Successful Treatment of Severe Pityriasis Rubra Pilaris with Cyclosporine A in An Infant
2022-07-06PingJiaoChenJingYaoLiangChangXingLiXiBaoZhang
Ping-Jiao Chen, Jing-Yao Liang, Chang-Xing Li, Xi-Bao Zhang,*
1Department of Dermatology of Southern Hospital, Southern Medical University, Guangzhou, Guangdong 510515, China;
2Institute of Dermatology, Guangzhou Medical University, Guangzhou, Guangdong 510095, China; 3Department of Dermatology,Guangzhou Institute of Dermatology, Guangzhou, Guangdong 510095, China.
Abstract
Keywords: pityriasis rubra pilaris, cyclosporine A, treatment
Intr oduction
Pityriasis rubra pilaris(PRP)is a rare chronic inflammatory disease,and its pathogenesis has not been fully elucidated.PRP is characterized as a papulosquamous disorder.1Some studies have suggested that PRP may be associated with infections, trauma, impaired immune responses, and dysfunction of vitaminA metabolismleadingtoa decreased supply of retinol in the epidermis. Furthermore, many studies have confirmed the involvement of an autosomal pattern of inheritance with a mutation of the Caspase Recruitment Domain Family Member (CARD)14 gene.1PRP can occur at any age.The diagnosis is based on both clinical and histopathological findings, but diagnosis is sometimes challenging because of the similarities between PRP and psoriasis.Many treatment options for PRP have been described in the medical literature, including topical agents, physical modalities, and multiple systemic agents.However, the responses vary among individual cases,particularly for severe cases and some cases of children.We herein describe an 18-month-old infant with severe PRP.The condition manifested as reddish follicular papules and inflammatory, infiltrated erythema over the whole body,including the trunk, legs, arms, face, and scalp, and the lesions showed a rapid and complete response to oral cyclosporine A(CsA).
Case report
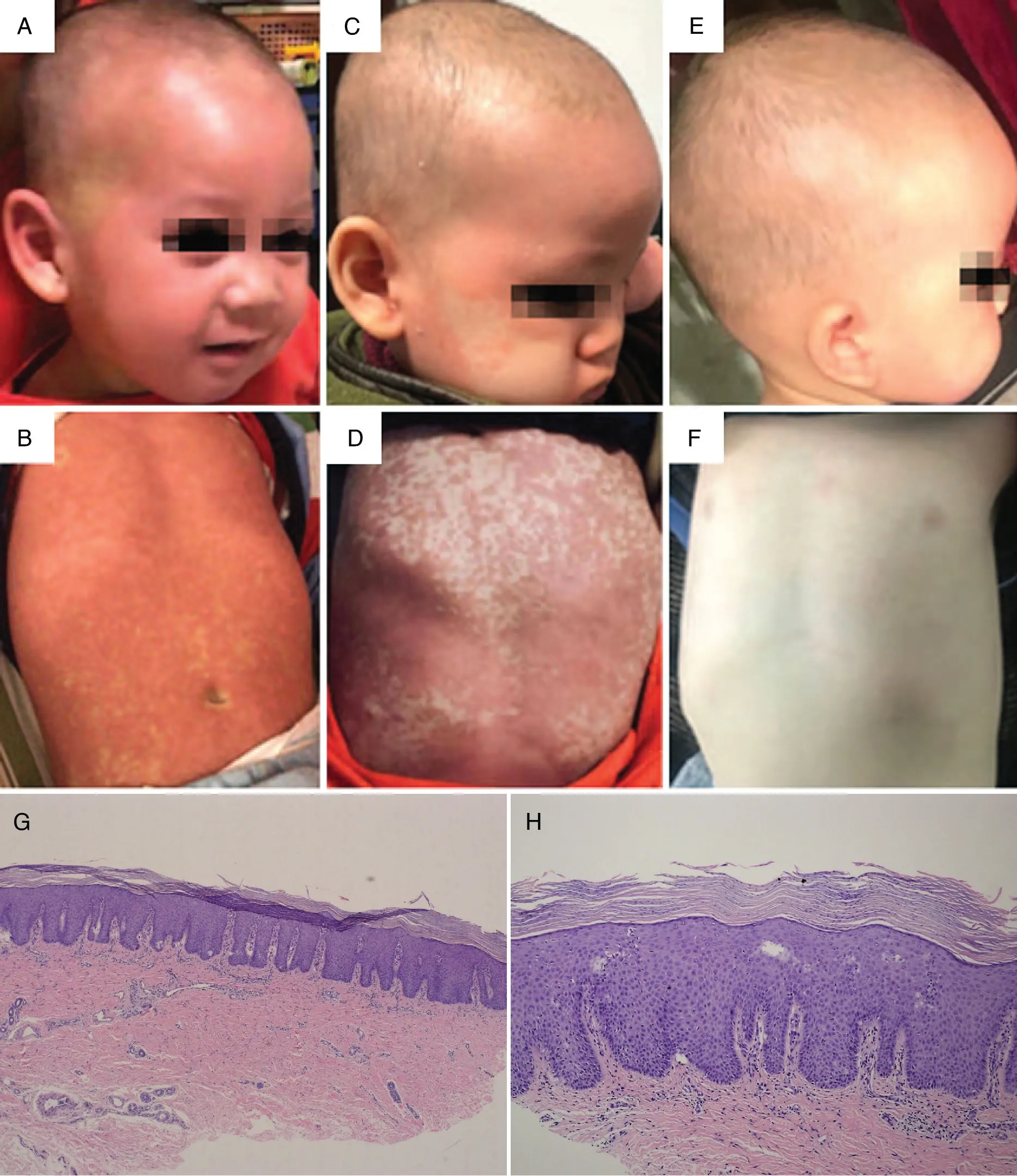
Figure 1. Clinical and histopathological features of the infant with severe pityriasis rubra pilaris.The thick scaly skin and erythroderma(A and B)regressed within 8weeks of treatment(C and D).Complete clearance of lesions was achieved within 16weeks of treatment(E and F),with no recurrence during 12 weeks of follow-up.The patient grew and developed normally,and his body weight increased by 3kg.Biopsy showed pityriasis rubra pilaris (G×100; H×200).
An 18-month-old boy was admitted to hospital with a 6-week history of an erythematous eruption affecting the trunk, legs, arms, scalp, and face. The lesions initially appeared as reddish-brown follicular papules with central keratosis. The papules tended to coalesce and form generalized hyperkeratotic, salmon-colored plaques with areas of uninvolved skin. On examination, follicular papules and plaques were found over the surface of the whole body(Fig.1A and B).The palms and soles showed waxy-yellow diffuse hyperkeratosis.A skin biopsy from the back showed psoriasiform acanthosis,alternating keratosis and para-keratosis, and a moderate lymphocytic perivascular infiltrate in the dermis(Fig.1G and H).
Type III PRP was diagnosed based on the clinical manifestations and histopathological findings.The infant was treated with topical low-potency corticosteroids and moisturizing cream. After 6 weeks, the skin lesions had shown no response, and the patient’s clinical condition rapidly progressed until the skin lesions covered the entire body surface.Treatment with retinoids and methotrexate(MTX), as recommended for adult-onset PRP, is problematic in prepubertal patients because of the toxic effects on the bone marrow and the patient’s growth and development.Therefore,for our young patient,oral CsA was chosen with an initiated dose of 3.0 mg/kg daily combined with topical emollients. The thick, scaly skin and erythroderma regressed within 8 weeks. During treatment, routine blood parameters, serum creatinine,serum urea nitrogen, liver function, and blood pressure were regularly monitored once a month and remained within the reference ranges. After remission of the skin lesions (Fig. 1C and D), the dose of CsA was gradually tapered and the treatment was stopped within 20 weeks(3 mg/day for 8 weeks,2 mg/day for 8 weeks,and 1 mg/day for 4 weeks).Complete clearance of lesions was achieved within 16 weeks (Fig. 1E and F), with no recurrence for 12 weeks of follow-up after stopping the CsA therapy.
Discussion
Several therapeutic approaches to PRP have been suggested, including vitamins, retinoids, antimetabolites,antibiotics, and psoralen plus ultraviolet A therapy.1-2Retinoids or MTX as the first-line treatment for adultonset PRP is recommended in many textbooks.3However,these recommendations are mainly focused on adults;the treatment of PRP in children remains problematic.1,4CsA is a systemic immunosuppressive agent that inhibits calcineurin. Adverse effects observed during therapy include nephrotoxicity,an increased risk of hypertension,hypertrichosis, and gingival hyperplasia. The dosage of CsA ranges from 2.5 to 5.0 mg/(kg·d)in clinical practice,and most patients respond to CsA therapy within 8 weeks.5Indeed, the efficacy and detailed mechanism of CsA for PRP remains unclear. The principal histological changes in patients with PRP are consistent with a proliferative pattern of keratinocytes, and some studies have also suggested that it inhibits the proliferation of keratinocytes in vitro.6Vujic et al.7described one patient who showed an excellent clinical response during prednisone and CsA therapy; however, treatment was discontinued because of severe adverse effects. Marsili et al.6described three patients who showed a good clinical response within 3 to 4 weeks of CsA therapy[5mg/(kg·d)],but two relapsed after dose reduction or discontinuation of CsA. Combination of CsA and extracorporeal photopheresis resulted in lesion resolution within several weeks.Our patient was only 18 months old,and the recommendation to administer retinoids and MTX in adults was not applicable. Moreover, the patient’s parents rejected the use of MTX,and the patient’s condition failed to respond to tacrolimus. With adequate communication and informed consent, CsA treatment was administrated,and provided excellent results. During more than 6 months of treatment and follow-up, the patient’s growth and development were normal and his body weight increased by 3 kg.
