Ventricular tachycardia induced by functional ventricular undersensing and AutoCapture®
2022-07-07ShingChingChiuSunYue
Shing Ching✉, Chiu Sun Yue
Division of Cardiology, Department of Medicine and Geriatrics, United Christian Hospital, Hong Kong, China
The implantable cardioverter-defibrillator(ICD) is a key component in the primary prevention of sudden cardiac death in patients with ischemic cardiomyopathy. Appropriate device programming is pivotal in maximizing the benefit as well as minimizing any proarrhythmic effect of device therapy. It is even more so for patients who require pacing for bradycardia, in which interaction of the device with intrinsic rhythm may generate unexpected consequences. We report a case of pacing induced ventricular tachycardia as a result of complex interplay of competitive atrial pacing, atrial and ventricular functional undersensing, ventricular functional loss of capture, and the AutoCapture®algorithm specific to Abbott devices.
A 70-year-old man with a dual chamber ICD (Ellipse™ DR 2377-36QC, St. Jude Medical [Abbott], St.Paul, MN) presented to the emergency department with his first ICD shock. He has a history of ischemic cardiomyopathy and sick sinus syndrome for which he received a dual-chamber ICD 4 years prior for primary prevention. On the latest follow up, the device parameters were nominal with no events. The patient reported that he was brisk walking at the time of ICD discharge.
The electrocardiogram (ECG) showed sinus rhythm and evidence of prior anterior myocardial infarct.Troponin was undetectable. ICD interrogation showed nominal device and lead parameters (Table 1). Auto-Capture®feature was activated.
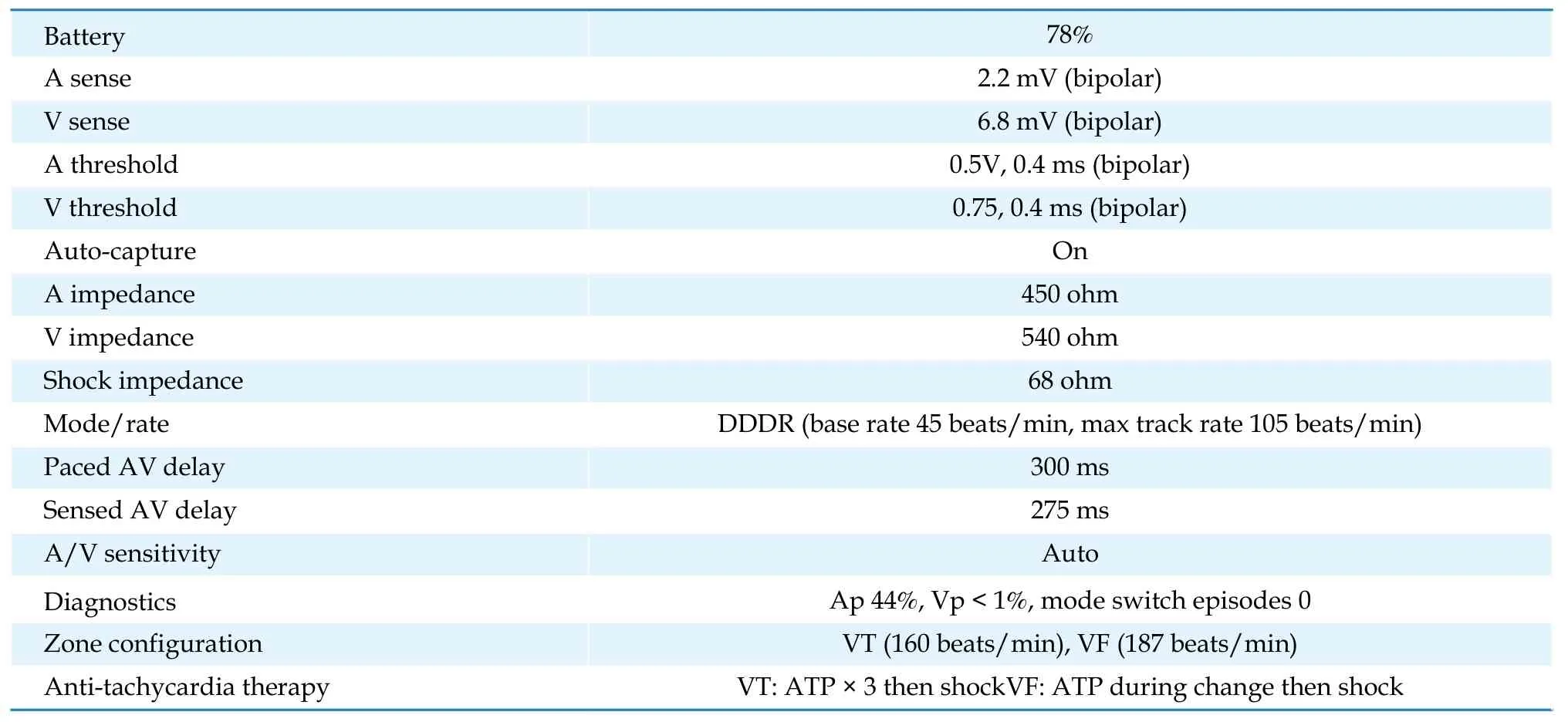
Table 1 Implantable cardioverter-defibrillator interrogation.
An episode of VT was recorded that correlated with patient’s time of symptom and was terminated witha shock (Figure 1A-1C). Analysis of the atrial channel revealed a regular intrinsic atrial signal at 150 beats/min interspaced with occasional atrial pacing (AP). The SIR marker indicates pacing at sensor indicated rate. Some of the intrinsic atrial signal was detected without AS marker (*) because they fell into the post ventricular atrial refractory period (PVARP)and would not reset the atrial timing cycle, resulting in competitive atrial pacing. As a consequence,some AP was delivered close to intrinsic atrial signal but did not capture due to refractoriness (*). In the ventricular channel, with the exception of the beat marked by (*), there was exclusively sensed ventricular signal. By studying the A-V relationship, it becomes clear that A-A cycle length variation always precedes that of V-V cycle length, favoring sinus or atrial tachycardia as the intrinsic rhythm. Importantly, VT was triggered after VP( →). On close scrutiny, as soon as an intrinsic atrial signal conducted to the ventricle ( ↘), an AP was delivered at sensor indicated rate due to functional atrial undersensing. The ventricular signal was blanked because it fell into the post atrial pacing ventricular blanking period (PAVB). Following a programed AV delay of 300 ms, a VP was delivered without capture due to ventricular refractoriness, which was identified by the AutoCapture®algorithm that delivered a backup pulse at high output (*), capturing the ventricle at a short R-R interval. It was followed by a premature ventricular beat(*) and monomorphic ventricular tachycardia ( →)that failed to terminate by anti-tachycardia pacing(ATP), only to be aborted by shock (Figure 1B-1C).

Figure 1 Functional ventricular undersensing in PAVB leading to R-on-T pacing followed by high output capture triggering VT.(A): Sinus or atrial tachycardia falling in the PVARP results in functional atrial undersensing (*) and fails to reset the atrial timer. AP is delivered at SIR rate with occasional LOC due to refractories (*). Coincidentally, a conducted ventricular beat ( ↘) encounters the post atrial PAVB and is not sensed. VP is delivered after a programmed AV delay but failed to capture because of refractoriness. AutoCapture® detects LOC with a high output backup stimulus (*) that captures but initiates a PVC (*) and VT ( →). (B): Anti-tachycardia pacing fails to terminate the VT ( →). (C): The VT is aborted by an ICD shock (HV). AP: atrial pacing; ICD: implantable cardioverter-defibrillator. LOC: loss of capture; PAVB: pacing ventricular blanking period; PVARP: post ventricular atrial refractory period; SIR: sensor indicated rate; VP: ventricular pacing; VPP: ventricular backup pacing; VS: ventricular sensed event.
Atrial functional undersensing and competitive atrial pacing is central to the events that led to VP in the ventricular vulnerable period inducing VT. The patient has sick sinus rhythm with atrial pacing but no ventricular pacing requirement. We increased the MTR to 155 bpm and deactivated AutoCapture®.Ventricular intrinsic preference®was activated to minimize ventricular pacing. The patient experienced no further VT episodes in the ensuing 6 months.
We described the first case of device induced VT due to inadvertent VP in the vulnerable period, contributed by an interplay of atrial and ventricular functional undersensing, competitive atrial pacing and the AutoCapture®feature of Abbott devices. Two factors favored the development of competitive atrial pacing. First, the patient was physically active with a sensor indicated rate at the programmed upper limit. Second, the intrinsic atrial rate exceeds the sensor indicated rate. Some of the intrinsic atrial signal would necessarily encounter PVARP and fail to reset the atrial timer, resulting in competitive atrial pacing. Although it does not induce VT directly, it would make the coincidental delivery of AP on a conducted ventricular beat a probable event in patients with intact AV node.
Following each AP, a post atrial pacing ventricular blanking period (PAVB) would elapse before a programmed AV delay is initiated (Figure 2). The duration of PAVB is not programmable in Abbott devices.It is a safety feature that prevents crosstalk and unintentional withholding of ventricular pacing. Any ventricular signal that fortuitously encounter PAVB is blanked and VP delivered upon expiry of AV delay.
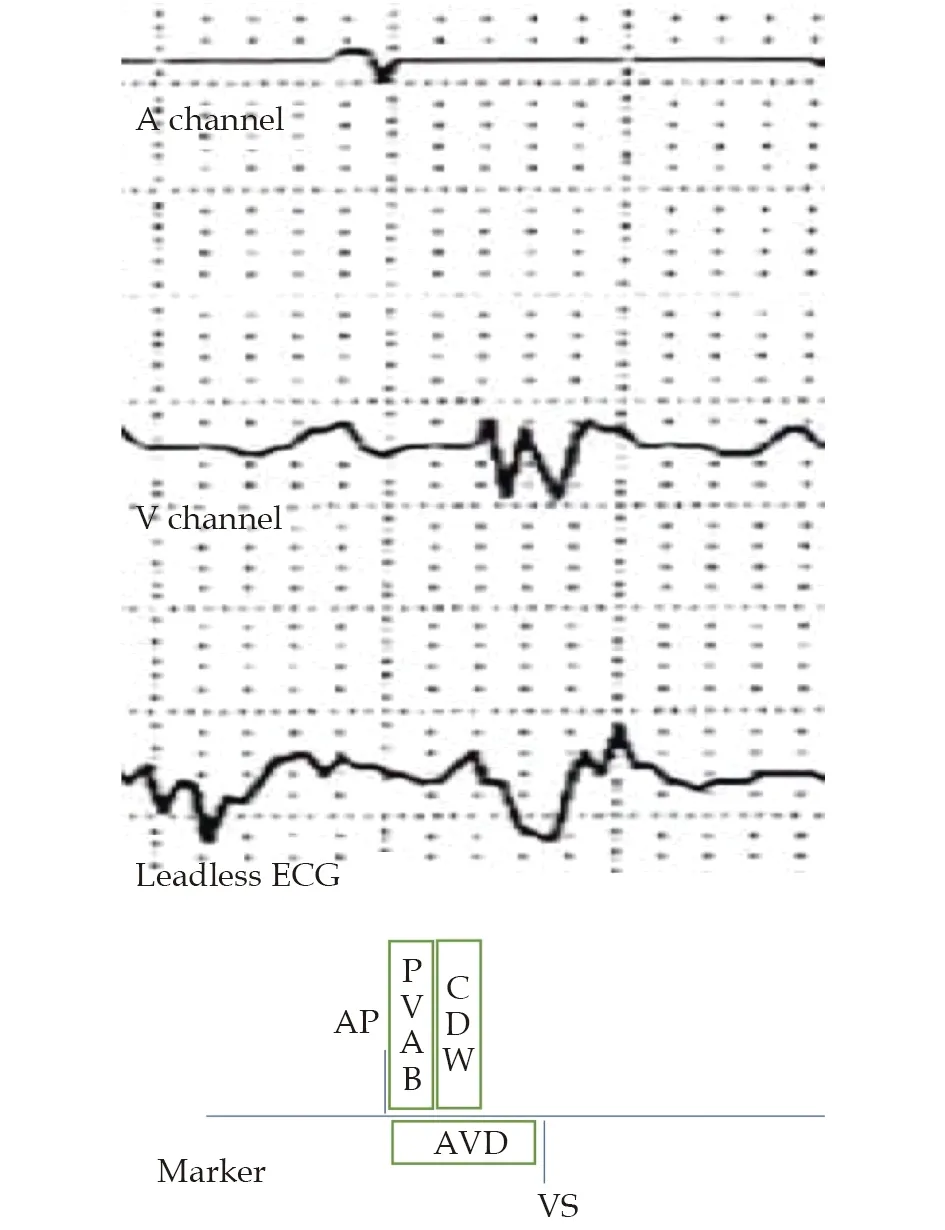
Figure 2 A fixed PAVB is initiated after each AP to prevent crosstalk from the AP stimulus, followed by CDW that prevents crosstalk from the atrial evoked response. AP: atrial pacing; AVD: AV delay; CDW: crosstalk detection window; PAVB:post atrial pacing ventricular blanking period; VS: ventricular sensed event.
Once an AP coincides with a ventricular beat, PAVB prevents the latter from being sensed and the AV delay ends with a VP, which predictably would land on the ventricular refractory period, given that the AV delay is programmed at 300 ms and the usual range of QT interval is 350 ms to 450 ms, resulting in ventricular loss of capture. The AutoCapture®algorithm is an Abbott device specific feature that continuously monitor the evoked response following VP to ascertain capture (Figure 3). In the event of loss of capture, a backup pulse of 5V would be delivered 80-100 ms after VP. Although intended to maintain constant ventricular capture, particularly for pacing dependent patients, the backup stimulus would find the ventricle in the relative refractory period and may capture, resulting in a short R-R coupling interval simulating an early coupled PVC that may encounter unidirectional block in the scarred myocardium, triggering monomorphic VT.

Figure 3 Inadvertent VP following an undersensed ventricular event. An intrinsically conducted ventricular event (*) encounters PAVB and is blanked. VP is delivered after an AV delay but did not capture due to refractoriness. LOC is sensed by AutoCapture® by the lack of ER, which responds with a high output stimulus 80-100 ms after VP that successfully captures the ventricle. ER: evoked response; LOC: loss of capture; P: backup stimulus; PAVB: pacing ventricular blanking period; VP:ventricular pacing.
The unintended blanking of ventricular signal in PAVB has been reported as a cause of device induced VT.[1-3]A common theme is frequent PVC that is blanked in PAVB with subsequent R-on-T pacing. In a background of post PVC pause and long-short cycle,polymorphic VT or torsade de pointe is triggered.However, pacing induced VT related to AutoCapture®has not been reported in sinus rhythm.
The issue of inappropriate blanking of ventricular signal by PAVB may be underrecognized with few reports in the literature. Our case shows that, in addition to PVCs, competitive atrial pacing in an active patient whose atrial rate is higher than the MTR/sensor rate can also seed the development of such phenomenon. To our knowledge, we reported the first case in the literature on pacing induced VT related to AutoCapture®in the absence of PVC.
Vogelgesang,et al.,[4]reported a similar case in a pacemaker patient with a fatal outcome unrelated to AutoCapture®. Although programming an MTR/sensor rate close to the estimated maximum heart rate is a solution, it can be challenging in patients who are prone to exertional atrial ectopics and short atrial runs. The atrial rate may easily exceed the programmed rate and be buried in the post ventricular atrial refractory period. In the presence of an intact AV node, the ventricular rate may accelerate with possible undersensing in PAVB. AutoCapture®aggravates the problem by ensuring capture in the relative refractory period, easily generating reentry in the diseased ventricle.
Reprogramming to AAI pacing is suggested to resolve the dilemma by avoiding ventricular pacing altogether.[4]However, up to 35% of patients with sick sinus syndrome may progress to atrioventricular block in the ensuring 5 years.[5]The development of atrioventricular block may be abrupt and unpredictable, making AAI a less favorable option. Algorithms that promote intrinsic conduction are preferred, such as Ventricular Intrinsic Preference®(VIP)by Abbott that periodically extends AV delay to search for conducted beats (positive AV hysteresis).They offer protection against unexpected atrioventricular block while reducing VP at the expense of possible increase in pacemaker mediated tachycardia (PMT), which is less likely in patients requiring little VP. VIP is particularly well suited for our patient who required predominantly AP and minimal VP. Indeed, since reprogramming to VIP the patient had had no further VT episodes in 6 months.
In conclusion, we presented the first case of pacing induced VT related to the AutoCapture®algorithm.High intrinsic atrial rate, low MTR and AutoCapture®contribute to inappropriate VP at a short coupling interval triggering VT. Proposed solution includes increasing MTR to approximate estimated maximum heart rate and activating VIP or similar algorithms that promote intrinsic conduction in patients with low VP requirement.
CONFLICT OF INTERESTS
None
杂志排行
Journal of Geriatric Cardiology的其它文章
- The coronavirus disease-19 pandemic and acute coronary syndrome: a specific impact in the elderly
- Fifteen-year mortality and prognostic factors in patients with dilated cardiomyopathy: persistent standardized application of drug therapy and strengthened management may bring about encouraging change in an aging society
- Comparison of the prognostic value of frailty assessment tools in patients aged ≥ 65 years hospitalized in a cardiac care unit with acute coronary syndrome
- Prospective application of a bleeding and ischemic risks-adjusted antithrombotic protocol in elderly patients revascularized with everolimus-eluting stents: EPIC05-Sierra75 study
- Using machine learning to aid treatment decision and risk assessment for severe three-vessel coronary artery disease
- Cardiovascular prevention in elderly patients
