H2S信号在拟南芥响应SO2胁迫过程中的作用
2022-06-29李利红郭宇茹侯俊鑫吴丽华
李利红,郭宇茹,侯俊鑫,吴丽华
H2S信号在拟南芥响应SO2胁迫过程中的作用
李利红1*,郭宇茹1,侯俊鑫1,吴丽华2
(1.晋中学院化学化工系,山西 榆次 030619;2.太原师范学院,山西 榆次 030619)
以拟南芥为实验材料,研究植物对H2S和SO2处理的转录响应及其关系,并利用外源喷施H2S及其清除剂的方法,检测SO2熏气后植株体内H2S的产生及其生理效应,探讨气体信号分子H2S在植物响应SO2胁迫过程中的作用.高通量测序结果发现,H2S和SO2处理诱导拟南芥植株多个基因转录水平改变,并有1220个基因在两种处理条件下均差异表达,其中包括多个硫代谢和谷胱甘肽代谢相关基因,表明H2S和SO2在调控硫代谢途径中具有交互作用.SO2熏气诱导拟南芥植株体内H2S合成酶基因和转录水平提高,胞内H2S含量增多.同时,超氧化物歧化酶(SOD)和过氧化氢酶(CAT)活性升高,含硫抗氧化物谷胱甘肽(GSH)含量及其相关酶谷胱甘肽硫转移酶(GST)和谷胱甘肽过氧化物酶(GPX)活性提高,活性氧H2O2含量增加,膜脂过氧化产物丙二醛(MDA)含量与对照相比无显著差异.外源喷施H2S可进一步提高SO2熏气下拟南芥植株GSH含量及其相关防御酶的活性, H2O2含量降低.但喷施亚牛磺酸(HT)清除H2S后,SO2熏气拟南芥植株GSH含量、抗氧化酶SOD、CAT和GST活性降低,MDA含量大幅增加.结果表明,SO2熏气诱导产生的H2S可作为信号分子,提高机体抗氧化防御能力,增强植物对SO2胁迫的抗性.
二氧化硫;硫化氢;拟南芥;含硫化合物;抗氧化酶
H2S是继一氧化氮、一氧化碳之后发现的第三种气体信号分子.在植物体中,产生H2S的途径有:直接通过叶片吸收大气中的H2S;通过半胱氨酸脱巯基酶(CDes)催化L/D-Cys降解生成H2S,这是植物体内源H2S形成的主要途径;在亚硫酸盐还原酶(SiR)的作用下, SO32–发生还原反应生成H2S[1-2].大量研究表明, H2S参与调节植物生长发育,如促进种子萌发、根形态建成,调节气孔运动,增强叶片的光合作用,延缓植物衰老等[3-5].此外, H2S信号还可诱导胁迫相关基因表达、激活体内的抗氧化系统、减小气孔孔径,从而帮助植物抵抗干旱、盐、温度、重金属等环境胁迫[6-9].
二氧化硫(SO2)是一种常见的全球性大气污染物.高浓度SO2会引起植物叶片褪绿或坏死,抑制光合作用, 影响植株的生长发育,还可诱发细胞核固缩、DNA断裂、染色体畸变,甚至导致细胞死亡[10-12].植物受到逆境胁迫时,会通过调节自身生理状态积极适应周围环境.研究表明, SO2熏气激活拟南芥胞内活性氧、植物激素等信号转导途径,诱导气孔关闭、防御基因转录上调和抗氧化酶活性增加[13-15],还可使植物产生交叉抗性,提高植物对其他环境刺激的耐受性[16-18].
大气中的SO2主要通过气孔进入植物体内,溶于细胞液形成HSO3-和SO32-.对植物有毒害作用的SO32-可被亚硫酸氧化酶(SO)氧化为SO42-,同时产生大量的活性氧[19-20],也可进入硫同化途径,被亚硫酸盐还原酶(SiR)催化生成硫化物(S2-),S2-在O-乙酰丝氨酸裂解酶(OAS-TL)作用下合成半胱氨酸(Cys),半胱氨酸可作为前体进一步合成GSH,在此过程中H2S作为副代谢产物释放[21-23].早在1982年, Hällgren等[24]研究发现SO2熏气后松树针叶中释放出H2S.近年来,有研究表明SO2熏气诱导植物体内生成的H2S可作为信号分子,介导植物对其他环境胁迫的抗氧化响应[25-26].此外, SO2与H2S缓解植物衰老的作用模式相似,并且SO2和H2S之间的关系是通过硫代谢途径建立起来的[27].这表明H2S和SO2对植物的影响存在一定的联系,提示信号分子H2S可能参与调控植物对SO2胁迫的响应过程.
本文以模式植物拟南芥为材料,利用高通量测序研究SO2和H2S处理后植株全基因组表达变化,并利用qRT-PCR检测H2S合成和硫代谢相关基因的转录水平,探讨植物对SO2和H2S处理的转录响应及其关系.同时,系统研究SO2熏气过程中植物体内H2S的产生机制及其生理效应,揭示信号分子H2S在拟南芥响应SO2胁迫过程中的重要作用,以期为提高植物对环境胁迫的耐受性提供新思路.
1 材料与方法
1.1 植株培养和胁迫处理
拟南芥(L.)Columbia生态型(Col-0).4℃春化2d后播种于营养土中,培养温度(22±1)℃,光/暗周期为16h/8h,光照强度140μmol/ m2/s,相对湿度约70%.
取4周龄生长一致的拟南芥植株,置于体积0.422m3的密闭箱中,适应1d后进行SO2熏气和H2S处理,温度和光照同上.前期预实验结果表明,0.1mmol/L NaHS处理和30mg/m3SO2熏气诱发拟南芥植株的抗氧化防御应答,且叶片无肉眼可见损伤.因此,本研究中选用0.1mmol/L NaHS和30mg/m3SO2,共设5个处理:
1) 对照组.
2) H2S处理: 0.1mmol/L NaHS.早、中、晚分别用NaHS处理液喷施拟南芥叶片,共处理3d.
3) SO2熏气: 30mg/m3SO2.根据K2S2O5+2HCl→ 2KCl+H2O+2SO2的原理,定量产生SO2气体,并采用甲醛吸收-副玫瑰苯胺分光光度法测定SO2浓度.前3d每天熏气12h (08:00~20:00),第4d熏气4h (8:00~ 12:00),共熏气40h.
4) H2S+SO2处理: 0.1mmol/L NaHS + 30mg/ m3SO2.每晚熏气结束后,在拟南芥叶片喷施NaHS处理液,共处理3d.
5) HT+SO2处理: 0.5mmol/L HT + 30mg/m3SO2.每晚熏气结束后,在拟南芥叶片喷施HT处理液,共处理3d.
每处理3次重复,每重复20株拟南芥.取各处理组拟南芥植株地上部分用于高通量测序、qRT-PCR和生理指标的分析.
1.2 转录组高通量测序
取对照组、H2S处理组和SO2熏气组拟南芥植株地上组织,用Trizol试剂提取总RNA,将mRNA Capture Beads置于四维旋转仪上充分混匀,平衡30min后使用.高通量测序由北京百迈克生物科技有限公司完成.
在处理组与对照组差异表达基因检测过程中,差异倍数(FoldChange, FC)表示两样品组间表达量的比值,错误发现率(False Discovery Rate, FDR)是通过对差异显著性P值(-value)进行校正得到的.以处理组与对照组信号比值的log2FC值表示处理后转录水平的改变,并用log2FC>1且FDR<0.01作为筛选差异表达基因的标准.
1.3 qRT-PCR检测
利用qRT-PCR技术检测H2S合成酶基因(、、)、氧乙酰丝氨酸(硫醇)裂解酶基因(、、)、亚硫酸氧化酶基因()、亚硫酸还原酶()基因表达水平.用Trizol试剂盒提取总RNA, PrimeScriptTMRT Master Mix (Takara)试剂盒合成cDNA.按照SYBR Premix Ex TaqTMII (Takara)的说明在ABI 7500Real- Time PCRSystem上进行相关基因的qRT-PCR分析,以基因作为内参基因,用2-∆∆CT方法计算相对表达量.引物序列使用见表1.
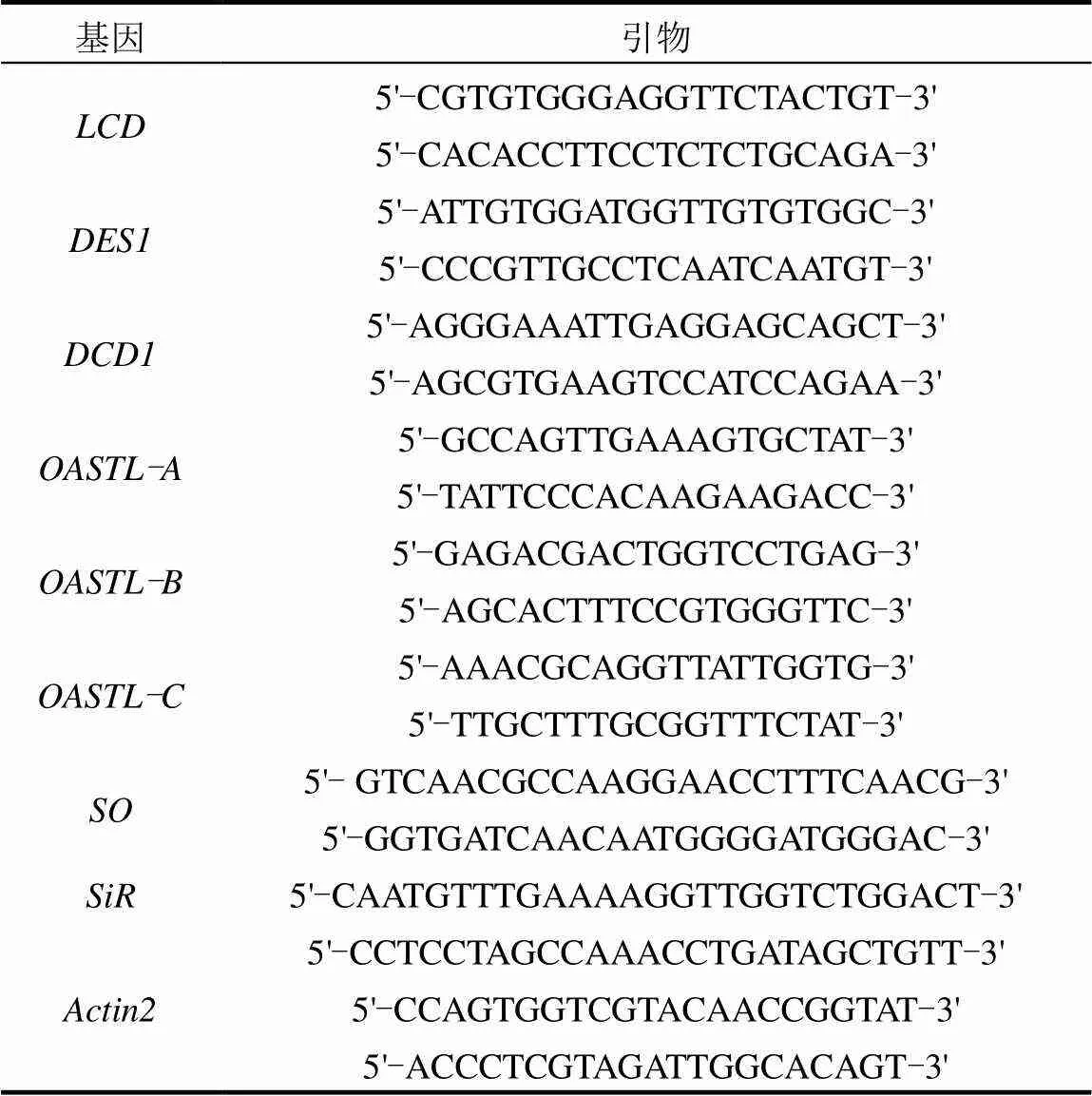
表1 qRT-PCR引物序列
1.4 生理指标检测
H2S、谷胱甘肽(GSH)、过氧化氢(H2O2)、丙二醛(MDA)、过氧化氢酶(CAT)、超氧化物歧化酶(SOD)、谷胱甘肽硫转移酶(GST)和谷胱甘肽过氧化物酶(GPX)测定利用南京建成生物工程研究所试剂盒.
1.5 数据分析
用SPSS 20.0进行统计分析, Origin lab 2019绘图,运用Duncan法比较样本间的差异显著性(<0.05).每个实验独立重复3次,数据均以3次重复的平均值和标准误差表示.
2 结果与分析
2.1 H2S和SO2处理对拟南芥植株基因表达的影响
外源H2S处理3d后,拟南芥全基因组中差异表达基因(DEG)共有3160个,其中上调表达基因1500个,下调表达基因1660个.在SO2熏气40h后,差异表达基因共有1892个,其中上调表达基因1034个,下调表达基因858个(表2).
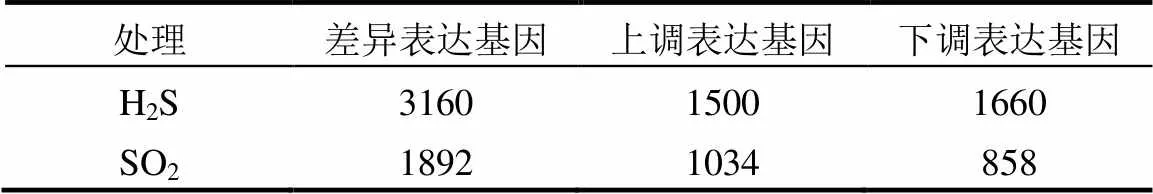
表2 差异表达基因数目统计
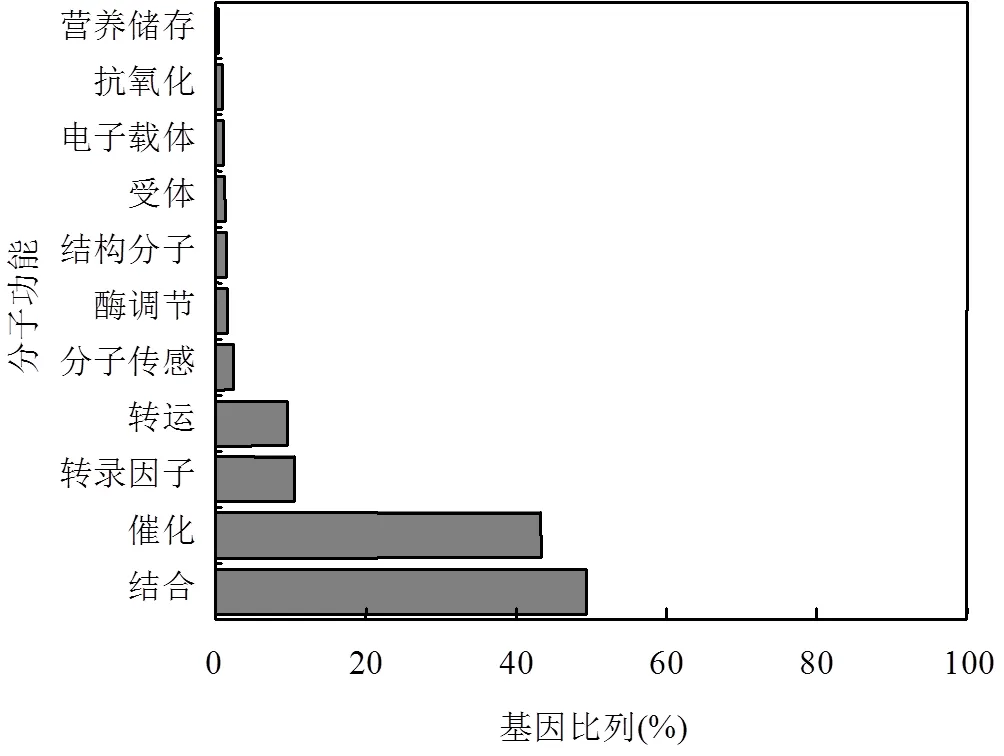
图1 H2S和SO2处理共有差异表达基因的功能分类
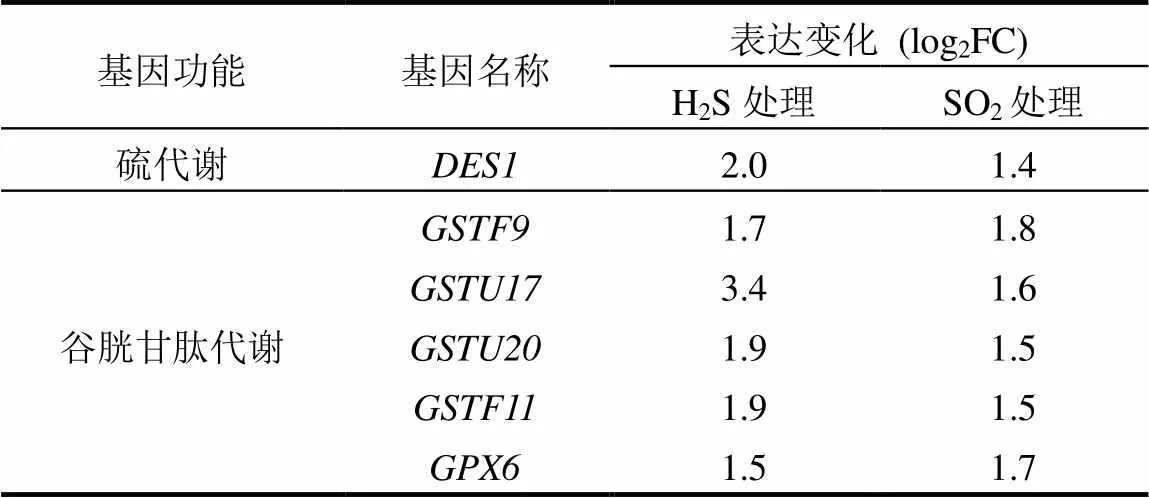
表3 H2S和SO2处理后部分差异表达基因
通过绘制维恩图,在H2S和SO2处理组中发现1220个共有差异表达基因,分别占各处理组差异表达基因的38.6%和64.5%.根据Gene Ontology (GO)分类法则对共有差异表达基因进行初步功能分类,发现其功能主要包括结合、催化、转录因子、转运、分子传感、酶调节、结构分子、受体、电子载体、抗氧化和营养储存(图1),其中有多个硫代谢和谷胱甘肽代谢相关基因差异表达,如、和(表3).
2.2 H2S和SO2处理后硫代谢相关基因表达水平
H2S和SO2处理后,拟南芥植株中硫代谢相关基因、、、、和表达水平发生改变. H2S处理后,H2S合成相关基因和表达量显著降低,而表达量显著升高.氧乙酰丝氨酸(硫醇)裂解酶基因表达量显著提高,而和表达量显著降低.亚硫酸氧化酶基因和亚硫酸还原酶基因表达量无明显变化(图2).
SO2熏气后, H2S合成相关基因和显著上调表达,而表达量无明显变化.氧乙酰丝氨酸(硫醇)裂解酶基因表达量显著降低,而和表达量没有明显变化.亚硫酸氧化酶基因表达量增加,亚硫酸还原酶基因表达水平无明显改变(图2).

图2 硫代谢相关基因表达水平
2.3 H2S对SO2胁迫下拟南芥含硫化合物水平的影响
SO2熏气后,拟南芥植株中H2S和GSH含量增加,GST和GPX活性显著高于对照组,说明SO2胁迫诱导胞内产生H2S,含硫抗氧化物GSH及其相关防御酶水平提高(图3).
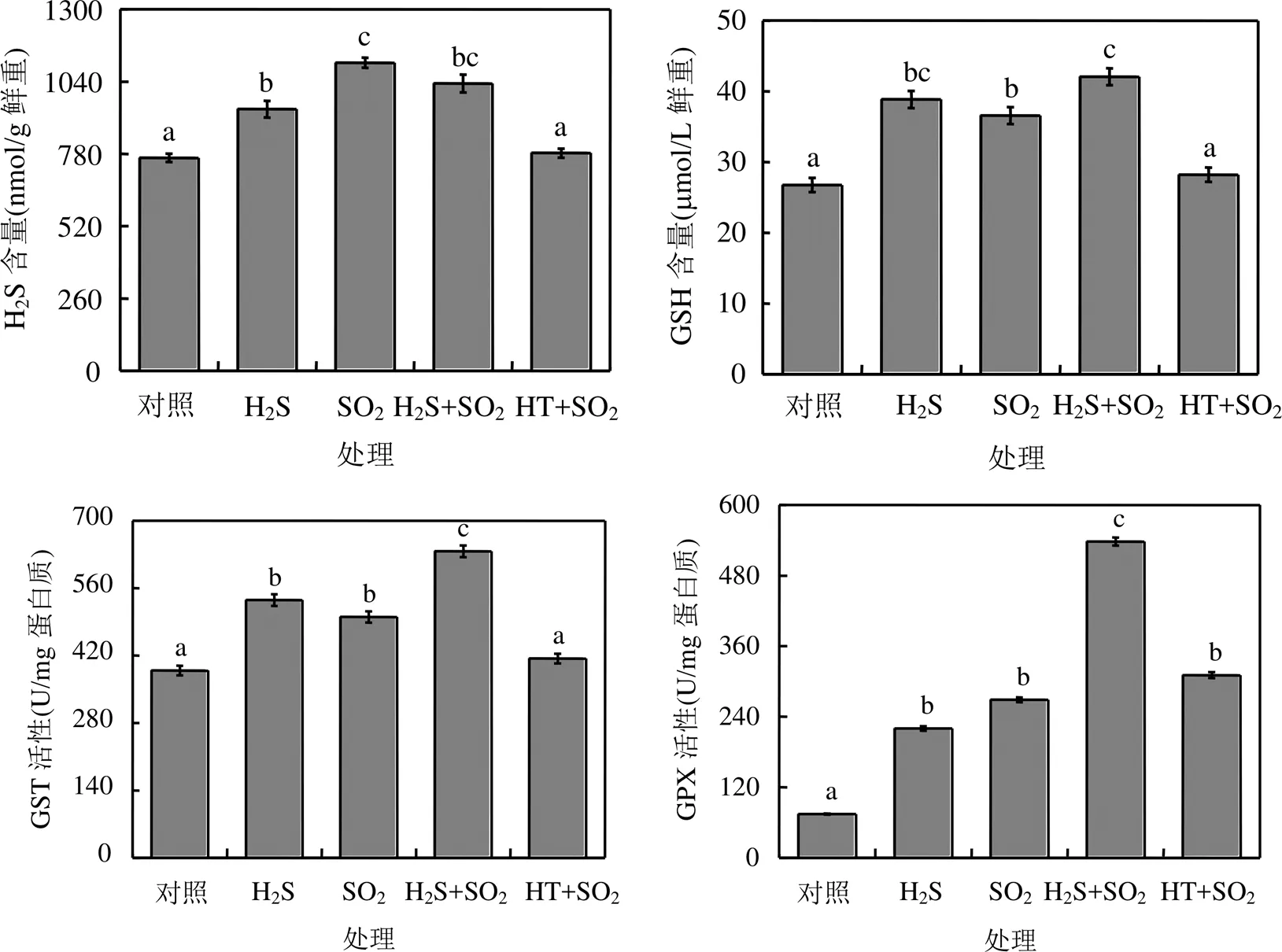
图3 H2S对SO2胁迫下拟南芥含硫化合物的影响
在SO2熏气过程中,分别用H2S供体NaHS或H2S清除剂HT喷施拟南芥叶片.结果发现,外源H2S处理显著提高了SO2熏气下拟南芥植株GSH含量、GST和GPX活性.相反,外源喷施HT后, SO2胁迫组拟南芥植株胞内H2S和GSH含量、GST活性均降低到对照水平,说明H2S信号参与调控SO2胁迫下拟南芥体内含硫化合物水平(图3).
2.4 H2S对拟南芥SO2胁迫的缓解作用
SO2熏气后,拟南芥植株中活性氧H2O2含量显著增加,抗氧化酶SOD和CAT活性显著升高,膜脂过氧化产物MDA含量与对照组相比没有显著差异(图4).
在SO2熏气过程中喷施H2S后,拟南芥植株中SOD和CAT活性维持在较高水平, H2O2含量降低,说明外源H2S可以有效缓解SO2熏气造成的氧化胁迫.相反,外源喷施HT后, SO2熏气拟南芥植株中H2O2含量维持在较高水平, CAT和SOD活性显著降低, MDA含量显著增加,说明清除H2S分子导致拟南芥植株发生膜脂过氧化,进一步说明H2S参与调控植物对SO2胁迫的抗氧化应答(图4).
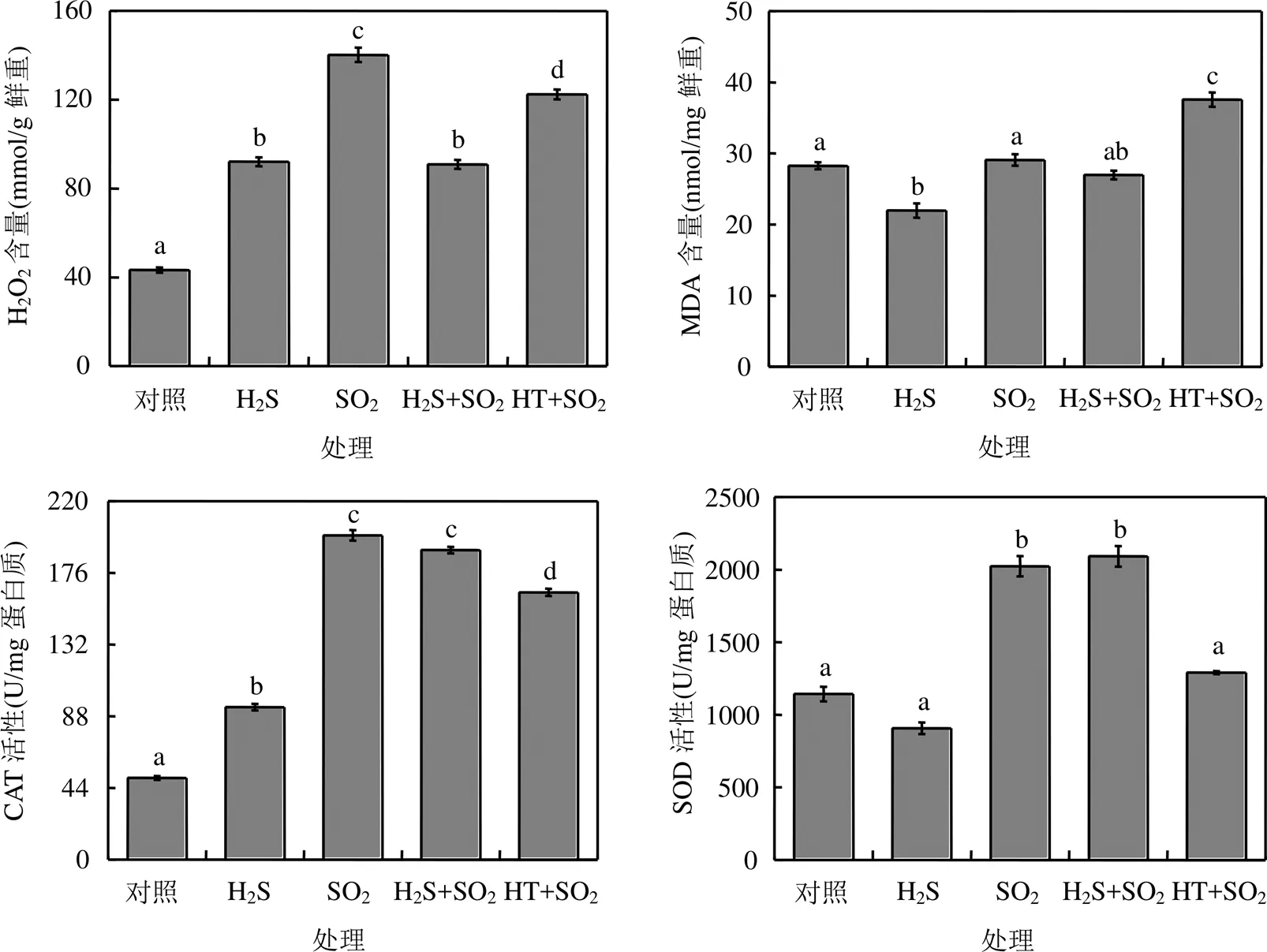
图4 H2S对SO2胁迫下拟南芥抗氧化系统的影响
3 讨论
SO2是一种有毒的大气污染物.H2S是硫代谢途径中重要的中间产物,也是一种新型气体信号分子,在植物生长发育及抵抗生物和非生物胁迫过程中发挥重要作用[28-30].本研究中,外源H2S和SO2处理诱导拟南芥植株中多个基因转录水平改变,并且有1220个基因在两种处理条件下均差异表达,涉及转录调节、信号传导、抗氧化应激等多种生理过程,其中包括多个硫代谢和谷胱甘肽代谢相关基因差异表达.拟南芥中多个基因既响应SO2胁迫又受H2S调节,证实SO2和H2S处理间存在一定的联系,并为信号分子H2S调节植物SO2胁迫应答机制研究奠定了基础.
SO2熏气诱导拟南芥植株中亚硫酸氧化酶基因表达量增加.亚硫酸氧化酶可催化SO32-+ O2+ H2O → SO42-+ H2O2反应,缓解SO32-对植物细胞的伤害,但同时也会产生大量的活性氧,导致胞内H2O2含量增多,造成氧化胁迫[31-32].同时,SO2熏气诱导胞内抗氧化酶CAT和SOD活性上升,抗氧化物GSH含量增加, GST和GPX基因表达水平和酶活性提高,有效清除胞内过量的活性氧,防止发生氧化损伤.
外源H2S处理降低了SO2熏气下拟南芥植株体内H2S含量,但是GSH含量增加,与外源H2S喷施Cr6+处理谷子中的研究结果类似[33],这可能是因为H2S处理不仅可诱导转录水平提高,同时氧乙酰丝氨酸(硫醇)裂解酶基因表达量显著增加,使部分H2S参与了Cys的合成,进而生成抗氧化物质GSH[34].因此,外源H2S对SO2熏气下拟南芥CAT和SOD活性没有明显影响,但可进一步提高植株抗氧化物GSH含量及其相关防御酶的活性,缓解SO2对植物造成的氧化胁迫.本研究表明,外源H2S处理影响植物体内的硫代谢过程,并可通过调控含硫化合物水平来提高植物对环境的适应能力.
对H2S合成相关基因表达水平研究发现, SO2熏气对拟南芥植株中亚硫酸还原酶基因转录水平没有明显影响,主要通过提高半胱氨酸脱巯基酶基因和表达水平来增加植株体内H2S含量.同时,氧乙酰丝氨酸(硫醇)裂解酶基因表达量降低,减少利用H2S合成Cys,从而使胞内积累大量H2S.外源喷施HT清除胞内产生的H2S后,拟南芥植株体内CAT和SOD活性显著降低, GSH含量及GST活性回落到对照水平,膜脂过氧化产物MDA含量大幅增加,说明SO2熏气诱导胞内产生H2S作为信号分子,参与调控植物体的抗氧化防御反应,有效缓解细胞氧化损伤效应.另外,有研究表明,SO2暴露诱导胞内产生H2S,促使植株产生抗氧化防御应答,提高后期干旱、Al胁迫期间植株的抗氧化酶活性,增强植物的逆境适应能力[25-26].因此,H2S信号分子不仅在植物响应SO2胁迫过程中发挥重要作用,同时还参与调控SO2暴露诱导植物体产生的对多种环境胁迫的交叉抗性.
本文不仅阐明H2S信号调节SO2胁迫应答的机理,还揭示了H2S是SO2诱导植物产生交叉适应性的重要基础,对其详细机制的深入研究将为植物适应复杂多变的环境条件提供一条新的有效途径.
4 结论
4.1 高通量测序研究发现, H2S和SO2处理组拟南芥中出现1220个共有差异表达基因,两者在调控硫代谢途径中具有交互作用.
4.2 外源喷施H2S可提高SO2胁迫下拟南芥GSH含量, GPX和GST活性增加,缓解SO2对植物造成的氧化胁迫.
4.3 SO2熏气诱导拟南芥H2S合成酶基因和转录水平提高,胞内H2S含量增加.H2S作为信号分子,通过提高抗氧化防御能力来调控植物对SO2胁迫的响应.
[1] Aroca A, Gotor C, Bassham D C, et al. Hydrogen sulfide: From a toxic molecule to a key molecule of cell life [J]. Antioxidants (Basel), 2020,9:621.
[2] Corpas F J, Palma J. H2S signaling in plants and applications in agriculture [J]. Journal of Advanced Research, 2020,24:131-137.
[3] Xuan L, Li J, Wang X, et al. Crosstalk between hydrogen sulfide and other signal molecules regulates plant growth and development [J]. Internaional Journal of Molecular Sciences, 2020,21:4593.
[4] 郭鸿鸣,肖天宇,谢彦杰.气体信号分子硫化氢在植物中的生理功能及作用机制[J]. 中国生物化学与分子生物学报, 2016,32(5):488- 495.
Guo H M, Xiao T Y, XieY J. The physiological function and molecular mechanism of signaling molecule hydrogen sulfide in plants [J]. Chinese Journal of Biochemistry and Molecular Biology, 2016, 32(5):488-495.
[5] Scuffi D, Alvarez C, Laspina N, et al. Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure [J]. Plant Physiology, 2014, 166:2065-2076.
[6] Shi H T, Ye T T, Han N, et al. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in[J]. Journal of Integrative Plant Biology, 2015,l57:628-640.
[7] Arif M S, Yasmeen T, Abbas Z, et al. Role of exogenous and endogenous hydrogen sulfide (H2S) on functional traits of plants under heavy metal stresses: a recent perspective [J]. Frontiers in Plant Science, 2021,11:545453.
[8] Jin Z, Wang Z, Ma Q, et al. Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in[J]. Plant and Soil, 2017,419:141-152.
[9] Li G, Shah A A, Khan W U, et al. Hydrogen sulfide mitigates cadmium induced toxicity inby modulating physiochemical attributes, osmolyte metabolism and antioxidative machinery [J]. Chemosphere, 2021,263:127999.
[10] Lee H K, Khaine I, Kwak M J, et al. The relationship between SO2exposure and plant physiology: A mini review [J]. Horticulture Environment and Biotechnology, 2017,58:523.
[11] Appalasamy M, Varghese B, Ismail R, et al. Responses ofleaves to sulphur dioxide pollution: A comparison of morphological, physiological and biochemical biomarkers [J]. Atmospheric Pollution Research, 2017,8:729-740.
[12] Weber J N, Kaufholdt D, Minner-Meinen R, et al. Impact of wildfires on SO2detoxification mechanisms in leaves of oak and beech trees [J]. Environmental Pollution, 2021,272:116389.
[13] Li L H, Yi H L. Differential expression ofdefense-related genes in response to sulfur dioxide [J]. Chemosphere, 2012,78:718- 724.
[14] Duan J, FuB, Kang H, et al. Response of gas-exchange characteristics and chlorophyll fluorescence to acute sulfur dioxide exposure in landscape plants [J]. Ecotoxicology and Environmental Safety, 2019, 171:122-129.
[15] 杨 丹,杨晓晓,钟霞飞,等.3种阴生地被植物对SO2胁迫的生理响应及净化能力 [J]. 西北植物学报, 2017,37(1):361-369.
Yang D, Yang X X, Zhong X F, et al. Resistant physiological response and purifying ability of three shady perennial plants to SO2stress [J]. Acta Botanica Boreali-Occidentalia Sinica, 2017,37(1):361-369.
[16] 李 蕊,仪慧兰.二氧化硫增强拟南芥植株对干旱的适应性[J]. 生态学报, 2018,38(6):2156-2162.
Li R, Yi H L. Sulfur dioxide improves drought adaptation inplants [J]. Acta Ecologica Sinica, 2018,38(6):2156-2162.
[17] Wang S S, Zhang Y X, Yang F, et al. Sulfur dioxide alleviates programmed cell death in barley aleurone by acting as an antioxidant [J]. PLoS ONE 2017,12(11):e0188289.
[18] Han Y, Wu M, Hao L, et al. Sulfur dioxide derivatives alleviate cadmium toxicity by enhancing antioxidant defence and reducing Cd2+uptake and translocation in foxtail millet seedlings [J]. Ecotoxicology and Environmental Safety, 2018,157:207-215.
[19] Lang C, Popko J, Wirtz M, et al. Sulphite oxidase as key enzyme for protecting plants against sulphur dioxide [J]. Plant Cell and Environment, 2007,30(4):447-455.
[20] Oshanova D, Kurmanbayeva A, Bekturova A, et al. Level of sulfite oxidase activity affects sulfur and carbon metabolism in[J]. Frontiers in Plant Science, 2021,12:690830.
[21] Yarmolinsky D, Brychkova G, Fluhr R, et al. Sulfite reductase protects plants against sulfite toxicity [J]. Plant Physiology, 2013,161(2):725- 743.
[22] Arif Y, Hayat S, Yusuf M, et al. Hydrogen sulfide: A versatile gaseous molecule in plants [J]. Plant Physiology and Biochemistry, 2021, 158:372-384.
[23] 尚玉婷,张妮娜,上官周平,等.硫化氢在植物中的生理功能及作用机制[J]. 植物学报, 2018,53(4):565-574.
Shang Y T, Zhang N N, Shangguan Z P, et al. Physiological function and mechanism of hydrogen sulfide in plants [J]. Bulletin of Botany, 2018,53(4):565-574.
[24] Hällgren J E, Fredriksson S A. Emission of hydrogen sulfide from sulfur dioxide-fumigated pine trees [J]. Plant Physiology, 1982,70: 456-459.
[25] Zhu D B, Hu K D, Guo X K, et al. Sulfur dioxide enhances endogenous hydrogen sulfide accumulation and alleviates oxidative stress induced by aluminum stress in germinating wheat seeds [J]. Oxidative Medicine and Cellular Longevity, 2015,612363,2015.
[26] Li L H, Yi H L, Liu X P, et al. Sulfur dioxide enhance drought tolerance of wheat seedlings through H2S signaling [J]. Ecotoxicology and Environmental Safety, 2021,207:111248.
[27] Liu H, Wang J, Liu J, et al. Hydrogen sulfide (H2S) signaling in plant development and stress responses [J]. aBIOTECH, 2021,2:32-63.
[28] 裴雁曦.植物中的气体信号分子硫化氢:无香而立,其臭如兰[J]. 中国生物化学与分子生物学报, 2016,32(7):721-733.
Pei Y X. Gasotransmitter hydrogen sulfide in plants: stinking to high heaven, but refreshing to fine life [J]. Chinese Journal of Biochemistry and Molecular Biology, 2016,32(7):721-733.
[29] Chen T, Tian M, Han Y. Hydrogen sulfide: a multi-tasking signalmolecule in the regulation of oxidative stress responses [J]. Journal of Experimental Botany, 2020,71:2862-2869.
[30] Zhang J, Zhou M, Zhou H, et al. Hydrogen sulfide, a signaling molecule in plant stress responses [J]. Journal of Integrative Plant Biology, 2021,63:146-160.
[31] Brychkova G, Xia Z, Yang G, et al. Sulfite oxidase protects plants against sulfur dioxide toxicity [J]. The Plant Journal, 2007,50:696- 709.
[32] Xia Z, Xu Z, Wei Y, et al. Over expression of the maize sulfite oxidase increases sulfate and GSH levels and enhances drought tolerance in transgenic tobacco [J]. Frontiers in Plant Science, 2018,9:298.
[33] Fang H, Liu Z, Jin Z, et al. An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in[J]. Environmental Pollution, 2016,213:870-877.
[34] Birke H, De Kok LJ, Wirtz M, et al. The role of compartment-specific cysteinesynthesis for sulfur homeostasis during H2S exposure in[J]. Plant and CellPhysiology, 2015,56:358-367.
Functions of H2S signal in response to SO2stress in.
LI Li-hong1*, GUO Yu-ru1, HOU Jun-xin1, WU Li-hua2
(1.Department of Chemistry and Chemical Engineering, Jinzhong University, Yuci 030619, China;2.Department of Biology, Taiyuan Normal University, Yuci 030619, China)., 2022,42(6):2904~2910
Sulfur dioxide (SO2) is one of the most common and harmful air pollutant. Hydrogen sulfide (H2S), as a new gasotransmitter, is involved in the regulation of plant development and stress adaptation. To reveal the role of H2S in response to SO2stress, the relation between transcriptional response to H2S and SO2treatments, and the production and physiological effect of H2S during SO2fumigation will be studied inplants. By using high-throughput sequencing, many genes were identified to be differentially expressed inplants exposed to 30mg/m3SO2or treated with 0.1mmol/L H2S. Among these differentially expressed genes (DEGs), 122, 0genes were overlapped between SO2and H2S treatments, including some genes encoding enzymes involved in sulfur and glutathione metabolism. These results suggested a interaction between H2S and SO2in regulating sulfur metabolism pathways in plants. Moreover, two H2S-generating genes, L-cysteine desulfhydrase () and L-cysteinedesulfhydrase1 () were significantly up-regulated, and endogenous H2S content was enhanced inplants under SO2stress. Meanwhile, the activities of superoxide dismutase (SOD) and catalase (CAT), the content of glutathione (GSH) and activities of GSH-related enzymes including glutathione S-transferase (GST) and glutathione peroxidase (GPX) were significantly increased inplants exposed to SO2. And that H2O2content was markedly increased in SO2-treatedplants, while malondialdehyde (MDA) content showed no significant difference compared with the control group. Exogenous application of H2S further increased the level of GSH and GSH-related defense enzyme, and reduced the content of H2O2inplants under SO2stress. When scavenged endogenous H2S by spraying hypotaurine (HT) during SO2fumigation, the content of GSH, the activities of SOD, CAT and GST were reduced, and the content of MDA was significantly increased inplants. Together, this study indicated that H2S played an important role in response to SO2stress through improving antioxidant capacity, which would be helpful for better understanding the adaptation mechanism of plants to environmental stress.
sulfur dioxide;hydrogen sulfide;;sulfur compounds;antioxidant enzymes
X171.5
A
1000-6923(2022)06-2904-07
李利红(1982-),女,山西吕梁人,副教授,博士,主要研究方向为环境污染物的生态毒理学.发表论文10余篇.
2021-11-10
国家自然科学基金项目(21307087);山西省应用基础研究计划项目(201901D111301);晋中学院“1331工程”创客团队项目(jzxycktd2019031)
* 责任作者, 副教授, lihongli19821129@163.com
