alkaline earth elements is high.Water type IV(Na-HCO3):The local aquifer in this zone is hosted in alluvial deposits,composed of volcanic material resulting from the erosion of the mountains.The concentration of sodium in this group is the highest;the temperature of the well water is also higher than that of the other wells,in the range of 30-48°C.Water type III(Na-Ca-HCO3):This water type is similar to water type I,but with higher calcium concentrations compared to those of water type IV and lower sodium concentrations(Tables 2 and 3).Wells representing this group are drilled in ignimbrites,rhyolitic tuffs,and breccias or vitreous matrix.These water types result from a mixing process with water rich in calcium and water hosted in volcanic material,as observed in a Piper diagram.Water type II(Ca-Na-HCO3):This water type is like water type III but with higher calcium concentrations compared to water type I and lower sodium concentrations.These water types are the result of a mixing process with calcium-rich water and water hosted in the sodium-rich volcanic material.Water type I(Ca-SO4-HCO3):This water is of the bicarbonate-calcium type with high sulfate proportions;the wells are located on limestone rocks of the Lower Cretaceous(Morales-Arredondo et al.2020).
The isotopic composition of the local wells is plotted in Fig.4 to verify the water origin and the physical processes occurring in the JR study area.The isotopic composition of the samples was compared with the meteoric water line reported by Wassenaar et al.(2009).
According to Craig(1961),in zones where evaporation occurs at ordinary temperatures,meteoric waters show enrichment inδ2H andδ18O consistent with a slope of about 5.According to Clark(2015),phreatic groundwaters in arid regions can also show evidence of evaporation,which can typically be characterized by a local evaporation line with a slope of near 5.6;similar slopes have been observed in other regions close to the study area(Gonza´-lez-Trinidad et al.2017).No evidence of geothermal footprint was observed as aδ18O enrichment typical of geothermal waters(Wassenaar et al.2009)caused by water-rock interactions(δ18O values more positive than meteoric water,deuterium isotopic composition like that of local meteoric water),as is the case in high-temperature geothermal sites in Mexico(Armienta et al.2014;Morales-Arredondo et al.2017).
Water samples from the study area demonstrated an isotopic composition similar to meteoric waters,with an isotope effect of evaporation mainly due to arid conditions in a confined aquifer(groundwater sampled in 2017).Some effect due to contamination processes is not ruled out(groundwater sampled in 2019).Figure 4c shows the equation obtained with the isotopic results of groundwater samples from the 2017 campaign(δ2H=5.5362*δ18O-18.961),while Fig.4d represents the equation obtained with isotopic results from groundwater samples collected in 2019(δ2H=4.7289*δ18O-26.902).Due to the lack of rainfall isotopic data in the study area,the Mexican meteoric water line was used as a reference.The isotopic data determined on the water samples collected in this study indicate that the groundwater is of meteoric origin as theδ2H,andδ18O values of the samples plot along or near the LMWL and GMWL and become more positive as they approach the surface.This process is possibly caused by a modification of the isotopic signature by evaporation of water during infiltration,as indicated by isotope values plotted below the meteoric water line(Clark and Fritz 1997).The slope modification in the18O versus2H plots in the groundwater of the studied area could be due to a series of processes,such as(1)The geothermal characteristics of the sampled water(Hoefs 2009),(2)The recycled meteoric water(used in agriculture)(Clark and Fritz 1997),and(3)The different samples analyzed during 2017 and 2019(not all the same samples were analyzed in both years).

Fig.4 Piper diagrams from JR and Vill for the years a 2017 and b 2019.Isotopic composition of water from wells at the study area for the years c 2017 and d 2019.Mexico groundwater line(dotted line,LWML)and global meteoric waterline(straight line,GWML),(Wassenaar et al.2009;Craig1961)are also shown
To identify hydrogeochemical reactions related to CO2interaction from natural processes or due to the incorporation of poor water quality into the aquifer of the study area,the behavior of major anions,nitrates,and stable isotopes in the years 2017 and 2019 was evaluated.The results are presented in Tables 2 and 3.
4.1.3 Incorporation of 13C in dissolution processes in the aquifer
From the isotopic evaluation ofδ13CVPDB andδ18-OVPDB in carbonate rocks and groundwater(Fig.3,4 and 5),several hydrogeochemical scenarios were inferred to explain the behavior ofδ13C in the aquifer(Fig.5):(a)The gaseous exchange of CO2between the atmosphere and water during the migration of water from an open system to a closed system(e.g.diffusive transport through pore or fracture networks),which generates sequential depletion of δ13C,This hypothesis is supported by the isotopic behavior of the water samples(water origin is meteoric)(Fig.4)and by previous studies that showed that there is a transition of groundwater from an open to a closed system in the aquifer,during this transition the generation of acidity occurs,subsequently buffering of acidity takes place,which in turn leads to an acceleration of mineral weathering and a release of very high amounts of As and F-(Morales-Arredondo and Armienta 2020),(b)The possible generation of CO2in soil by biological activity of plants through their roots and the activity of microorganisms and decomposition processes,mainly because the vertisol soils of the area(with an alkaline-very alkaline pH and accumulation of salts)are cultivated from 4 to 8 continuous cycles every 2 to 4 years,organic matter is added to these soils which produces organic acids reacting with the mineral fraction of the soil in the first 30 cm,generating an accumulation of soluble organic carbon,mainly when erosion,mineralization and leaching is lower(Ba´ez-Pe´rez et al.2017;Sabagh et al.2021).In the case of these soils,a higher CO2 emission occurs when the soluble organic matter content increases,in greater proportion during the wet season(Ba´ez-Pe´rez et al.2011).and(c)The dissolution of minerals,process reported in the study area and which is strongly related to the high alkalinity of the water in the region and the presence of elements of a toxic nature(Morales-Arredondo and Armienta 2020;Mora´n-Ramirez et al.2020;Landa-Arreguı´n et al.2021).All these actions incorporate carbonic acid into the water and generate a modification ofδ13C at specific sites by C incorporation and in turn produce an isotopic signature that can be correlated with its origin,considering the ranges proposed by Clark and Fritz(1997),Kalin(2000),Clark(2015),and Han&Plummer(2016)(Fig.5a and b).According to the obtained results and considering that the dominant anion is HCO3-in all the analyzed water samples,the origin of CO2 is the product of the following.

--watertype;WT4=Na-HCO3 thegroundwatersamplingin2017,majoranions,nitrateandstableisotopesof13C,18Oy2H;WT5=Ca-Na-HCO3 Table2Hydrogeochemicaldatafrom watertypeWT3=Na-Ca-HCO3-watertype;WT2=Ca-Na-Mg-HCO3-watertype;WT1=Ca-Mg-HCO3--SO42-watertype(Morales-Arredondoetal.2020b)d13CVPDB(‰)d18OVSMOW‰d2HVSMOW‰NO3-(mg/L)I.B HCO3-(mg/l)SO42-(mg/l)Cl-(mg/l)K+(mg/L)Ca2+(mg/L)Mg2+(mg/L)Na+(mg/L)Eh(V)depth(m)pH C.E.(μS/cm)2019 T(°C)Family Water type ID Well 2017-9.0-10.50-7.50-7.5-9.0-9.57-10.3-9.7-8.80-69.3-60.0-76.5 45.54<D.L 44.8 35-70.1 0.53<D.L-71.7 7.2 231.55-8.7-22.8 13.9 11.7 3.5 4.8-1.1-21.1 1.1 1.7 266.4 303.8 607.5 443.18 443.2 435.71-9.5 278.9 318.7 209.14 203.26 57.2 78.81 97.93 118.04 120.02 104.75 119.6 32.79 41.87 28.18 23.11 34.3 27 49.5 51.6 68.08 111.6 49.6 25.1 33.6 13.5 11.15 15.62 19.24 22.67 27.1 23.45 25.55 22.35 10.97 17.95 9.09 7.31 14.14 33.8 56.15 103.51 59.15 74.71 53.44 4.71 25.15 10.89 10.12 1.91 3.34 1.91 16.05 23.6 23.64 21.92 1.91 5.72 2.36 2.36 108.5 76.3 145.2 193.3 152 158.3 114.7 131.2 86.85 91.2 83.75 1.05 0.39 0.4 1.01 0.63 0.65 300 200 70 200 101 200 300 270 300 8.17 7.73 7.91 7.22 7.64 7.65 7.45 8.34 7.99 8.35 8.16 665 729 872 1414 1116 1253 1056 621 688 462 426 23.8 23.4 21.2 25.5 23.1 26 42.2 42.9 47.8 Na-Ca-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3-Cl Ca-Na-HCO3-Cl Ca-Na-HCO3 Ca-Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 5 5 5 5 5 5 5 4 4 4 4 WT WT WT WT WT WT WT WT WT WT WT WT5-1 WT5-2 WT5-3 WT5-4 WT5-6 WT5-7 WT5-8 WT4-1 WT4-2 WT4-3 WT4-4 18-Mzo 12 RBar 13 14 CG SJG15 JUM01 SJR02 DIF04 03 PV RCap 05 PTol06 PTol2 07-9.7-8.59-9.75-73.0-72.1 11.02<D.L 4.3-6.4 0.4 2.7 244 248.98 313.7 268.9 286.27-0.8 38.08 45.99 70 50.92 26.83 16.8 22.8 29.8 20.8 6.74 16.09 11.54 15.34 14.44 3.664 20.43 25.93 36.15 15.72 16.23 4.76 10.01 3.81 2.86 1.545 92.35 74.05 123.4 118.75 99.33 1.14 1.2 300 250 180 300 7.94 8.06 7.6 7.79 6.82 534 559 753 624 590 40.8 29.4 26.5 36 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 4 4 4 4 4 WT WT WT WT WT WT4-5 WT4-6 WT4-11 WT4-12 WT4-13 STorr 08 SRosa 09 Such10 RTov 11 10JR-8.04-8.04-10.0-9.75 6.82 3.81-73.1 8.06-72.0 280.23-0.9 280.23-5.6 258.49-3.4 257.28-2.8 265.74-1.6 26.59 40.3 29.1 21.8 33.96 8.37 6.26 9.79 9.41 9.66 6.791 4.791 1.69 9.382 15.91 19.06 18.08 4.526 10.83 16.41 6.511 3.614 0.648 2.204 1.902 83.46 84.76 102.5 85.2 86.95 1.2 0.71 0.87 0.75 0.47 300 280 300 250 7.32 7.11 8.04 7.52 7.36 595 648 577 494 576 37 48 38 47 30 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 4 4 4 4 4 WT WT WT WT WT WT4-14 WT4-15 WT4-16 WT4-17 WT4-18 Pozos Rom SJMer Gpe FT SM

d13CVPDB(‰)-d18OVSMOW‰-9.67-10.02-9.45-9.74-d2HVSMOW‰-NO3(mg/L)5.62<D.L-71.9<D.L-75.4<D.L-72.0<D.L-73.2-0.05 I.B-HCO3(mg/l)2-SO4(mg/l)Cl-(mg/l)K+(mg/L)Ca2+(mg/L)Mg2+(mg/L)Na+(mg/L)270.57-0.3 22.94 11.7 10.38 12.4 2.173 95.36-2.5 275.4 25.7 9.03 11.26 13.81 2.9 87.97 218.63-2.6 23.06 3.68 10.76 28.73 7.428 37.55 1.7 289.9 38.75 21.5 8.081 35.75 6.084 89.46 346.67-0.6 8.6 2.64 26.03 44.68 9.987 49.39 343.04-1 12.9 3.93 11.78 43.42 8.411 62.69 294.73-0.6 23.87 5.18 9.466 32.44 7.787 66.74 323.72-5.7 46.44 17.1 14.18 37.29 6.068 76 207.76-0.8 32.72 6.18 11.3 33.64 10.37 31.51 352.71-1 46.17 4.4 3.388 62.88 21.44 40.03 314.05-2.7 183.04 26.5 4.501 118.5 26.74 22.11-0.0005 0.00009 0.0005 0.0005 0.0008 0.0008 0.001 Eh(V)0.96 0.9 1.4 1.27 1.42-depth(m)300 280 280 300 250 250 250--pH 7.32 7.14 6.31 7.27 6.86 6.7 6.61 7.16 6.42 6.76 6.84-Table2continued C.E.(μS/cm)2019 T(°C)Family Water type ID Well 2017 517 660 459 751 629 660 594 646 473 696 955--42.3 33 34.5 34.5 28 32 33.5 24 34 29.5 29-Na-HCO3 Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-Mg-HCO3 Ca-Mg-Na-HCO3 Ca-Mg-HCO3-SO4--4 4 3 3 3 3 3 3 2 2 1 WT WT WT WT WT WT WT WT WT WT WT--WT4-19 WT4-20 WT3-1 WT3-2 WT3-5 WT3-6 WT3-7 WT3-8 WT2-1 WT2-2 WT1-1-Tej Val1 DN 05JR 11JR 12JR 13JR EZ NR SJCruz Cen2 D.L:=identification;D.L.=detectionlimit;I.B.IonicBalance ID

thegroundwatersamplingin2019,majoranions,nitrateandstableisotopesof13C,18Oy2H;WT5=Ca-Na-HCO3-watertype;WT4=Na-HCO3-2-watertype--SO4-watertype;WT1=Ca-Mg-HCO3 Table3Hydrogeochemicaldatafrom-watertype;WT2=Ca-Na-Mg-HCO3 watertypeWT3=Na-Ca-HCO3 d13CVPDB(‰)d18OVSMOW(‰)d2HVSMOW(‰)NO3-(mg/L)I.B HCO3-(mg/L)SO42-(mg/L)Cl-(mg/L)K+(mg/L)Ca2+(mg/L)Mg2+(mg/L)Na+(mg/L)Eh(V)depth(m)pH C.E.(μS/cm)2019 T(°C)Family Water type ID Well 2019-8.8-9.8-8.8-8.4-8.6--11.2-8.6-10.6-8.7-9.8-10.2-9.6-9.5-67.6-65.7-67.6-67.9-67.9-74.3-73.1-72.6-73.2 32.02 15.02 24.67 14.13 10.47 0.56 227.59 16.1 12.63-1.3-1.2-2.5-2.4-0.9-25.5-0.3 1.3 246.4 255.9 454.67-4.9 444 441.58 247.57 213 266.61 277.33 59.36 95.1 94.75 104.64 111.37 27.08 18.19 39.91 37.9 42.6 56.7 100 62.2 43.4 30.9 10.9 18.65 13.9 15 20.42 25.06 22.02 21.35 9.99 7.04 13.98 10.97 14.38 42.19 73.91 57.91 54.86 4.57 9.59 21.33 24.93 3.2 5.23 25.41 25.87 24.02 1.38 4.07 4.62 11.63 130.25 155 143 139.75 131.5 129.8 88 95.4 82.8 0.3276 0.2846 0.2257 0.2207 0.1963 0.1737 0.1418 0.1829 0.1755 300-100 200 200 300 270 300 250 7.58 8.1 7.51 7.43 7.35 8.19 7.94 7.61 7.85 773 1011 1313 1184 1123 862 634 649 627 23.86 23.4 24.8 25.5 26 42.2 42.9 40.8 29.4 Na-Ca-HCO3 Ca-Na-HCO3 Ca-Na-HCO3-Cl Ca-Na-HCO3 Ca-Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 5 5 5 5 5 4 4 4 4 WT WT WT WT WT WT WT WT WT WT5-1 WT5-3 WT5-5 WT5-6 WT5-8 WT4-1 WT4-3 WT4-5 WT4-6 18-Mzo 12 14 CG JUM02 JUM01 DIF04 03 PV PTol06 STorr 08 SRosa 09-9.6-71.9 5.56 1.8 252.33 19.62 11.2 12.45 12.19 2.77 98.7 0.1892 295 7.93 545 27.96 Na-HCO3 4 WT WT4-7 Carac-9-9.2-9.4-8.8-9.4-9.2-9.7-9.5-9.4-9.6-9.6-8.8-9.4-10.1-9.5-9.2-9.9-72.3-71.1-70.6-73.2-71.5-72.5-71-70.8 11.69 9.87 5.75 8.66 8.29-73.6 11.66 5.41 15.85 5.46 5.62-74.6 0 2.6 1 2.8 1 2 3.5 5.4 2.9 0.7 270.2 272.6 273.76 281 278.52 234.5 254.71 257 272.6 226.15 27.42 29.42 27.88 25.6 41.4 39.52 19.96 38.72 20.46 20.22 11.3 9.52 7.28 7.33 5.26 16.3 10.6 7.46 11.8 3.61 10.35 2.64 3.36 6.54 4.97 4.69 9.92 16.28 10.29 10.74 16.78 11.03 15.88 19.28 19.66 15.34 10.55 17.39 13.42 32.13 6.98 3.78 1.83 7.56 5.5 6.98 5.52 3.21 4.65 8.94 88 112.2 100.1 89.6 94.8 101.1 94.5 98.3 99.4 38.6 0.1508 0.1393 0.1555 0.1576 0.1645 0.1734 0.1368 0.1569 0.1642 0.2132 520 520 300 250 300 280 300 250 300-7.79 7.67 7.48 7.72 7.42 8.01 7.74 7.84 7.88 6.67 550 840 675 540 538 702 687 584 737 427 32.91 51.28 36 37 48 38 47 30 42.3 33 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 Na-HCO3 4 4 4 4 4 4 4 4 4 4 WT WT WT WT WT WT WT WT WT WT WT4-8 WT4-9 WT4-13 WT4-14 WT4-15 WT4-16 WT4-17 WT4-18 WT4-19 WT4-20 Provid Salit 10JR Gpe FT Pozos Rom SJMer SM Tej Val1
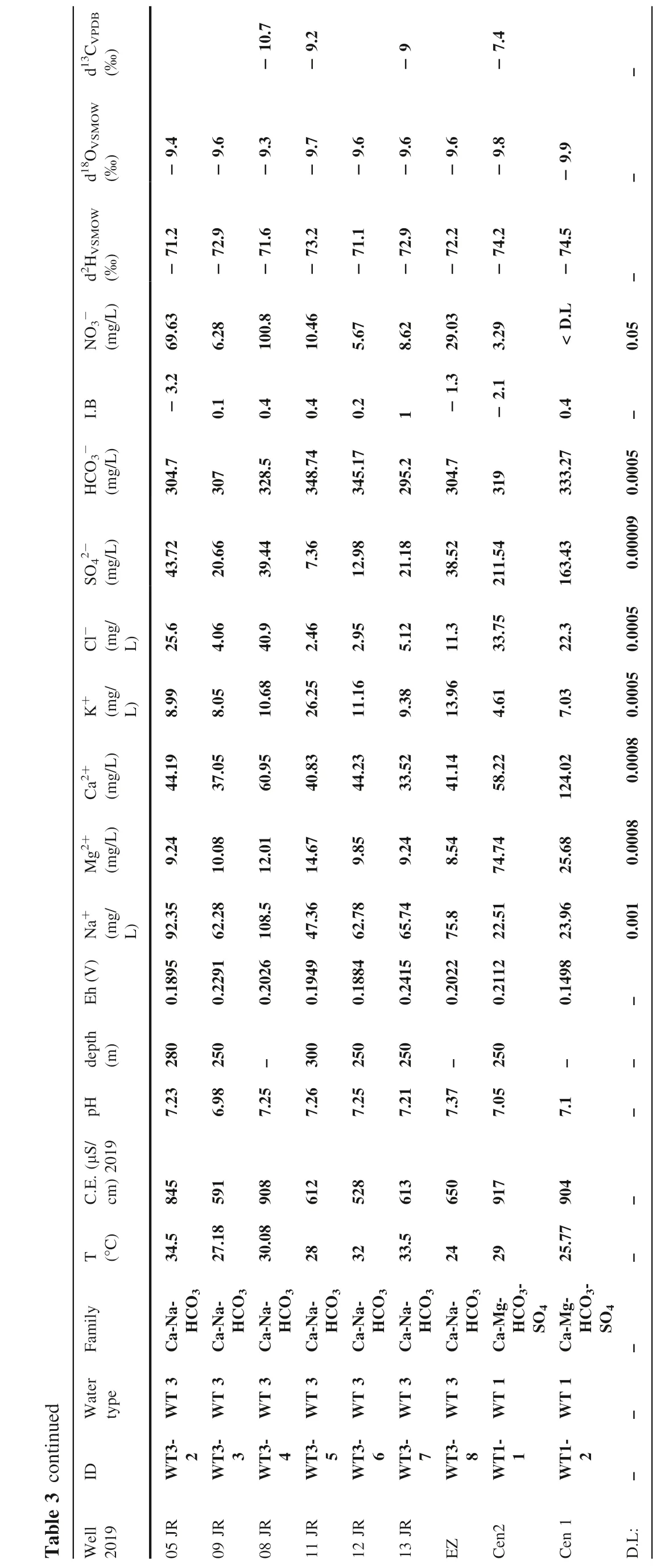
d13CVPDB(‰)d18OVSMOW(‰)d2HVSMOW(‰)-NO3(mg/L)I.B-HCO3(mg/L)2-SO4(mg/L)Cl-(mg/L)K+(mg/L)Ca2+(mg/L)Mg2+(mg/L)Na+(mg/L)Eh(V)depth(m)pH C.E.(μS/cm)2019 T(°C)-9.4-71.2 69.63-3.2 304.7 43.72 25.6 8.99 44.19 9.24 92.35 0.1895 280 7.23 845 34.5-9.6 6.28-72.9 0.1 307 20.66 4.06 8.05 37.05 10.08 62.28 0.2291 250 6.98 591 27.18-10.7-9.3-71.6 100.8 0.4 328.5 39.44 40.9 10.68 60.95 12.01 108.5 0.2026-7.25 908 30.08-9.2-9.7-73.2 10.46 0.4 348.74 7.36 2.46 26.25 40.83 14.67 47.36 0.1949 300 7.26 612 28-9.6-71.1 5.67 0.2 345.17 12.98 2.95 11.16 44.23 9.85 62.78 0.1884 250 7.25 528 32-9.6-9 8.62-72.9 1 295.2 21.18 5.12 9.38 33.52 9.24 65.74 0.2415 250 7.21 613 33.5-9.6-72.2 29.03-1.3 304.7 38.52 11.3 13.96 41.14 8.54 75.8 0.2022-7.37 650 24-7.4-9.8-74.2 3.29-2.1 319 211.54 33.75 4.61 58.22 74.74 22.51 0.2112 250 7.05 917 29-9.9-74.5<D.L 0.4 333.27 163.43 22.3 7.03 124.02 25.68 23.96 0.1498-7.1 904 25.77---0.05-0.0005 0.00009 0.0005 0.0005 0.0008 0.0008 0.001-----Family Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Na-HCO3 Ca-Mg-HCO3-SO4 Ca-Mg-HCO3-SO4-Table3continued Water type ID Well 2019 3 3 3 3 3 3 3 WT WT WT WT WT WT WT WT3-2 WT3-3 WT3-4 WT3-5 WT3-6 WT3-7 WT3-8 05JR 09JR 08JR 11JR 12JR 13JR EZ 1 WT WT1-1 Cen2 1 WT WT1-2 Cen1--D.L:
(1) Dissolved inorganic carbon in groundwater(DIC),mainly due to deep input from interaction with carbonate rocks and metamorphic carbonate rocks(RC1701,DD1705,and D1706)mainly in wells PTol02,SRosa09,SJMer,SMGpe,PV03,FT,10JR,SMGPe,11JR,Salit,13JR,Pozos,and Rom.These processes have already been observed in previous studies in the study area(Moran-Ramı´rez et al.2020;Morales-Arredondo and Armienta 2020)and have been confirmed by Landa-Arreguı´n et al.(2021)who observed in some hot springs the reprecipitation of carbonates on the surface,and b).The Ptol02 well is located near a deep fault.Most of the wells are drilled to depths between 250 and 300 m.Only the Salit well reaches a depth of 520 m.Near this well,the piezometric level is located at 1590 m.a.s.l.In these wells,a deep contribution of CO2is suggested due to the presence of a carbonate basement that is affected by geothermal.
(2) The proportion of carbon isotopes in shallow groundwater is related to a carbon source dominated by incorporation of surface water or due to processes occurring in the soil(e.g.C4 plants habitually with small additions of dissolved carbon in groundwater),principally in the upper horizons(mainly in wells CG14,DIF04,JUM02,JUM 01,and 08JR),or even the incorporation of poor-quality surface water.Most of the wells are drilled at depths between 100 to 200 m.Near these wells,the piezometric level is located at 1660 m.a.s.l.According to the suggested possible sources,it is observed that the wells that present a higher isotopic modification ofδ18O and δ2H(indicating a certain degree of evaporation)are of the WT-5 water type and the WT-3 type.These are the same wells that present a more negativeδ13C isotopic signature(Figs.4 and 5).Around the world,severalδ13C results obtained in shallow groundwater and nitrate-contaminated water are in the range of-12‰to-19‰,these studies have linked this isotopic behavior to the interaction of water with atmospheric and soil CO2,where carbon comes from organic and inorganic sources in an open system,and where recharge is rapid with a lower reaction rate between rock and water(Warner et al.2013;Nowak et al.2017;Ogrinc et al.2019;Li et al.2020).In addition,degradation of OM releases CO2from the soil,which can influence the concentration and isotopic signature of DIC for both an open and a closed system,or even in an isotopic versus kinetic equilibrium system(Cronin et al.2005).Considering that in the study area C4 type plants dominate,the influence of plants on the isotopic signature may be due to the influence of corn and sorghum that are grown in the region,since they have a C4 photosynthetic tracer(Fig.5a and b)(Clark and Fitz 1997),as observed in a study conducted in groundwater where C3 or C4 plants dominate in the region.(Awad 2014;Ogrinc et al.2019).In the case of corn,theδ13C isotopic signature varies approximately from-15‰-10‰because these plants have a third and fourth carbon atom in the glucose chain enriched inδ13C(Hana et al.2010),always taking into account the kinetic isotopic fractionation of approximately 4,4‰during CO2diffusion(Cerling et al.1991).

Fig.5 Behavior of the isotopic signature ofδ13C of the sampled groundwater wells(red squares,green triangle,purple diamonds,yellow square with cross)and collected rocks(green rhombuses)in the study area during years a 2017 and b 2019.The Fig.shows various environments according to isotopic behavior ofδ13C(Clark,2015),samples WT4-1,WT4-3,WT4-6,WT4-17,WT4-18 and WT5-8 were collected in both years
4.1.4 Isotopic behavior ofδ18O,δ13C and the hydrogeochemistry of NO3-
In the groundwater samples,the behavior ofδ18O with respect to the NO3increase andδ13C depletion was compared to identify the different hydrogeological environments in which all these elements may or may not interact,such as the contribution of atmospheric and soil CO2in groundwater,evaporation affecting theδ18O signature,dissolutionweathering reactions in mineral rocks,the transformation of nitrogen species,or a mixture of these various processes.Some research usingδ13C to define sources of nitrate contamination suggests that the isotopic signature of surface water that is rapidly incorporated into a shallow aquifer is in the range of-13‰and-10‰,this behavior is related to the presence of organic matter and its degradation or the incorporation of atmospheric CO2(Ogrinc et al.2019).It was also considered that throughout the region,agricultural and livestock activities are intense and generally included the application of chemical fertilizer or urea,in addition to soil nitrification and atmospheric deposition.The fertilizers mainly used in the agricultural fields in the study area are ammonium nitrate,ammonium sulfate,and urea(SEMARNAT 2019;Morales-Arredondo et al.2020).There is also a wastewater treatment plant that disposes of its waste on the same land(Morales-Arredondo et al.).These activities can incorporate organic matter into the aquifer and generate a change in isotopicδ13C and hydrogeochemical behavior(Yue et al.2018),mainly due to the degradation of organic matter and the generation of carbonic acid(Ogrinc et al.2019;Akbar et al.2020;Sabagh et al.2021).
It is necessary to consider that the groundwater acquired its hydrogeochemical properties by processes such as silicate weathering(due to the hydrothermal influence)and ion exchange(because of the water that circulates through the clay materials in the valley)in the study area(Moran-Ramirez et al.2021;Landa-Arreguı´n et al.2021).The CO2present in groundwater may interact,accelerating some of these processes(Clark 2015).In addition,three flow systems were identified:local(WT1,WT2,and WT5),intermediate(WT3),and regional(WT4),which were mixed in the center of the Valley.The presence of geological faults facilitates mixing with the thermal flow(regional flow)(Morales-Arredondo and Armienta 2020;Moran-Ramı´rez et al.2020).
According to Fig.6a and b,an isotopic enrichment of δ18O from the year 2017 to the year 2019 occurred(In addition to that observed in samples collected in 2017).This enrichment coincides with wells that show high values of NO3-in WT4 water samples,although previous studies suggest that theδ18O isotopic enrichment is a product of evaporation processes at ordinary temperatures(Morales-Arredondo and Armienta 2020;Landa-Arreguı´n et al.2021).This coincidence suggests that water with high nitrate content has undergone evaporation and could be water from recent infiltration only in well PV03.Water samples from WT 4 do not show much variation to LMWL and GMWL,as their variation is controlled by water-rock interaction(more negative values),even in wells with lowtemperature geothermal features(Morales-Arredondo and Armienta 2020).
Close to regions dominated by WT 4 wells,the piezometric level is located at 1590 m.a.s.l.The greatest withdrawal is observed in this area,moreover for wells from the groundwater samples of WT 4 that show a depth between 170 to 300 m.The deepest wells reach 520 m depth;likewise,several deep faults are observed near some wells.For 2019,δ18O isotopic enrichment still occurs,but unlike 2017,two completely different trends are observed as NO3-content increases,specifically for WT3 and WT5 water samples(Fig.6a and b).As observed in Fig.5a and b,the WT3 and WT5 water samples could represent the intervention of biogenic carbon related to processes occurring in the soil(e.g.microbial decomposition or organic matter)or due to photosynthetic cycles of C4-type plants,typical of maize and sorghum crops,which are quite common in the study area.Another possibility could be that theδ13C is a product of the dissolved inorganic carbon common in groundwater due to superficial inputs(e.g.,atmospheric in addition to the soil contribution),which may contribute substantially to the incorporation of carbon dioxide(CO2).Both situations would imply that there is infiltration of surface water(e.g.,irrigation water),which would have an important role in mineralization in an environment dominated by C4-type vegetation(Vogel 1993;Hu 2010;Ogrinc et al.2019;Akbar et al.2020;Sabagh et al.2021)or other sources that are not yet clarified for shallow wells(150 m).Further studies are necessary to define the specific source.In addition,the rocks that hosted the aquifer are volcanic with high permeability(by fracturing)(Fig.1).
Another important point is that some water samples of the WT3(e.g.,05 JR,08JR)exceed the maximum allowable limit for NO3-concentrations according to the Mexican drinking water standard NOM-127(45 mg/L).These wells are located next to a wastewater treatment plant that disposes of its wastewater without any environmental control,as previously reported(Morales-Arredondo and Armienta 2020,Morales-Arredondo et al.2020)(Fig.6c and d).These sampled wells reach a depth of nearly 250 m,and here the piezometric level is close to 1660 m.a.s.l.
The assessment of theδ18O value generated by nitrification is complex,mainly due to the complexity involved in biological processes in soils(Mengis et al.2001;Yue et al.2018).However,Vandekerckhove et al.(2019)reported that during the ammonia and nitrite oxidation,a slight increase inδ13C abundance is generated from the biomass attributed to autotrophic patterns other than nitrification.Moreover,various authors have identified changes in the HCO3-concentration in water from biogeochemical sources,which even impact carbon behavior and control the occurrence of denitrification in groundwater(Hartland et al.2011),limiting the nitrification range and possibly modifying theδ13C isotopic signature(Zhang et al.2014;Mohammadzadeh and Clark 2011).Van Breukelen et al(2003)observed increasedδ13C values in the landfill leachate plume(where degradation and denitrification take place in redox environments),andδ13C isotopic depletion was observed in water samples showingδ18O enrichment by evaporation processes(Fig.6c and d).To confirm or rule out whether any of these processes occur in groundwater,it is vital to conduct a more detailed study to evaluate the behavior of reactive nitrogen species and15N isotopic behavior.
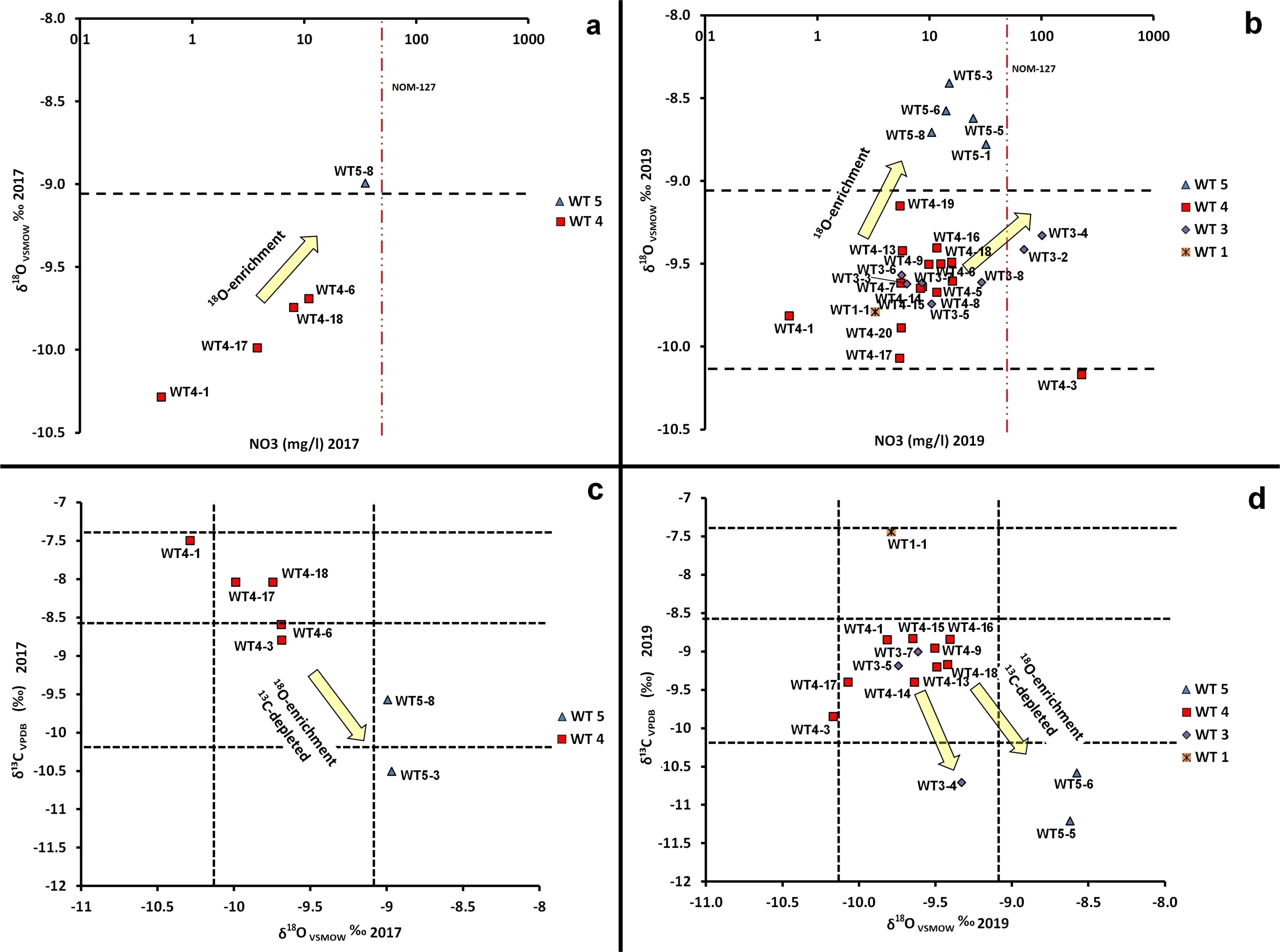
Fig.6 Isotopic signature ofδ18O andδ13C of the groundwater samples considering a nitrate concentration versusδ18O in 2017,b nitrate concentration versusδ18O in 2019,c behavior ofδ13C versusδ18O in 2017,d behavior of theδ13C versusδ18O isotopes in 2019.Red dotted line represents the maximum limit allowed for the concentration of NO3-in drinking water,according to NOM-127,black dotted lines represent a reference within the graph to visualize the isotopic modification ofδ13C andδ18O
The suggestion to use15N isotopes is because,during the transformation of the reactive N species,carbon participates in the reactions listed below(Eqs.1 to 4),as reported by several research studies(Sabagh et al.2021),and which are more intense due to climate change,as the increase in CO2has a significant influence on most of the important biological processes such as photosynthesis,respiration,hormone signaling,and antioxidant defense,as well as other significant tributary metabolic processors in crop plants(Sing and Agrawal 2015;Sabagh et al.2021).Furthermore,the isotopic and hydrogeochemical characteristics could be related to bacterial participation in the nitrification-denitrification processes and modify theδ13C isotopic signature as other studies have noted(Warner et al.2013;Nowak et al.2017;Ogrinc et al.2019;Li et al.2020).

Dissolved inorganic carbon dominates as a species in groundwater(Aravena et al.1995);however,in some cases,the presence of dissolved organic carbon(fulvic acids,humic acids,amino acids,proteins,etc.)and methane(CH4)become important carbon species(Hounslow,1999),which regularly come from microbial processes in the soil,organic matter oxidation,and even methane oxidation(Han and Plummer 2016).During organic matter oxidation(O2consumption)or respiration by microorganisms and plants,CO2(g)is generated,which is released or dissolved in the water producing carbonic acid H2CO3(aq),in turn leading to precipitation of dissolved carbonate as insoluble carbonate(Wigley,1975).The elevated presence of CO2can affect plant metabolic processes(Singh et al.2015;Rae et a.2017;Sabagh et al.2021).Within these processes,isotopic fractionation occurs,which modifies the isotopic behavior ofδ13C(Fontes and Garnier 1979)increasing the heavy isotopes ofδ13C in groundwater contaminated with nitrogen species(Zhang et al.2014).Because the excessive agricultural activity is taking place in the studied area,it is necessary to consider the possible modification of theδ13C isotopic signature in the entire region.
In reducing environments,nitrate is consumed by anaerobic bacteria that use NO3-as electron acceptors,since nitrate reduction is an alternative respiratory pathway that helps to maintain the redox and energy balance of the cell under hypoxia(George et al.2012).These bacteria actively participate in the evolution of carbon in groundwater,fixing it as an energy source and accelerating reactions that would not occur without their presence(e.g.,Eq.5)(Clark 2015).These bacteria also participate in the isotopic fractionation ofδ13C(Clark and Fitz 1997).Some reactions that account for the contribution of C to the medium through the oxidation of organic matter are summarized below:

Oxidation of organic matter can generate more negative values ofδ13C in groundwater(Coetsiers and Walraevens 2009),as degradation of dissolved organic carbon(DOC)product of various organic sources that depend on the surface environment of each region as other works have reported (e.g.,aquatic photosynthesis,photooxidation,DOC degradation,as well as microbial respiration)(Salifu et al.2020).In some cases,C contributions to the water are very high,especially when FeOOH,SO42-and to a lesser extent MnO2reduction occurs,including carbonate dehydration by acidity from Fe3+hydrolysis due to mixing of groundwater with surface water and subsequent diffusive loss of CO2(g)(Salifuf et al.2020).However,when only nitrate reduction occurs,the modification of the isotopic behavior ofδ13C values is very low and even null(Clark and Fitz 1997)by physical and biological processes causing slight but often consistent variations in natural abundance(Doods and Whiles 2020),except when manure is applied or when contamination is related to wastewater incorporation into groundwater(Eqs.1 and 5),two situations that occur in the study area(Fig.1).
During 2017 and 2019,δ13C depletion was observed,mainly in the WT5 water samples and in one WT3 sample(Fig.6e and f).These water samples showδ13C isotopic signatures similar to those associated with a C4 plantdominated carbon source(Cronin et al.2005;Awad 2014;Ogrinc et al.2019).Therefore,it is suggested that theδ13C modification could be related to the sum of several of the processes mentioned above.These processes include first evaporation,then nitrification and denitrification,which generate isotopic modification(Eqs.1 to 4),and subsequently the involvement of carbon during nitrate reduction in the medium(Eq.5),which results inδ13C depletion as has been observed in other studies,as mentioned above(Doods and Whiles 2020;Salifuf et al.2020).These processes do not necessarily have to be simultaneous,such as the oxygen depletion that occurs when water migrates from the upper recharge zones(even by irrigation or precipitation)to the confined areas of the aquifer(Craig 1961;Wassenaar et al.2009;Awad 2014).
To evaluate the reactions linked to the increased nitrate concentration and isotopic modification ofδ13C,an evaluation of the behavior ofδ13C versus NO3-was conducted for samples collected in 2017 and 2019.Initially,it was observed that samples with the higher NO3-content presented aδ13C depletion in both years,mainly for water samples from WT5 in 2017,and one sample from WT3 and another for WT4.
It was also observed that for 2019,δ13C depletion showed three different directions,with the most intense depletion for WT5 water samples and the least intense depletion for water samples with higher nitrate content(Fig.7c and d).This behavior indicates a possible relationship between increasing NO3-concentration andδ13C depletion in the aquifer.It has been proposed that anaerobic respiration during microbial metabolism processes could be involved in the isotopic modification ofδ13C(denitrification)through carbon fixation processes as an energy source since organic carbon is required for these reactions to occur(Aravena et al.1995;Han and Plummer 2016),an evaluation usingδ13C andδ15N isotopes is recommended to confirm or rule out this process,as they have done in environments whereδ15N,13C,and2H values have been interpreted together to identify dissolved inorganic C uptake,N2fixation,NH4and NO3assimilation by phytoplankton communities(Musat et al.2012),mixotrophy(Terrado et al.2017),symbioses(Thompson et al.2012),C processing by cable bacteria(Vasquez-Cardenas et al.2015)and consortia of microbes in sediments(Hatzenpichler et al.2016).This generally does not occur in oxygenated water,where aerobic bacteria outperform denitrifiers for available carbon substrates(Clark 2015).Importantly,denitrification does not occur in water with low nitrate concentration and the transformation of nitrogen species is highly dependent on the oxidation-reduction conditions of the groundwater.
Some authors have also noted that higherδ18O enrichment can be attributed to strong silicate weathering(Paces and Sˇmejkal 2004),In the State of Guanajuato,the use of the13C isotopic signature in groundwater has helped to define that the dissolution of carbonates influences the presence of CO2in the aquifer,and that shallow water infiltration also occurs through faults and fractures in specific zones(Horst et al.2007),in other studies,C isotopes have been used to determine vertical transmissivity of the aquifer,using geochemical corrections of solute evolution(Phillips et al.1989).Mahlknecht et al(2004;2006)use hydrogeochemical and isotopic tools to evaluate water quality in the Independence aquifer,considering the geochemical evolution and mineralization throughout the area where they consider carbonate dissolution,silicate alteration,manganese-containing silicate alteration,and contamination from agricultural and livestock activities.Horst et al(2008)identified with13C a CO2 influence by exchange with soil and with carbonate dissolution,along with the flow patterns.Silicate weathering processes have been reported in the study area using hydrogeochemical tools(Morales-Arredondo et al.2017),but it must be considered that the slow infiltration of rainwater can produce significant evaporation before reaching the aquifer(Burns and Kendall 2002)and even the arid characteristics of a site can affect the isotopic signature by increasing evaporation processes,affecting the behavior ofδ18O and generating a slope in the local meteoric line of 5.6(this situation was observed in 2017,but in 2019,the value decreased to a value of 4.73).
Another process that can affect the isotopic signature of δ13C in groundwater,in addition to biochemical reactions,is the precipitation of CaCO3in alkaline waters(Eq.6),as well as other studies near the study area,have observed(Horst et al.2007;2008;Mahlknecht et al.2004;2006).The saturation index obtained in a previous study in the region(Morales-Arredondo and Armienta 2020)confirms calcite precipitation in the open system of WT5 water samples,and that this process plays an important role in water mineralization-demineralization.

To evaluate the occurrence of reactions related to carbonate formation,the behavior ofδ13C versus HCO3-was compared(Fig.7).From 2017 to 2019,depletion ofδ13C was observed in all water types,but it was more marked in WT4 water samples(Fig.7a and b).On the other hand,it is evident that with increasing HCO3-content in the environment,δ13C depletion occurred in both years,mainly for WT5 water(in 2017 and 2019)and in one WT3 water sample(in 2019)(Fig.7a and b).The isotopic depletion of δ13C can be explained considering the products of Eq.5,where it was observed that during the oxidation of organic matter through the reduction of nitrates,HCO3-increased and CO2was generated(Fig.7c and d).

Fig.7 Isotopic behavior ofδ13C of the groundwater samples considering a the concentration of HCO3-versusδ13C in 2017,b the concentration of HCO3-versusδ13C in 2019,c the concentration of NO3-versusδ13C in 2017,d the concentration of NO3-versusδ13C in 2019.The red dotted line represents the maximum limit allowed for the concentration of NO3-in drinking water,according to NOM-127.Black dotted lines represent a reference within the graph to visualize the isotopic modification ofδ13C andδ18O
All these evaluations allow inferring that there is an incorporation of CO2to the aquifer due to superficial processes coming from the soil and the atmosphere,but the dissolution of carbonates and silicate weathering,incorporates CO2to the aquifer,all these processes modify the isotopic signature of the groundwater in the zone.
5 Conclusions
The behavior of theδ2H andδ18O values of the groundwater samples indicate a meteoric origin,and their changes to the global and Mexican meteoric water line indicate surface evaporation processes,although some other processes are not ruled out,such as the incorporation of gases from processes occurring in the soil or surface water migration(or even CO2from the atmosphere),which would mainly affect the behavior ofδ13C.In addition,the isotopic origin ofδ13C was defined in the rocks and groundwater of the volcanic thermal sedimentary aquifer of the western portion of the aquifer,where carbonate rocks(mainly metamorphic)contribute carbon dioxide to deep wells(mainly WT1 and WT4),as evidenced by the enrichment ofδ13C closer to this type of rock,besides atmospheric contribution,there is no evidence of isotopic modification resulting from thermalism in the region.
In the superficial aquifer(mainly in some wells of WT5 and WT3),decomposition processes of organic matter in the soil can be a contribution to the water,generating a modification in the isotopic signature of the stable isotopes of C,H,and O(e.g.the bacterial metabolic reactions can be involved in nitrification-denitrification processes to produce NO3-),and it is possible that some bacteria reduce nitrate by organic matter decomposition,generating carbon dioxide.An evaluation withδ13C andδ15N isotopes is recommended to confirm or rule out these processes.The13C isotopic results may suggest the participation of C4-type plants in carbon assimilation-release processes in groundwater.The isotopic signature also suggests thatδ13C is a product of the dissolved inorganic carbon common in groundwater due to superficial inputs such as atmosphere and soils.Therefore,this process should be confirmed or ruled out in future research in the area,since it would provide evidence that the excessive agriculture developed in the region generates contamination to the aquifer and that this incorporation of CO2into the environment can accelerate some mineral dissolution-alteration processes.
From 2017 to 2019,the analyzed water samples from the Celaya Valley Aquifer showed depletion inδ13C from one year to the next.More detailed studies need to be developed to define whether agriculture affects water quality.Theδ13C depletion could be due to calcite precipitation in a specific region of the study area.It is suggested that the joint application of trace elements as Sr or Br,as well as the isotopic values ofδ13C,and NO3-and HCO3-concentrations,can be used to initiate an assessment to identify possible carbon sources related to contamination by agriculture and wastewater treatment or by the natural environment.The use of this hydrogeochemical tool can reduce costs in the study of environmental problems.
It is necessary to consider an evaluation related to groundwater age since the depletion ofδ13C in the Celaya Valley Aquifer can be related to this factor.It is recommended to include information about the infiltration time of the water in the Celaya Valley Aquifer in the different studied locations.This point is important since the depth of the wells is generally shallow or intermediate(between 150 and 300 m),and different infiltration times may be identified between them that would help clarify the influence of the infiltration of contaminants on groundwater.
AcknowledgementsThe study carried out in the region was financed by PAPIIT,and the grant number is IA101019.The authors would like to thank PAPIIT for its support and the scholarships provided to students Cuellar Ramı´rez E.The authors are thankful for the support of O.Cruz,and A.Aguayo from the Analytical Chemistry Laboratory at the Instituto de Geofisica,UNAM and Giron P.from the X-ray Fluorescence Laboratory.LANGEM.Institute of Geology-UNAM.We also thank R.Flores-Vargas for their help in the field campaigns.
Data AvailabilityAll data generated and analyzed during this study are included in this published article and its supplementary information files.
Declarations
Conflict of interestOn behalf of all authors,the corresponding author states that there is no conflict of interest.
杂志排行
Acta Geochimica的其它文章
- A molecular simulation study of Cs-Cl and Cs-F ion pairs in hydrothermal fluids
- Hydrocarbon distribution along the Soapaga thrust(Eastern Cordillera,Colombia)based on new strategic geochemistry samples
- Genesis and magma fertility of gold associated high-K granites:LA-ICP-MS zircon trace element and REEs constraint from Bakoshi-Gadanya granites in NW Nigeria
- Organic geochemistry characteristics of Jurassic black shales from the Amdo area,northern Tibet,China
- Using zircon saturation thermometry of source magma in strongly altered volcanic ashes
- Rare earth element(REE)geochemistry of different colored fluorites from the Baoshan Cu-Pb-Zn deposit,Southern Hunan,South China
