Double Side Interfacial Optimization for Low-Temperature Stable CsPbI2Br Perovskite Solar Cells with High Efficiency Beyond 16%
2022-07-04JingMaJieSuZhenhuaLinJianHeLongZhouTaoLiJinchengZhangShengzhongLiuJingjingChangandYueHao
Jing Ma, Jie Su, Zhenhua Lin*, Jian He, Long Zhou, Tao Li, Jincheng Zhang, Shengzhong Liu,Jingjing Chang* , and Yue Hao
1. Introduction
Organic–inorganic hybrid perovskite materials have attracted so much attention and have been considered as one of the alternatives to silicon for next-generation solar cells due to their excellent optical absorption, tunable bandgap, favorable carrier mobility, and long lifetime.[1–4]Up to now, the certified power conversion efficiency (PCE) of perovskite solar cells (PSCs) has rapidly progressed to 25.5%from unstable 3.8%in the past decade.[5]Even so, the inevitable organic cations (FA+, MA+)induced thermal and moisture instability is one of the major limitations to the commercialization of PSCs.[6,7]To address this issue,the all-inorganic cesium lead halide perovskite(CsPbX3) debuted and achieved considerable progress in recent years because of their remarkable thermal stability with Cs as an alternative to organic cations.[8–11]
Among all the inorganic perovskites,the PSC based on CsPbI3with a favorable bandgap of~1.7 eV has made a high PCE of 20.37% by Yoon et al.[12]However, CsPbI3is easy to degrade from α-phase(black phase)to δ-phase(yellow phase)at room temperature or trace moisture owing to its low Goldschmidt tolerance factor(0.81),[13]and the temperature of phase transformation is over 315 °C.[14,15]In contrast, Goldschmidt tolerance factor of the mixedhalide CsPbI2Br increases to 0.84,[16]which promotes the stability of α-phase and decreases the phase transform temperature to 150 °C.[17,18]Therefore, the CsPbI2Br perovskite with a bandgap~1.9 eV shows a promising prospect for highly stable all-inorganic PSCs. Nevertheless, the limited short-circuit current density (Jsc) and large open-circuit voltage(Voc)loss(Elossdefined as Eloss= Eg/e - Voc)are two issues that need to be solved urgently. Interface engineering is an effective and common strategy to reduce the Eloss.[19]Up to now,Cao’s group proposed a dual interfacial engineering by combination with PN4N and PDCBT to obtain a great PCE of 16.2%with a high Vocof 1.30 V.[20]Our group modified SnO2surface with short-period deep-ultraviolet (DUV) photoactivation, decreasing the work function and achieving an elevated efficiency of 15.1% with a superior Vocof 1.22 V.[21]Shen et al.[22]reported a remarkable PCE of 16.42%with a Vocof 1.28 V via adopting ZnO by CsCO3modification which narrowed the ohmic loss to a negligible level.Very recently,Guo et al.[23]found the aging of SnCl2precursor solution as electron transport layer(ETL)could reduce the Elossto less than 0.50 V,bringing the champion Vocof 1.43 V. In summary, these researches similarly reduced the energy mismatching between CsPbI2Br perovskite and ETL/HTL to improve Vocas well as the quality of perovskite film, which is conducive to the enhancement of device performance.
Besides, it has been widely recognized that the defect sites at grain boundary and surface of perovskite film would become the trap/recombination centers.[24]Thus, surface passivation is also a universal method to suppress nonradiative carrier recombination within the perovskite film itself for high efficiency and stability of PSCs.[25,26]For instance, Zhao’s group reported that PTABr could effectively passivate CsPbI3film to obtain a record efficiency of 17.09% and high stability.[27]Zhang et al.[28]introduced rubidium (Rb) to CsPbI2Br perovskite with guanidinium bromide(GABr)post-treatment,leading to a high PCE of 15.6%. The much bigger inorganic cation could enlarge the value of tolerance factor even beyond 1 to form 2D-perovskite,like PEA2PbI4,which not only promoted the moisture stability of perovskite film but also effectively reduced trap density, suppressed the interface recombination,and improved the performance of PSCs.[29]Herein,we reduce the Elossvia a CsPbI2Br/ETL interface engineering approach by ZnO insert layer thickness modulation between ITO and SnO2, which is also beneficial to the growth of CsPbI2Br perovskite film.In addition,guanidinium iodide (GAI) interfacial layer is introduced to passivate CsPbI2Br perovskite top surface, align the energy levels, which further suppresses the interface charge recombination at CsPbI2Br/HTL(Spiro-OMeTAD) interface. Consequently, by optimizing the double side heterojunction interfaces, the optimized PSC based on CsPbI2Br perovskite delivers the highest PCE of 16.25%with an extraordinary high fill factor (FF) of 82.15%, a high Vocof 1.27 V, and an Elossas low as 0.61 V, while the thermal stability and long-term storage stability are largely promoted.
2. Results and Discussion
As well known, the energy barrier between cathode and conduction band of perovskite could lead to the accumulation of interface charges,and delay the rate of extraction and transfer of photogenerated carriers,resulting in severe interfacial charge recombination.SnO2is commonly used as an electron transporting material with ~4.3 eV of the conduction band,inevitably resulting in energy barrier at the interface,which would restrict the carrier transfer and extraction. Here, a lowtemperature solution-processed ZnO with low work function is applied to modify the surface work function of cathode, passivate the defects from the bottom of SnO2layer,and affect the morphology of SnO2ETL layer, which will be discussed later (Figure 1a). The possible reason should be the charge transfer between underneath ZnO and SnO2which can cause a nonstoichiometric ratio of SnOxdue to high oxygen-deficiency of ZnO.[30]The device structure is confirmed by cross-sectional scanning electron microscopy (SEM) of the device section(Figure 1b).The energy levels of SnO2and ZnO/SnO2bilayer ETL layer are characterized by Ultraviolet photoelectron spectroscopy(UPS)(Figure S1), and the results show that the fermi level of bilayer ETL is significantly upshifted to 3.92 eV compared to SnO2ETL,which could improve the interface band matching between CsPbI2Br and ETL. The corresponding energy bands are illustrated in Figure 1c,and the energy band of perovskite has been reported in our previous works.[17]The transmittance and bandgap of ETL are not influenced by ZnO insert layer (Figure S2). A better band matching has formed at the interface of ETL/CsPbI2Br by introducing ZnO insert layer, which could efficiently suppress interfacial recombination caused by energy barrier and enhance the built-in potential of devices.
To deeply investigate the beneficial effect of ZnO insert layer,the allinorganic PSCs with a planar structure of ITO/ZnO/SnO2/CsPbI2Br/Spiro-OMeTAD/Ag are prepared. Different thicknesses of ZnO insert layers are utilized and marked as A-ZnO(~36 nm),B-ZnO(~19 nm),C-ZnO (~10 nm), and D-ZnO (~6 nm) for convenience, respectively.The current density–voltage (J–V) characteristics of PSCs based on ZnO/SnO2ETLs are shown in Figure 2a,the extracted device parameters are summarized in Table S1, and other device parameter distributions are described in Figure S3.The devices based on ZnO/SnO2ETLs exhibit obvious enhancement in Voc, while the thick ZnO film(~36 nm)could seriously deteriorate the device FF,which might result from the poor electrical conductivity (Figure S4). Finally, the devices based on D-ZnO (~6 nm) give the best performance ascribing to the improvement of Voc(from 1.13 to 1.25 V) and FF (from 71.41% to 75.11%)without Jscloss.This enhancement could attribute to the beneficial effect of ultrathin ZnO insert layer which is commonly used in photovoltaic devices to suppress the carrier recombination.[31,32]The relationship between device response and light intensity is investigated(Figure S5),and the results demonstrate that the devices with or without ZnO insert layer both show a linear Jscversus light intensity relationship, indicating reduced trap-assisted recombination. In addition, the slope of the device with ZnO insert layer (1.49 KBT/q) is smaller than that without ZnO insert layer (1.95 KBT/q), suggesting that ZnO/SnO2bilayer definitely and effectively suppresses the trapassisted recombination in the devices. Moreover, to explain the Vocimprovement, the built-in potential (Vbi) is extracted based on Mott-Schottky equation via capacitance-voltage (C–V) measurement (Figure S6).[33]The Vbiof ZnO-modified device is dramatically enhanced to 1.22 V compared to that of the control device (1.08 V), implying that the ZnO-modified device possesses greater charge driving force to accelerate the extraction and transfer of photogenerated carriers.
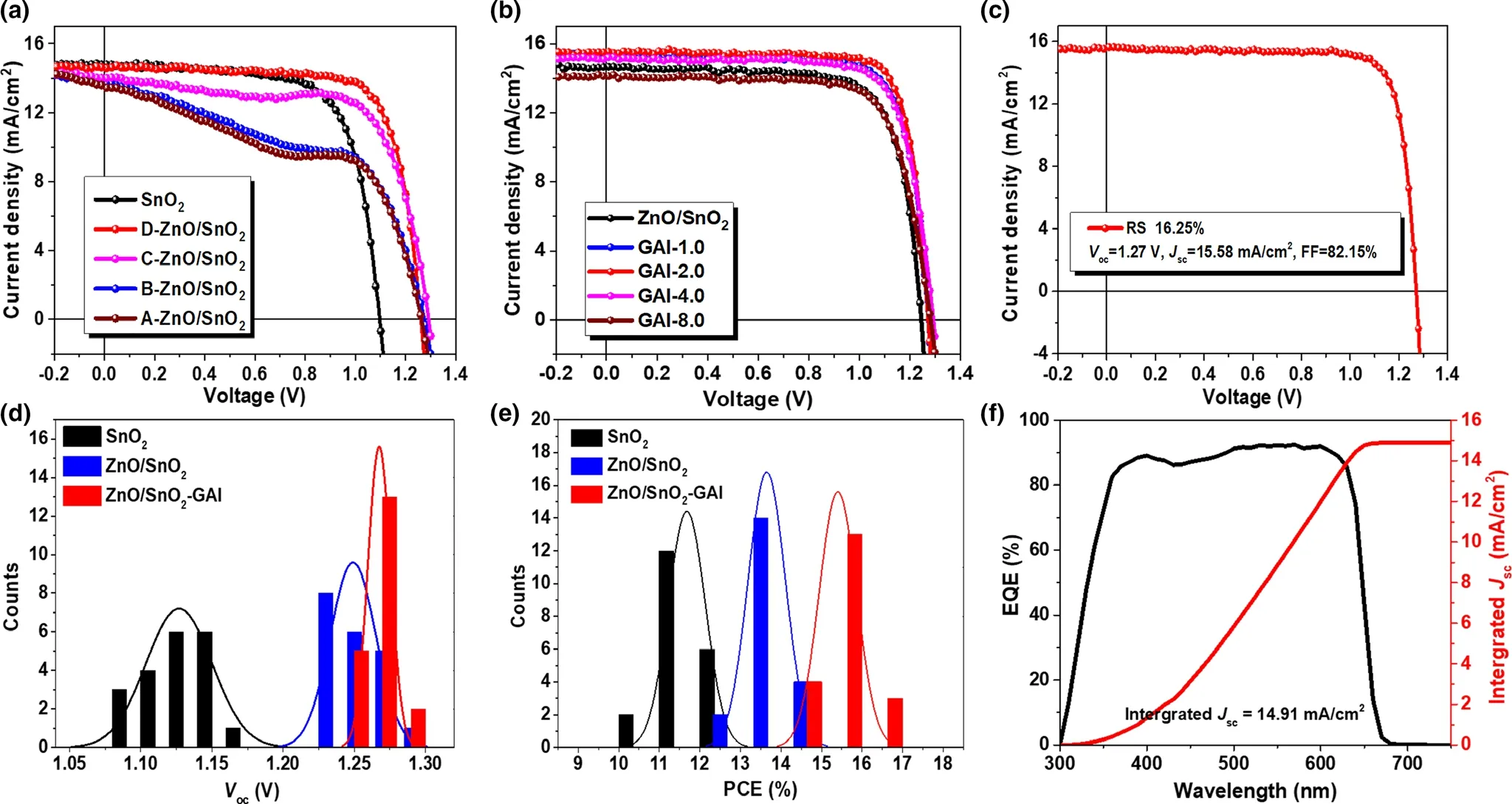
Figure 2. a) J–V characteristics of inorganic CsPbI2Br PSCs with and without ZnO interlayer. b) J–V characteristics of inorganic CsPbI2Br PSCs based in ZnO/SnO2 with and without GAI passivation. c) J–V characteristic of the best inorganic CsPbI2Br PSC. d) Voc and e) efficiency histogram of inorganic CsPbI2Br PSCs based on different conditions: SnO2 (dark), ZnO/SnO2 (blue), and ZnO/SnO2–GAI (red). f) The incident photo-to-current conversion efficiency (IPCE)spectrum.
To further improve the performance of CsPbI2Br inorganic PSCs, it is prevalent to optimize the quality of perovskite film via surface passivation for defects-related recombination reduction, as we referred above. It has been reported that guanidinium (GA) can inactivate under-coordinated halide species etc. to decouple electron-hole pairs,suppressing the recombination within perovskite film itself and enhancing the performance of PSCs.[34,35]Therefore, GAI solutions with different concentrations (1.0,2.0,4.0,and 8.0 mg mL-1) are treated on the top surface of CsPbI2Br perovskite films based on ZnO/SnO2ETLs after the annealing process.Noteworthy,the device with GAI treatment shows better performance with comprehensive improvement (Figure 2b, Figure S7, and Table S2). It is thought that the grain boundaries related defects of CsPbI2Br perovskite film are passivated by GAI,and energy levels are also aligned between perovskite and Spiro-OMeTAD, so nonradiative recombination is efficiently suppressed by GA passivation, causing the improvement of device performance. As a result, the best device of PSC deposited on ZnO/SnO2ETL with GAI passivation exhibits an excellent PCE of 16.25%with Voc,Jsc,and FF of 1.27 V,15.58 mA cm-2and 82.15%,respectively(Figure 2c).Moreover, the performance of devices shows satisfactory reproducibility(Figure 2d,e). The incident photo-to-current conversion efficiency(IPCE) spectrum shows an improved integrated Jscof 14.91 mA cm-2compared to pristine device (14.12 mA cm-2), as illustrated in Figure 2f and Figure S8,closely matching the measured Jsc.In addition,J–V hysteresis behavior has also been characterized, and the hysteresis index (HI) (decided by (PCEreverse- PCEforward)/PCEreverse) is calculated to 15.2%, as shown in Figure S9, which is effectively decreased comparing to that in our previous works.[17,21]The beneficial energy level alignments contribute to reducing ion accumulation at the interface,triggering the decreased HI and the enhanced Vocand FF.A stabilized power output (SPO) is also achieved with a PCE of 14.98% at maximum power point(Figure S10).
In order to elucidate the performance-enhancing mechanism behind the double side heterojunction optimization, the film characterizations are performed. The top-view scanning electron microscopy (SEM)images and water contact angle measurements of CsPbI2Br perovskite films with different conditions are shown in Figure 3a–c and Figures S11 and S12.The grain size of CsPbI2Br film based on ZnO/SnO2ETLs markedly increases to 300–600 nm with more uniform surface observed at low resolution compared to SnO2ETL, which attributes to the decreased root mean square (RMS) of ITO/SnO2with ZnO insert layer (Figure S13). Interestingly, the morphology of the CsPbI2Br film is hardly affected by the concentration of ZnO precursor (Figure S14).The better CsPbI2Br film deposited on ZnO/SnO2ETL contributes to the enhancement of efficiency and stability.Moreover,the grain boundaries show little blurry after GAI top surface treatment,as we estimated before, suppressing the uncoordinated ions at the surface of perovskite film.Many small particles remain on the surface,which should be precipitated GAI residue because of the sudden vaporization of the IPA solution annealing at 150 °C. This phenomenon would be more pronounced,and thus deteriorates the CsPbI2Br film at high concentrations(Figure S15), leading to the degradation of device performance as mentioned in Figure 2b.The absorptions of CsPbI2Br films at different conditions are characterized by UV-vis spectroscopy,and the results are shown in Figure S16. The light absorption of CsPbI2Br film is slightly improved by ZnO and combining ZnO with GAI passivation, respectively, and the bandgap of CsPbI2Br perovskite is determined to be 1.88 eV from the absorption onset. Hence, the Vocloss of the device remarkably decreases to 0.61 V via ZnO modification and GAI passivation from 0.74 V of the control device.
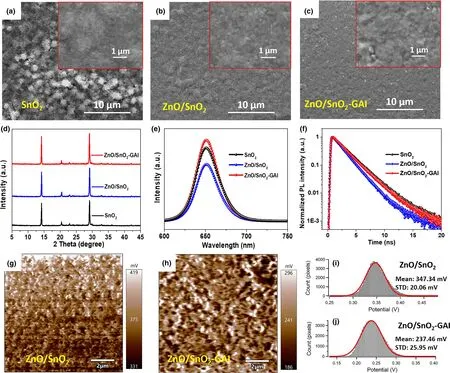
Figure 3. SEM images and magnified SEM images (inset) for the CsPbI2Br PSCs based on different conditions: a) SnO2, b) ZnO/SnO2, and c) ZnO/SnO2–GAI.d) X-ray diffraction (XRD) patterns of inorganic CsPbI2Br thin films based on different conditions. e) Steady-state photoluminescence (PL) spectra and f)normalized transient PL decay curves of inorganic CsPbI2Br PSCs based on different conditions: SnO2 (dark), ZnO/SnO2 (blue), and ZnO/SnO2–GAI (red). g–j)Surface potential maps and values from KPFM measurements for perovskite surface without and with GAI treatment.
The X-ray diffraction(XRD)patterns of the CsPbI2Br films with different conditions are shown in Figure 3d and Figure S17.Compared to the perovskite film deposited on SnO2ETL as a control film,the typical peak of CsPbI2Br film shows an obvious enhancement after ZnO insert layer introduction, while the crystalline of perovskite film is slightly improved by GAI passivation because the passivation position of GAI is at the surface of CsPbI2Br with low concentration. Nevertheless, as the effective radius rAeffof GA is too large(278 pm)to form 3D perovskite,it is possible that trace 2D-perovskite containing GA cation is formed on the surface of CsPbI2Br film after GAI passivation, which is conducive to stabilize the black phase and improve the stability of device performance.[36,37]This can be confirmed by the new 1D peaks that appeared in the XRD spectra (Figure S18). In addition, steady-state photoluminescence (PL) and time-resolved PL (TRPL) decay measurements have been characterized at different conditions from the side of perovskite films, as shown in Figure 3e,f and Figure S19. As a result,PL and TRPL patterns both show a trend of decrease first and then increase corresponding to the conditions of ZnO/SnO2bilayer ETLs and GAI-passivated CsPbI2Br perovskite. The quenching of PL intensity and reduction of carrier lifetime can be responsible for the beneficial energy level alignment between CsPbI2Br and ETLs,accelerating the rate of electron extraction and transfer from ETL to electrode(Figure S19a).Besides,the fitting results of TRPL curves are listed in Table S3.Nevertheless,when the perovskite film based on ZnO/SnO2condition is passivated by GAI, the quality of CsPbI2Br is improved as we analyzed above, leading to the enhancement of PL intensity and carrier lifetime.The surface potentials of the perovskite film surface before and after GAI treatment are further investigated by Kelvin probe force microscopy (KPFM) measurements (Figure 3g–j). The results show that a uniform surface potential decreases about 109 mV,corresponding to a work function increase after GAI treatment,which indicates an upward band bending occurred and beneficial energy level alignment between perovskite and Spiro-OMeTAD layer.Hence,better charge carrier transfer and extraction are observed.
To further understand the passivation mechanism of GAI,the microstructure and electronic properties of CsPbI2Br (001) surface which is the most stable perovskite surface with and without GAI are calculated by density functional theory (DFT). Both the I-interstitial (Ii) and Ivacancy (VI) at the CsI-terminated surface and Pb-vacancy (VPb) at the PbI-terminated surface are considered since these defects generally occur in the perovskite film surface. These defects usually introduce some defect states in the bandgap and serve as the nonradiative recombination center, and then prejudice the efficiency of PSC. However,upon introducing GAI passivation,its I ion could occupy the VI,meanwhile obvious charge transfers from the H atoms of GA+ cation to I ions of perovskite surface and from Pb atom of perovskite surface to the N atoms of GA+ cation (Figure 4a–c). As a result, the trap states are eliminated (Figure 4d–f), suggesting the reduced nonradiative recombination for GAI-passivated perovskite.To further confirm the interface interaction between GA cation and perovskite surface,X-ray photoelectron spectroscopy(XPS)measurements are taken.As we expected,an N 1s signal is present in the film with GAI, as shown in Figure S20a. Of course,the other elements,Pb,Br, and I are all detected to analyze the influence of GAI on the interaction of them (Figure S20b–d). It is found that peaks of all elements exhibit a slight shift to the higher binding energy after GAI passivation.The localized charge transfer from the H atoms of GA+cation to I ions of perovskite surface and from Pb atom of perovskite surface to the N atoms of GA+cation leads to the peak shift of XPS patterns.[35,38]
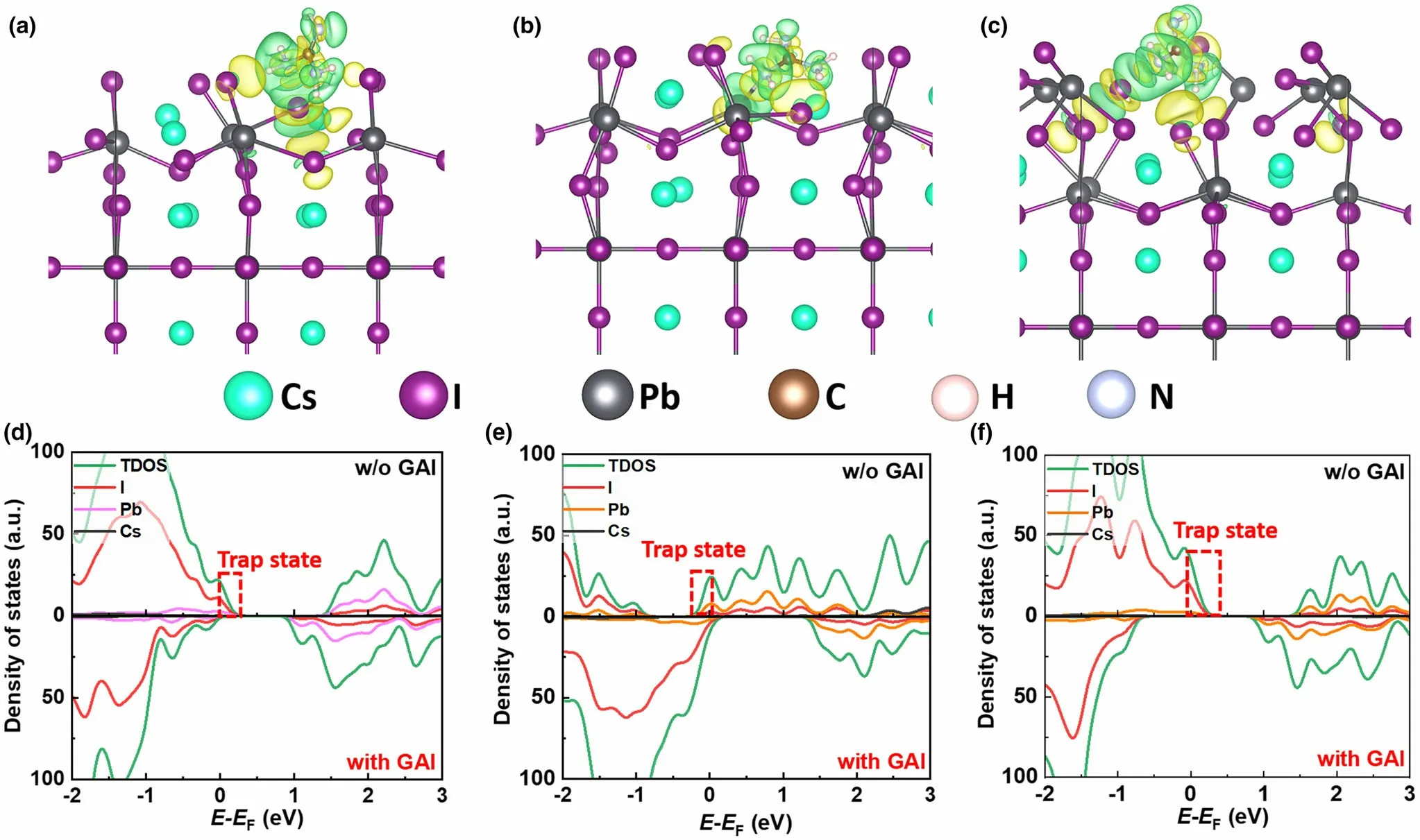
Figure 4. Charge density difference of GAI-passivated perovskite surface with a) Ii, b) VI, and c) VPb. The green and yellow regions represent the electron depletion and accumulation, respectively. Density of states of GAI-passivated and unpassivated perovskite surface with d) Ii, e) VI, and f) VPb.

Figure 5. a) Transient photocurrent, b) transient photovoltage measurements, c) Nyquist plots results of inorganic CsPbI2Br PSCs based on different conditions. d) Dark J–V curves for the electron-only devices with structure (ITO/ETL/perovskite/PCBM/Ag) based on different conditions: SnO2 (dark), ZnO/SnO2(blue), and ZnO/SnO2–GAI (red).
The mechanisms of charge transfer and recombination of devices at different conditions have been explored by transient photocurrent(TPC), transient photovoltage (TPV), and electrochemical impedance spectroscopy (EIS) measurements (Figure 5a–c). The fitting results of TPV and TPC show that the charge-transfer lifetimes of SnO2,ZnO/SnO2,and ZnO/SnO2–GAI are 1.68, 0.98, and 0.71 µs, respectively, while the recombination lifetimes of them are 82.17, 104.36,and 131.88 µs, respectively. The significantly reduced transfer lifetime and increased recombination lifetime of the device with both ZnO and GAI treatment indicate that the efficiency of extraction and transport photogenerated charge carriers is accelerated and the recombination of carriers at the interface is effectively inhibited. Moreover, the recombination resistance(Rrec)of devices based on different conditions is fitted and the fitting parameters are summarized in Table S4. It is found that the device based on ZnO/SnO2–GAI shows largest Rrecvalue compared to others,consistent with TPV results.Besides,the space-charge-limited current(SCLC)technique is carried out to estimate the trap density(Nt)of perovskite films. According to the equation for the relationship between Ntand trap-filled limit voltage (VTFL): Nt= 2VTFLεrε0/eL2,where L (≈270 nm) is the thickness of CsPbI2Br perovskite film observed from Figure 1b, εr(= 8.6) is the relative dielectric constant for CsPbI2Br,[39]and VTFLis extracted from the measured dark J–V curves of the electron-only devices (Figure 5d), the trap densities of SnO2, ZnO/SnO2,and ZnO/SnO2–GAI are calculated to be 4.3 × 1015, 2.87 × 1015, and 2.09 × 1015cm-3, respectively. The reduction of trap density is mainly aided by the improved quality of perovskite based on ZnO/SnO2–GAI compared to the control device.
Finally, the unencapsulated CsPbI2Br PSCs based on SnO2, ZnO/SnO2,and ZnO/SnO2–GAI are tested under heating process and continuous light illumination of AM 1.5G in ambient air, respectively(Figure 6a–c).As a result,the PCE of device based on ZnO/SnO2–GAI remains 86%and 79%of initial values after continuous light illumination for 600 min in ambient air and continuous heating for 5 days at 85 °C in glove box, while the PCE of control device rapidly decreases to 40%and 50%,respectively.The improved light and thermal stability could be ascribed to the optimized quality of CsPbI2Br film by ZnO modification on ITO electrode and stabilized α-phase of perovskite film by GAI surface passivation. Moreover, we also recorded the long-term stability of the device by storing it in a drying oven(RH < 20%).The device based on ZnO/SnO2–GAI manifests brilliant stability compared to the control device, with the efficiency loss of 8% (modified) versus 40% (controlled) after the 60-day storage, as shown in Figure 6c. Therefore,the combination of ZnO and GAI passivation can effectively promote the light-soaking,thermal,and air stability of the device. The enhanced device stability could be confirmed by the defect formation energies and ion migration energy. The defect formation energies of Ii,VI,and VPbgenerally occurred at perovskite film surface with or without GAI is calculated (Figure 6d),and the results show that GAI passivation could efficiently suppress the formation of defects that existed in perovskite surface, which could powerfully contribute to the stability improvement of PSCs. Moreover, the migration of halogen atoms has been one of the critical reasons for hysteresis behavior and device performance deterioration. After GAI passivation,hydrogen bonding between hydrogen atoms from GA and Ii(N–H......I) can increase the migration energy of Ii(Figure 6e,f), thereby inhibiting the migration of halogen atoms, reducing hysteresis behavior (Figure S9), and improving the stability of devices.
3. Conclusion
In conclusion, a solution-processed ZnO interlayer is introduced into all-organic CsPbI2Br PSCs to reduce the work function and the roughness of cathode.At the same time,GAI is also used to align the energy levels between perovskite and HTL and passivate the top surface defects of perovskite film.As a result,through double side heterojunction engineering,a satisfying PCE of 16.25%has been realized with an excellent FF of 82.15%and a Vocof 1.27 V,attributing to the better band matching and enhanced charge extraction and transfer. Meanwhile, due to the significantly improved stability with obviously improved PCE of allorganic CsPbI2Br PSCs beyond 30%, we believe that the key to the development of inorganic perovskite is to promote the quality of perovskite film and reduce the Vocloss, which would facilitate the commercialization of inorganic PSCs.
4. Experimental Section
Materials: Lead iodide (PbI2, 99.999%), lead bromide (PbBr2, 99.999%) Spiro-OMeTAD(99.5%),tris[2-(1H-pyrazol-1-yl)-4-tertbutylpyridine]cobalt(III)tris[bis-(turfluoromethylsuflonyl)imide] (FK209), bis-(trifluoromethance) solfonimide lithium salt(LiTFSI)and all were purchased from Xi’an Polymer Light Technology Corp.Other materials,such as cesium iodide(CsI,99.998%)and SnO2colloid precursor (tin (IV) oxide) were purchased from Alfa-Aesar, while Dimethyl sulphoxide(DMSO, 99.8%), zinc acetate dihydrate (Zn(CH3COO)2∙2H2O, 99.99%) and 4-tBP (96% purity) were purchased from Sigma-Aldrich. All materials were used as received without further purification.
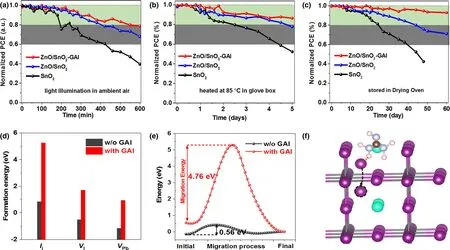
Figure 6. Stability of devices based on different conditions under a) continuous light illumination, b) continuous heating at 85 °C, and c) aging in drying oven (RH < 20%). d)Formation energies of Ii, VI, and VPb at the perovskite surface. e) The migration energy curves of I-interstitial at perovskite surface with and without GAI passivation, where the migration energy is marked by the arrows. f) The corresponding schematic of I-interstitial migration at perovskite surface (The lead atoms in the black circle are possible post-migration locations in red circle).
PVSC fabrication and characterization: The indium tin oxide (ITO) glass substrates (10 Ω sq-1) were cleaned by ultrasonic treatment with detergent,deionized water, acetone, and ethyl alcohol for 20 min and dried with nitrogen.Then, the ITO substrates were treated with UV-ozone for 20 min to remove organic residues.Preparation process of sol-gel ZnO is that zinc acetate and ethanolamine were dissolved in 10 ml 2-methoxyethanol solution in a 1:1 molar mass ratio to get 0.456 mol/L sol-gel ZnO solution marked as A-ZnO and rigorously stirred for 12 h at room temperature.Other concentrations of ZnO were diluted by volume ratio to 0.228,0.115,and 0.046 mol/L with 2-methoxyethanol solution and were labeled B-ZnO, C-ZnO, and D-ZnO for convenience, respectively. SnO2colloid precursor was diluted to 5% with deionized water. A thin layer of ZnO film was firstly spin-coated at 124.88 × g for 30 s with annealing process at 150 °C for 30 min. The following steps are SnO2nanoparticle layer was spincoated at 222.00×g for 45 s with annealing process at 150 °C for 30 min.And then PbI2:PbBr2:CsI(0.6 M:0.6 M:1.2 M)were dissolved in DMSO(1 mL)and stirred all night. The different concentrations of GAI were dissolved in IPA solution for later use. The prepared precursor solution was filtered with filter (0.22 µm). The precursor solution was spin-coated on the ZnO/SnO2substrate at 500 rpm for 5 s and 124.88 × g for 30 s. Then the CsPbI2Br film annealed via sequential graded thermal annealing process at 50 °C for 3 min and 150 °C for 10 min.After cooling down to the room temperature, the prepared GAI solutions were spin-coated on the surface of perovskite film and annealed at 150 °C for 3 min.Then, the Spiro-OMeTAD film was spin-coated on the CsPbI2Br perovskites film at 13.88×g for 10 s and 222.00×g for 30 s.The Spiro-OMeTAD solution contained 72.5 mg Spiro-OMeTAD,18 µL of LiTFSI stock solution(520 mg mL-1in acetonitrile) and 29 µL of FK209 solution (300 mg mL-1in acetonitrile) and 29 µL of tBP. Finally, 100 nm Ag electrodes were thermally evaporated to finish the device fabrication. All the devices had an effective area of 7.5 mm2defined by the metal shadow mask.
Materials and device characterization: The thicknesses of ZnO layers were determined by spectroscopic ellipsometer and step profiler. All the devices were measured using Keithley 2400 under standard solar simulator with an intensity of 100 mW cm-2. All the measurements of the solar cells were performed under ambient atmosphere at room temperature without encapsulation.IPCE measurements were carried out on the SCS10-X150 systems (Zolix Instrument. Co. Ltd).XRD measurements were performed by Bruker D8 Advance XRD. PL and TR-PL spectra were recorded using the Pico Quant Fluotime 300 with a 510 nm picosecond pulsed laser.The morphology characterization of the films was measured by SEM (JSM-7800F). XPS and UPS measurements were performed by the Escalab 250Xi with a source of monochromatic Al-Ka(1486.6 eV).Transient photocurrent(TPC)measurement was performed with a system excited by a 532 nm(1000 Hz,3.2 ns) pulse laser. Transient photovoltage (TPV) measurement was performed with the same system excited by a 405 nm (50 Hz, 20 ms) pulse laser. A digital oscilloscope (Tektronix, D4105) was used to record the photocurrent or photovoltage decay process with a sampling resistor of 50 Ω or 1 MΩ,respectively.
Simulation section: first-principles calculations: All calculations were based on density functional theory (DFT)[40]as implemented in the Vienna ab initio simulation package (VASP)[41,42]code with projector augmented wave (PAW)method.[43–45]The Perdew-Burke-Ernzerhof(PBE)functional within the generalized gradient approximation (GGA) was employed to describe the exchangecorrelation interaction.[46]The migration barriers were calculated using the climbing image nudged elastic band(CI-NEB)method[47,48]which is an efficient way to find saddle point configuration as implemented in VASP through the VTST tools.The plane-wave basis cutoff energy was set to be 400 eV.All structures were relaxed until the residual force on each atom less than 0.01 eV°A-1.The self-consistent convergence accuracy was set at 10-5eV atom-1in the structural calculation, and 10-7eV atom-1in the CI-NEB calculation. To simplify the calculation of CsPbI2Br with GAI,2 × 2 × 1 CsPbI3(001)surface with five atomic-layer was employed. A vacuum region more than 15°A in the zdirection in conjunction with the dipole correction to avoid the fictitious interaction with its periodic images.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (61704131 and 61804111), National Key Research and Development Program of China(Grant 2018YFB2202900),Key Research and Development Program of Shaanxi Province (Grant 2020GY-310), the Joint Research Funds of Department of Science & Technology of Shaanxi Province and Northwestern Polytechnical University(2020GXLH-Z-018),the Fundamental Research Funds for the Central Universities and the Innovation Fund of Xidian University.
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Informationis available from the Wiley Online Library or from the author.
Keywords
CsPbI2Br, dual interfacial optimization, high performance, low temperature,perovskite solar cells
Received: March 1, 2021
Revised: April 15, 2021
Published online: May 10, 2021
[1] Z. Hu, Z. Lin, J. Su, J. Zhang, J. Chang, Y. Hao, Sol. RRL 2019, 3, 1900304.
[2] J.Di,J.Du,Z.Lin,S.(Frank)Liu,J.Ouyang,J.Chang,InfoMat 2021,3,293.
[3] J. Di, J. Chang, S. (Frank) Liu, EcoMat 2020, 2, 1.
[4] B. Zhang, J. Su, X. Guo, L. Zhou, Z. Lin, L. Feng, J. Zhang, J. Chang, Y.Hao, Adv. Sci. 2020, 7, 1903044.
[5] National Renewable Energy Laboratory (NREL) 2020. https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200925.pdf (accessed:1 April 2021).
[6] S.Aharon,A.Dymshits,A.Rotem,L.Etgar,J.Mater.Chem.A 2015,3,9171.
[7] F. Lang, O. Shargaieva, V. V. Brus, H. C. Neitzert, J. Rappich, N. H.Nickel, Adv. Mater. 2018, 30, 1702905.
[8] T. Zhang, M. I. Dar, G. Li, F. Xu, N. Guo, M. Gr¨atzel, Y. Zhao, Sci. Adv.2017, 3, e1700841.
[9] L. Zhou, X. Guo, Z. Lin, J. Ma, J. Su, Z. Hu, C. Zhang, S. (Frank) Liu, J.Chang, Y. Hao, Nano Energy 2019, 60, 583.
[10] M. Yue, J. Su, P. Zhao, Z. Lin, J. Zhang, J. Chang, Y. Hao, Nano-Micro Lett. 2019, 11, 91.
[11] J. He, J. Su, Z. Ning, J. Ma, L. Zhou, Z. Lin, J. Zhang, S. Liu, J. Chang, Y.Hao, ACS Appl. Energy Mater. 2020, 3, 5173.
[12] S.M.Yoon,H.Min,J.B.Kim,G.Kim,K.S.Lee,S.I1 Seok,Joule 2021,5,183.
[13] W. Ahmad, J. Khan, G. Niu, J. Tang, Sol. RRL 2017, 1, 1700048.
[14] L.Protesescu,S.Yakunin,M.I.Bodnarchuk,F.Krieg,R.Caputo,C.H.Hendon,R.X.Yang,A.Walsh,M.V.Kovalenko,Nano Lett.2015,15,3692.
[15] A. K. Jena, A. Kulkarni, Y. Sanehira, M. Ikegami, T. Miyasaka, Chem.Mater. 2018, 30, 6668.
[16] Z. Zeng, J. Zhang, X. Gan, H. Sun, M. Shang, D. Hou, C. Lu, R. Chen, Y.Zhu, L. Han, Adv. Energy Mater. 2018, 8, 1801050.
[17] J. Ma, J. Su, Z. Lin, L. Zhou, J. He, J. Zhang, S. Liu, J. Chang, Y. Hao, Nano Energy 2020, 67, 104241.
[18] C. Liu, W. Li, C. Zhang, Y. Ma, J. Fan, Y. Mai, J. Am. Chem. Soc. 2018,140, 3825.
[19] L. Yan, Q. Xue, M. Liu, Z. Zhu, J. Tian, Z. Li, Z. Chen, Z. Chen, H. Yan,H. L. Yip, Y. Cao, Adv. Mater. 2018, 30, 1802509.
[20] J. Tian, Q. Xue, X. Tang, Y. Chen, N. Li, Z. Hu, T. Shi, X. Wang, F. Huang,C. J. Brabec, H. L. Yip, Y. Cao, Adv. Mater. 2019, 31, 1901152.
[21] L. Zhou, J. Su, Z. Lin, X. Guo, J. Ma, L. Feng, J. Zhang, S. Wang, S.(Frank) Liu, J. Chang, Y. Hao, Sol. RRL 2020, 4, 2000001.
[22] E. C. Shen, J. De Chen, Y. Tian, Y. X. Luo, Y. Shen, Q. Sun, T. Y. Jin, G.Z. Shi, Y. Q. Li, J. X. Tang, Adv. Sci. 2020, 7, 1921952.
[23] Z. Guo, A. K. Jena, I. Takei, G. M. Kim, M. A. Kamarudin, Y. Sanehira, A.Ishii,Y.Numata,S.Hayase,T.Miyasaka,J.Am.Chem.Soc.2020,142,9725.
[24] A. Abate, M. Saliba, D. J. Hollman, S. D. Stranks, K. Wojciechowski, R.Avolio, G. Grancini, A. Petrozza, H. J. Snaith, Nano Lett. 2014, 14, 3247.
[25] Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z.Yin, J. You, Nat. Photonics 2019, 13, 460.
[26] X. Guo, J. Su, Z. Lin, X. Wang, Q. Wang, Z. Zeng, J. Chang, Y. Hao, iScience 2021, 24, 102276.
[27] Y.Wang,T.Zhang,M.Kan,Y.Zhao,J.Am.Chem.Soc.2018,140,12345.
[28] W. Zhang, J. Xiong, J. Li, W. A. Daoud, Sol. RRL 2020, 4, 2000112.
[29] L. Zhou, Z. Lin, Z. Ning, T. Li, X. Guo, J. Ma, J. Su, C. Zhang, J. Zhang, S.Liu, J. Chang, Y. Hao, Sol. RRL 2019, 3, 1900293.
[30] J. Chang, Z. Lin, C. Jiang, J. Zhang, C. Zhu, J. Wu, ACS Appl. Mater. Interfaces 2014, 6, 18861.
[31] P. Zhang, J. Wu, T. Zhang, Y. Wang, D. Liu, H. Chen, L. Ji, C. Liu, W.Ahmad, Z. D. Chen, S. Li, Adv. Mater. 2018, 30, 1703737.
[32] Y. Li, L. Meng, Y. Yang, G. Xu, Z. Hong, Q. Chen, J. You, G. Li, Y. Yang,Y. Li, Nat. Commun. 2016, 7, 10214.
[33] N. De Marco, H. Zhou, Q. Chen, P. Sun, Z. Liu, L. Meng, E.-P. Yao, Y.Liu, A. Schiffer, Y. Yang, Nano Lett. 2016, 16, 1009.
[34] J.He,W.-H.Fang,R.Long,O.V.Prezhdo,ACS Energy Lett.2018,3,2070.
[35] X. Hou, Y. Hu, H. Liu, A. Mei, X. Li, M. Duan, G. Zhang, Y. Rong, H.Han, J. Mater. Chem. A 2017, 5, 73.
[36] G. Kieslich, S. Sun, A. K. Cheetham, Chem. Sci. 2014, 5, 4712.
[37] J. Ma, M. Qin, Y. Li, T. Zhang, J. Xu, G. Fang, X. Lu, J. Mater. Chem. A 2019, 7, 27640.
[38] S. Wu, Z. Li, J. Zhang, T. Liu, Z. Zhu, A. K. Y. Jen, Chem. Commun.2019, 55, 4315.
[39] Z. Yang, A. Surrente, K. Galkowski, A. Miyata, O. Portugall, R. J. Sutton,A. A. Haghighirad, H. J. Snaith, D. K. Maude, P. Plochocka, R. J. Nicholas,ACS Energy Lett. 2017, 2, 1621.
[40] P. Hohenberg, W. Kohn, Phys. Rev. 1964, 136, B864.
[41] G. Kresse, J. Furthm¨uller, Comput. Mater. Sci. 1996, 6, 15.
[42] G. Kresse, J. Furthm¨uller, Phys. Rev. B 1996, 54, 11169.
[43] W. Kohn, L. J. Sham, Phys. Rev. 1965, 140, A1133.
[44] G. Kresse, D. P. Joubert, Phys. Rev. B 1999, 59, 1758.
[45] P. E. Blochl, Phys. Rev. B 1994, 50, 17953.
[46] J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 865.
[47] G.Henkelman,B.P.Uberuaga,H.J′onsson,J.Chem.Phys.2000,113,9901.
[48] G. Henkelman, H. J′onsson, J. Chem. Phys. 2000, 113, 9978.
杂志排行
Energy & Environmental Materials的其它文章
- Progress of Pb-Sn Mixed Perovskites for Photovoltaics:A Review
- Development Strategies in Transition Metal Borides for Electrochemical Water Splitting
- Polymer-/Ceramic-based Dielectric Composites for Energy Storage and Conversion
- Controllable Construction of Bifunctional CoxP@N,P-Doped Carbon Electrocatalysts for Rechargeable Zinc–Air Batteries
- Unveiling the Underlying Mechanism of Transition Metal Atoms Anchored Square Tetracyanoquinodimethane Monolayers as Electrocatalysts for N2 Fixation
- Rational Design of High-Performance Bilayer Solar Evaporator by Using Waste Polyester-Derived Porous Carbon-Coated Wood
