ssDNA functionalized nanodiamonds for uranium decorporation
2022-07-09QinglongYanYuMiaoXiaomeiWangJifeiMaJuanDiwuYingZhuaShuaoWangChunhaiFan
Qinglong Yan, Yu Miao, Xiaomei Wang, Jifei Ma, Juan Diwu, Ying Zhua,,*,Shuao Wang, Chunhai Fan
a Interdisciplinary Research Center, Shanghai Synchrotron Radiation Facility, Zhangjiang Laboratory, Shanghai Advanced Research Institute, Chinese Academy of Sciences, University of Chinese Academy of Sciences, Shanghai 201210, China
b Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China
c State Key Laboratory of Radiation Medicine and Protection, School for Radiological and Interdisciplinary Sciences (RAD-X) and Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, Soochow University, Suzhou 215123, China
d School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and National Center for Translational Medicine,Shanghai Jiao Tong University, Shanghai 200240, China
ABSTRACT The hunt for agents that are suitable for actinide decorporation to reduce the whole-body load of actinide in accidental internal exposure is the ever-lasting goal in radiation protection and medical treatment in nuclear emergency.All current decorporation agents can be categorized as two groups, one is the molecular ligands, and the other is the nanoparticles decorated with molecular ligands.Here in this work, functional nanodiamonds (fNDs) with ssDNA (the endogenous biomacromolecule rich in phosphate groups) loaded on the NDs is reported, which poses good uranyl adsorption selectivity, high cellular uptake, fast excretion, and effective decorporation of uranyl from rat renal proximal tubular epithelial cells(NRK-52E).All those results corroborate that fNDs can potentially serve as a brand new family of chelators for actinide decorporation.
Keywords:Nanodiamond ssDNA Uranium Decorporation
Accidental internal exposure of actinides especially uranium is one of the health risks that practitioners in the nuclear industry and the researchers in related fields are facing due to the chemo and radio toxicity of actinides [1–5].To deal with the actinide internal contamination, chelation therapy has been the most common approach by using decorporation agents that selectively complex with actinide ionsin vivo.Currently, molecular chelators have been the most developed agents, but the drawbacks of those molecular chelators include but not limited to poor uranium decorporation ability, fast clearancein vivo, or high toxicity [6].For instance, the only clinical approved diethylenetriamine pentaacetic acid (DTPA) salts fail to remove uranium efficiently [7].Besides, hydroxypyridinone (HOPO) ligands that are permitted for clinical trials show limited uranium bone decorporation efficacy [8,9].Catecholates (CAMs), hydroxamates, and polyphosphonate ligands have been evaluated for their actinide decorporation efficacies, but toxic side effects have been observed, thereby restraining their application in actinide decorporation [10–13].
Functional nanoparticles modified with the aforementioned ligands have been considered as effective solutions to improve the decorporation performance.There have been several successful outcomes.The DTPA modified liposomes exhibit greatly extended blood circulation time comparing with the DTPA ligands [14].The chitosan oligosaccharide (COS) nanoparticles with HOPO ligand decorated on the surface are reported with much lower cytotoxicity and improved decorporation performance compared with the HOPO ligand [15].Fe3O4magnetic nanoparticles grafted with bisphosphonate could effectively remove uranyl ions from the blood[16].However, improvements of factors such as biocompatibility,toxicity, and decorporation ratio are still required, which is the goal for the development of the next generation agents.
The endogenous macromolecules such as nucleic acids, peptides, proteins, have already been used in thein vitroquantification of uranium, the decontamination of actinide in the environment, and the selective extraction of uranium from seawater.For instance, deoxyribonucleic acids (DNA) are abundant in phosphonate groups, the metal binding affinity of which toward actinide ions is superior to that of HOPO ligands [17].Therefore, Wanget al.developed a DNA based sensor for highly sensitive uranyl detection in water [18].Luet al.reported a uranyl-specific DNAzyme(single strand DNA fragments) modified AuNP (gold nanoparticles)and demonstrated that the material could serve as an intracellular uranyl sensor with high specificity [19].Recently, Wanget al.polymerized DNAzyme into DNA-based uranium extraction hydrogel(DNA-UEH), and achieved high adsorption capacity (6.06 mg/g) and selectivity for uranium in natural seawater [20].Detailed theoretical and synchrotron radiation characterization of DNA and uranyl binding modes demonstrated that DNA strands bind with uranyl using the phosphonate moieties [21].However, to our best knowledge, decorporation of actinide using DNA based materials remains to be an unexplored area.
Furthermore, an appropriate carrier is required to facilitate the uptake of DNA by cells, as well as avert its degradation in organism.Nanodiamonds (NDs) have been proven to be a new multifunctional drug delivery system and used in biomedical and pharmaceutical applications due to its high stability, biocompatibility,and low toxicity [22].Besides, we have previously illustrated that functional NDs, with individual particle sizes of 2–10 nm and the cluster size of ~200 nm [23–25], loaded therapeutic nucleic acids could enter cells with an increased rate of three orders of magnitudes compared with the control groups without NDs and accumulate in kidneys due to the protection of “sponge-like” NDs [26].
Herein, the uranium removal effect of single strain deoxyribonucleic acid (ssDNA) fragments was investigated, demonstrating their high uranyl binding affinity and selectivity.Then functional nanodiamonds (fNDs) were obtained by loading ssDNA fragments on NDs to improve the stabilityin vivoand the cell uptake rate.The selectivity, cellular metabolic kinetics, and uranium removal efficiency of fNDs was investigated and the results show that fNDs could sufficiently enter the cells and decorporate uranyl effectively,suggesting the potential of ssDNA functionalized NDs as a brandnew type of actinide decorporation agents.
Firstly, a NDs-based decorporation agent was obtained by complexing NDs with negatively charged ssDNA (rich in phosphate groups)viathe simple electrostatic interaction with the NDs/ssDNA w/w ratio of 10:1 (Fig.1a) [26].After the ssDNA adsorption, transmission electron microscopy (TEM) images showed that DNA complexation made the sharp surface of NDs attained a more rounded morphology (Fig.1b).The mean size of functionalized NDs (fNDs) was increased from ~230 nm to ~242 nm, and their zeta potential was shifted from 31.1 eV to -16.5 eV (Fig.S1 in Supporting information).Ultraviolet (UV) spectra further confirmed the encapsulation of NDs by ssDNA (Fig.S2 in Supporting information).
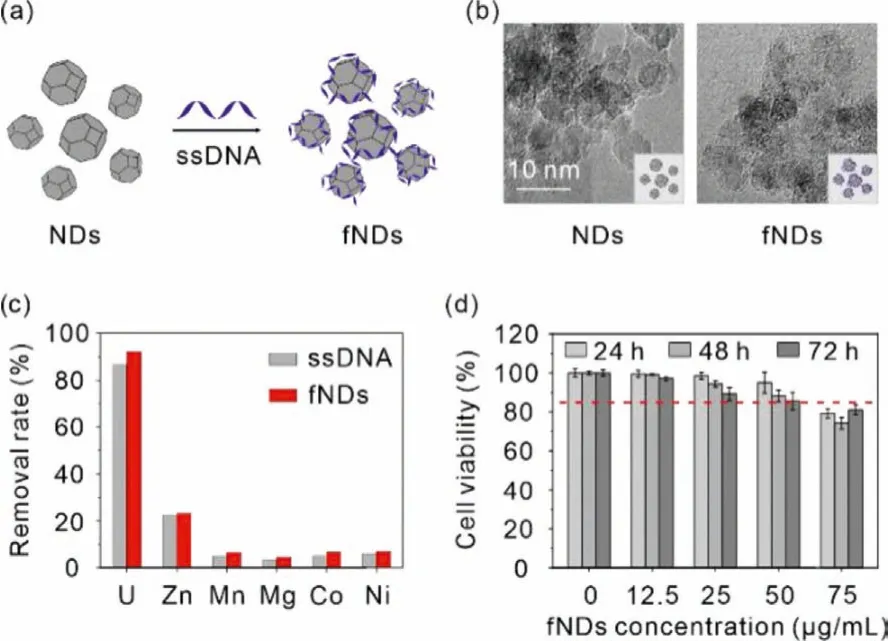
Fig.1.(a) Schematic showing of the preparation of fNDs.(b) High magnification TEM images of fNDs.Scale bar: 10 nm.(c) Adsorption behavior of ssDNA and fNDs toward uranium and essential metal ions (pH 7.4 tris-HCl buffer, m/v=0.04 g/L for ssDNA and m/v=0.44 g/L for fNDs, T=310 K, the initial concentration of uranium and other metal ions were 4.2 ppm and 40–50 ppm, respectively).(d) Dosagedependent cell growth rate of NRK-52E cells treated with fNDs for 24, 48, 72 h.Results are expressed as the mean ± SD.
Then,in vitrocompetitive adsorption experiment was carried out to evaluate the selectivity of fNDs for uranyl over the essential metal ions (Zn(II), Mn(II), Mg(II), Co(II), Ni(II)) in Tris–HCl buffer(pH 7.4).As shown in Fig.1c, ssDNA could remove 86.7% of uranyl in the presence of excess (approximately 10 times) divalent metal ions, and a slightly higher uranyl removal efficiency of 92.1% of fNDs was obtained.In both system, no more than 7.0% of essential metal ions were adsorbed except for Zn(II) (22.6%).These results conclude that both ssDNA and fNDs exhibit high selectivity toward uranylin vivo, indicating that the removal of uranyl is highly effi-cient without pathogenic loss of essential metals.
Furthermore, to better understand the adsorption behavior of uranyl, TEM mapping was performed by incubating fNDs with uranyl aqueous solution for 12 h.As presented in Fig.S3 (Supporting information), TEM images and the corresponding energydispersive X-ray spectroscopy (EDX) mapping images reveal the homogeneous elemental distribution of C and P in fNDs with and without uranium adsorption.Hence, it is concluded that the uranyl is evenly adsorbed and distributed on fNDs particles.
Having demonstrated the efficient adsorption of uranium by fNDs, we then assessed the cytotoxicity of fNDs to rat renal proximal tubular epithelial cells (NRK-52E) through MTT assay to determine the appropriate concentration for the subsequent assays.Specifically, NRK-52E cells were co-treated with 12.4 μmol/L uranium and different concentrations of fNDs ranging from 0 to 75.0 μg/mL for 24, 48 and 72 h, respectively.For the cells treated for 24 h, the cell viability slightly decreased from 100% to 79.2%with the increase of the concentration of fNDs.Prolonging the treatment time to 48 h, cell viability showed a similar trend as the cells treated for 24 h, and decreased to 74.3% at the point of 75.0 μg/mL.The cell viability of NRK-52E cells treated for 72 h continued to decrease in comparison to the cells treated for 24 and 48 h.All the three groups showed dose-dependent drug toxicity.Since no apparent cytotoxicity was observed after being treated with 50 μg/mL fNDs (Fig.1d), this concentration was chosen for subsequent intracellular uranium removal analysis.
The cellular metabolic kinetics of fNDs were studiedviathe cellular uptake and clearance assays.The cellular uptake assays of fNDs were performed firstly to certify the advantage of NDs loaded with ssDNA on the cellular uptake by culturing NRK-52E cells in the medium containing 50.0 μg/mL fNDs labeled with a red-color fluorophore Alexa647 and then quantified using confocal laser scanning microscope (CLSM) [26].As shown in Fig.2a, bright fluorescent dots were observed throughout the cytoplasm of cells when incubated with fNDs for 12 h, implying the efficient cellular uptake of fNDs.The mean fluorescence intensity (MFI) in cells increased over time until the cytoplasm was saturated by red fluorescence at 24 h (Fig.2b).In contrast, the uptake of ssDNA was extremely limited for the NRK-52E cells under the identical experimental conditions (Fig S4 in Supporting information).Further assays were carried out to investigate the clearance rate of fNDs from the NRK-52E cells.After incubated with Alexa647 labeled fNDs for 24 h, the cells were washed twice to remove extracellular fNDs and fluorescently imaged at 0, 24 and 48 h, respectively, during which the fresh medium was changed every 12 h (Fig.2c).The fluorescence intensity of fNDs decreased sharply as extending the cells culture time to 48 h, corresponding to the quantitative analysis of MFI that the intracellular retention of fNDs was 31.5% and 17.5% of the initial fNDs at 24 and 48 h, respectively, as illustrated in the Figs.2d and e.
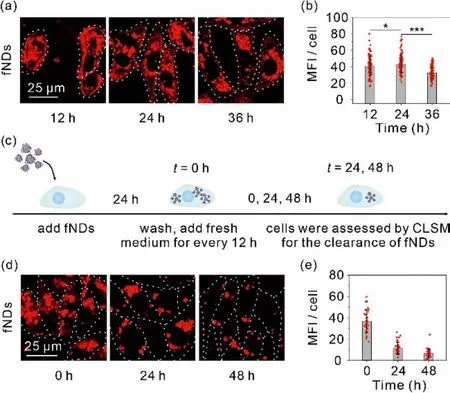
Fig.2.Cell uptake and clearance of fNDs.(a) Confocal images of cells and (b) MFI inside the cells after 12, 24 and 36 h incubation.(c) Schematic showing of cells clearance of fNDs.(d) Confocal images of cells and (e) MFI inside the cells after 24 h incubation and at the intervals of 24 and 48 h after the initial 24 h incubation with fNDs.Scale bar: 25 μm.
Based on the cellular metabolic kinetics of fNDs, the fNDsmediated intracellular uranium removal efficiency was assessed by pre-incubating NRK-52E cells in the medium containing uranium(12.4 μmol/L) for 24 h, and then incubating in the medium containing 50.0 μg/mL of fNDs or DTPA-ZnNa3for 24 h, respectively, while the control group was only treated with medium (Fig.3a).By ICPMS analysis, we found the addition of fNDs can remove 61.5% and 77.9% of uranium from the NRK-52E cells at 24 and 48 h after the initial 24 h incubation, respectively, which is consistent with the efficiency of fNDs excretion from the cell by itself (Figs.3b and c).In contrast, the commercial DTPA-ZnNa3can remove only 22.9% at 24 h and 24.3% at 48 h of uranium under the identical experimental condition.
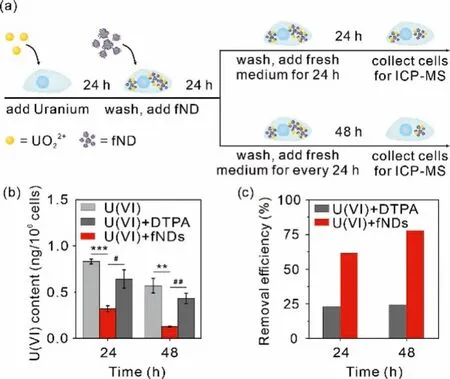
Fig.3.Intracellular uranium decorporation by fNDs.Cells were treated with 12.4 μmol/L uranium for 24 h, followed by 50 μg/mL fNDs for 24 h.(a) Schematic showing of intracellular uranium decorporation by fNDs.(b) Intracellular uranium content and (c) the uranium removal efficiency by fNDs.
In conclusion, we presented a NDs loaded with ssDNA (fNDs)as a new generation uranium chelator with high uranium removal efficiency at the cellular level for the first time.The competitive adsorption experiments demonstrate the good adsorption selectivity of fNDs for uranyl in the presence of essential metal ions under physiological pH.A series of cellular uptake and clearance analyses illustrate that NDs significantly prompt the uptake of ssDNA by NRK-52E cells and fNDs can be gradually excreted from cells.More importantly, the results ofin vitrodecorporation experiment show that fNDs can remove 77.9% uranium from the cells, which is remarkably higher than that of the clinical actinide decorporation agent DTPA.This work demonstrates the superiority of fNDs in uranyl chelation and its potential as the next generation actinides decorporation agents, which also opens a broad avenue for the utilization of macrobiomolecules in the field of decorporation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos.82050005, 21976127,22022410), the Youth Innovation Promotion Association of CAS(No.2016236), the Natural Science Foundation of Jiangsu Province(Nos.BK20190044, BK20210736), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the China Postdoctoral Science Foundation (No.2020M681431).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.052.
杂志排行
Chinese Chemical Letters的其它文章
- Efficient photoreduction strategy for uranium immobilization based on graphite carbon nitride/activated carbon nanocomposites
- Synergy of photocatalytic reduction and adsorption for boosting uranium removal with PMo12/UiO-66 heterojunction
- Carbon dots and carbon nitride composite for photocatalytic removal of uranium under air atmosphere
- Effect of ligand initial conformation and counteranion on complexation behaviors of R-BTBP toward Pd(II) contained in highly active liquid waste
- Temperature-responsive alkaline aqueous biphasic system for radioactive wastewater treatment
- Efficient gaseous iodine capture enhanced by charge-induced effect of covalent organic frameworks with dense tertiary-amine nodes
