Hematopoiesis reconstitution and anti-tumor effectiveness of Pai-Neng-Da capsule in acute leukemia patients with haploidentical hematopoietic stem cell transplantation
2022-06-23JiaoJiaoYuanYingLuJunJieCaoRenZhiPeiRuiLanGao
lNTRODUCTlON
Allogeneic hematopoietic stem cell transplantation is an effective therapy for hematologic malignancies. Over recent years, haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has shown similar outcomes to identical-sibling transplants. Moreover, haplo-HSCT is an efficient post-remission treatment for patients lacking an identical donor[1-3]. Nonetheless, in haplo-HSCT, the human leukocyte antigen (HLA) disparity between the donor and the recipient can lead to an intense bidirectional alloreactivity predisposition for developing primary graft failure. Graft failure includes graft rejection and primary poor graft function (PGF). The reported incidence of primary PGF is 5-27%[4,5]. PGF results in prolonged hospitalization, expensive medical costs, and high transplant-related mortality owing to infectious complications or hemorrhage. According to recent studies, improving the bone marrow (BM) microenvironment represents a promising therapeutic approach to promote hematopoietic reconstitution in PGF patients[6]. Nonetheless, the pathogenesis of primary PGF is yet unclear, and its effective therapies need to be explored.
In this story the mirror represents:(a) the male gaze which objectifies the female,(b) the husband/father who is otherwise absent from the story and for whose approval both Snow White and her mother compete. IRReturn to place in story.
The old man was fond of all creatures, and every morning he used to open the cage door, and the sparrow flew happily about until it caught sight of a cat or a rat or some other fierce beast, when it would instantly return to the cage, knowing that there no harm could come to it
Panaxadiol saponin component (PDS-C) is a biologically active component isolated from total saponins of ginsenosides and currently available in the form of capsules named as Pai-Neng-Da (PND) Capsule[7]. Preclinical studies have suggested that PDS-C can be used to treat multiple hemocytopenia in mice, including idiopathic thrombocytopenic purpura (ITP), aplastic anemia (AA), myelosuppression, and hemocytopenia caused by chemotherapy or radiation[8,9]. PDS-C possesses hematopoietic growth factor-like function that promotes proliferation and differentiation of hematopoietic progenitor cells in myelosuppressed mice, probably mediated by regulating phosphorylated mitogen-activated protein kinase, extracellular signal-regulated kinases protein kinases, receptor tyrosine kinase and globin transcription factor (GATA) 1 transcription factors[8]. PDS-C could modulate T lymphocyte immune functions by increasing CD4
cells, downregulating T-bet protein expression, and upregulating GATA-3 protein expressions[8,9]. Moreover, panaxadiol showed anti-tumor activity in human leukemia cell lines by arresting the cell cycle at the G1/S phase[10,11].
So far, two certificates of new class-five Chinese patent medicine have been authorized and granted by China Food and Drug Administration, including PDS-C and its PND capsule. PND capsule for treating hemocytopenia has successfully passed Phase I clinical trial and has shown to be effective without significant side effects in Phase II trial[7]. On the other hand, the efficacy of PND capsule in hematopoietic recovery after haplo-HSCT has not yet been reported.
In this study, we evaluated the role of PND capsule in acute leukemia (AL) patients with haplo-HSCT. To the best of our knowledge, this is the first study that evaluates the efficacy of PND capsule in the treatment of AL patients following haplo-HSCT.
MATERlALS AND METHODS
Patients and donors
A total of 60 patients diagnosed with AL who underwent haplo-HSCT at the Department of Hematology, Affiliated People’s Hospital of Ningbo University, were included in this retrospective study. Twenty-nine consecutive patients received oral PND capsule from the sixth day to the first month after haplo-HSCT between April 1, 2018 and June 30, 2020 were included in the PND group. In addition, 31 patients who did not receive PND capsule during haplo-HSCT between April 1, 2015 and March 31, 2018 were included in the non-PND group. All patients were included in the analysis, and none were excluded on account of severe toxicity.
Primary PGF was defined as persistent neutropenia (≤ 0.5×10
/L), thrombocytopenia (platelets ≤ 20 × 10
/L), and/or hemoglobin concentration ≤ 70 g/L after engraftment with hypocellular BM and full donor chimerism without concurrent acute graft-versus-host disease (aGVHD) or disease relapse. Neutrophil engraftment was defined as the first step in the three consecutive days when the absolute neutrophil count was 0.5 × 10
/L without granulocyte colony-stimulating factor (G-CSF) stimulation. Platelet engraftment was defined as the first 7 consecutive days when the platelet count was ≥ 20 × 10
/L, independent of platelet transfusion. The hematological recovery was defined as neutrophils > 0.5 × 10
/L, platelets > 20 × 10
/L, and hemoglobin concentration > 70 g/L without transfusion support or GCSF stimulation[13-16]. The red blood cells (RBC) transfusions were performed in patients with symptomatic anemia or hemoglobin concentration < 70 g/L or hematocrit level < 0.2. Platelet transfusions were performed considering a threshold level of ≥ 20×10
platelets/L in patients with bleeding or fever.
Hematopoietic stem cells were derived from both BM and peripheral blood. Potential haploidentical donors included both first- and non-first-degree relatives. The haploidentical donor was selected based on the status of donor-specific anti-HLA antibodies (DSA). In the presence of DSA, patients received plasmapheresis or rituximab therapy. The other factors that were considered were sex, age, cytomegalovirus (CMV) serostatus, and weight ratio between donors and recipients.
According to CM symptom scores, the therapeutic efficacy of patients in the PND group was significantly better than the non-PND group. The adverse events of PND capsule were mild and spontaneously alleviated without intervention. Thus, these results indicated that PND capsule could improve the clinical effect and quality of life by alleviating the CM symptoms.
Drugs
PND capsule was provided by the First Affiliated Hospital of Zhejiang Chinese Medical University and Ningbo Tianzhen Pharmaceutical Co. Ltd., China (Batch No. 20150401, each capsule contains PDS-C 40 mg with a purity of 92.44%, analyzed and defined by high-performance liquid chromatography using specific monomers of ginsenosides as the reference standards)[12].
PND capsule exerts dual effects of promoting hematopoiesis recovery and regulating immunity[7,9,20]. Phase I clinical trials have demonstrated that PND capsule is safe and does not induce side effects; the recommended safe dose range for clinical studies is 6-10 capsules (240-400 mg) daily[21]. State Food and Drug Administration of China has recommended PND capsule for treating ITP and unexplained leucopenia. Moreover, Zhu
[22] found that PND capsule maintains peripheral hemograms, appropriately reducing the androgen dosage and improving the quality of life of patients with AA. However, the application of PND in haplo-HSCT has not yet been reported. Therefore, this study was conducted to evaluate the role of PND in promoting the recovery of hematopoietic function after haplo-HSCT and the correlation between PND and the prognosis of AL patients who underwent haplo-HSCT.
Definition of western medical evaluation indexes
It might be an Italian or a Spaniard, remarked the clergyman.But to the fisherman s wife these nations seemed all the same, andshe consoled herself with the thought that the child was baptized as aChristian.
Evaluation of therapeutic efficacy according to Chinese medical standards
The therapeutic efficacy was assessed according to the rate of decrease in Chinese medical (CM) symptom score: (1) Cured: the CM symptoms disappeared, and the score decreased more than 95%; (2) Favorably effective: CM symptoms were improved, and the score decreased more than 75%; (3) Effective: CM symptoms were improved, and the score decreased more than 30%; and (4) Ineffective: No significant improvement or an even worse condition was observed, and the score reduced to less than 30%. CM symptoms included pale lips and nails, palpitation and dizziness, weakness and fatigue, soreness and weakness of waist and knees, dry mouth and thirst, hot flashes, night sweats, cold limbs, dry or thin feces, epistaxis, and bleeding. CM symptoms were evaluated and recorded from 0 to 3 according to severity[12].
Monitoring of adverse drug reactions
According to the criteria established by the Center for Adverse Drug Reaction Monitoring of the Ministry of Health and the five-level classification, adverse drug reactions were assessed using the following levels: Certain, probable, possible, suspicious, and impossible; the first four levels were judged as possibly related to the investigational drug.
Conditioning regimen, graft-vs-host disease and viral infection prophylaxis
The conditioning therapy was as follows: Cytarabine (4 g/m
/d) intravenously on days -10 to -9, busulfan (3.2 mg/kg/d) intravenously on days -8 to -6, cyclophosphamide (1.8 g/m
/d) intravenously on days -5 to -4, semustine (250 mg/m
/d), orally once on day -3, and thymoglobulin (2.5 mg/kg/d, Sang Stat, Lyon, France) intravenously on days -5 to -2. Graft-versus-host disease (GVHD) prophylaxis regimen consisted of cyclosporine A, mycophenolate mofetil, and short-term methotrexate. Patients received prophylaxis against bacterial, viral, and fungal agents. The reactivation of CMV infection and Epstein-Barr virus (EBV) infection was monitored by polymerase chain reaction twice/week during the early period (days +15 to +100) and then once/week (from days +100 to +180).
Statistical analysis
All clinical data were analyzed in this study using SPSS 26.0 software. The patient characteristics between the two groups were compared using the Mann-Whitney test for continuous variables and the chi-square test or Fisher’s exact test for categorical data. Measurement data is shown with mean ± SD. The survival analysis was carried out using the Kaplan-Meier method. All statistical tests were 2-sided.
value < 0.05 was considered statistically significant.
RESULTS
Comparison of the baseline characteristics between the two groups
The baseline characteristics are summarized in Table 1. No differences in the baseline data, including sex, age, diagnosis, high cytogenetic risk, disease status at transplantation, peripheral blood cell counts (pre-transplantation), the number of courses of chemotherapy before transplantation, donor’s age, female donor-male recipient pairs, CMV IgG-positive recipients, and the infused mononuclear cells (MNCs) and CD34+ cells of the grafts were observed between the PND and the non-PND groups (all
> 0.05).

Comparison of therapeutic efficacy according to western medical evaluation indexes
Compared with the non-PND group, the PND group achieved higher hemoglobin concentration on the eighteenth day (
= 0.029), the twenty-fourth day (
= 0.003), and the first month (
= 0.043) after haplo-HSCT, as well as the platelet count on the eighteenth day (
= 0.002) and the twenty-fourth day (
= 0.046) after haplo-HSCT (Table 2 and Figure 1).

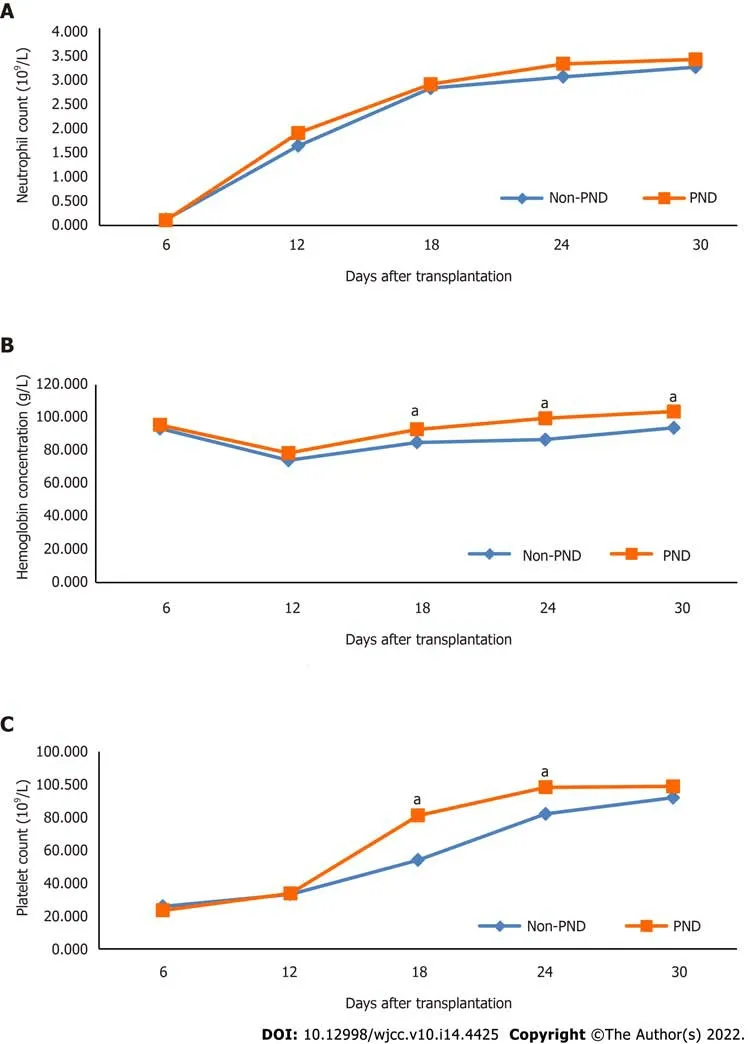
The duration of platelet engraftment was shorter in the PND group than in the non-PND group [median 12 (range, 8-152) days
median 13 (range, 9-145) days, respectively,
= 0.039]. Also, patients in the PND group received a lower frequency of RBC and platelet transfusions compared to the non-PND group (
= 0.033 and
= 0.035, respectively). Furthermore, compared to the non-PND group, patients in the PND group experienced a lower incidence of PGF (3.4%
25.8%,
= 0.027). In addition, the relapse rate after haplo-HSCT was reduced in patients using PND compared to those without PND (6.9%
29.0%,
= 0.043). However, the duration of neutrophil engraftment [median 12 (range, 10-14) days
, median 12 (range, 10-15) days, respectively,
= 0.454], the rate of infectious complications within 100 days after haplo-HSCT (79.3%
87.1%,
= 0.500) and the occurrence of aGVHD (58.6%
45.2%,
= 0.316) were similar in the PND and non-PND groups (Table 3).

The survival analysis showed a statistically significant benefit for the 3-year relapse-free survival (RFS) (69.1%
61.9%,
= 0.046) and the 3-year progression-free survival (PFS) (69.1%
61.7%,
= 0.049) with the use of PND. Nonetheless, PND failed to improve the 3-year overall survival (OS) (69.1%
65.6%,
= 0.069) (Figure 2).

Comparison of therapeutic efficacy according to CM symptom scores
The therapeutic efficacy was assessed according to the rate of decrease in CM symptom score. In the PND group, 2 cases (6.90%) were cured, 9 cases (31.03%) were favorably effective, 16 cases (55.17%) were effective, 2 cases (6.90%) were ineffective; the effective rate was 93.10% (27/29). In the non-PND group, 0 cases were cured, 6 cases (19.35%) were favorably effective, 15 cases (48.39%) were effective, 10 cases (32.26%) were ineffective, and the effective rate was 67.74% (21/31). Thus, the therapeutic efficacy of the PND group according to CM symptom scores was significantly better than that of the non-PND group (
= 0.022) (Table 4).
Climbing the wall was rather a difficulty, but when he at length found himself inside it he was charmed with the peaceful beauty of the little domain6 it enclosed, and still more delighted when he perceived a slender, lovely maiden7 wandering among the flowers

Adverse events
During the administration of the PND capsule, the laboratory examinations of urine routine, electrocardiogram, hepatic and renal functions were normal. The hot flush, insomnia, dry mouth, sweating, and mild stomach discomfort at the early stage of medication were respectively observed in 1 case (3.4%), 2 cases (6.9%), 2 cases (6.9%), 1 case (3.4%) and 4 cases (13.8%) with PND capsule, and they spontaneously alleviated without any intervention.
DlSCUSSlON
With the rapid development of haplo-HSCT, primary PGF has become a life-threatening complication. The clinical risk factors of PGF include the number of infused CD34+ cells, donor-specific anti-HLA antibodies, GVHD, age, donor-recipient blood-type matching, and CMV infection[17-19]. Previous studies have found that patients with PGF have an inferior 2-year OS compared to those with good graft function[15]. However, reports related to the pathogenesis of primary PGF are limited. Effective therapies are inconclusive and need to be explored further.
Pediatric patients (< 14 years old) were given PND capsule 4 mg/kg/day (integer capsules were taken according to clinical operability). Adult patients were given two capsules at a time, threetimes/day.
Our results showed that PND capsule markedly reduces the duration of platelet engraftment, the frequency of RBC and platelet transfusions, and the incidence of primary PGF. PND capsule can decrease the myelosuppression caused by chemotherapy, accelerate hematopoietic function recovery, especially for erythroid and megakaryocytic lineages, following haplo-HSCT. T cells have a crucial role in the immune destruction of BM. Patients with PGF showed significantly higher proportions of stimulated CD4+ and CD8+ T cells that produced IFN-gamma but notably decreased proportions of IL-4-producing T cells, resulting in a shift of the IFN-gamma/IL-4 ratio towards a type 1 response and an elevated percentage of activated CD8 + T cells[23]. PND-C could reduce peripheral blood CD8+ cells and increase CD4+ cells, reverting the ratio of CD4+/CD8+ cells to normal level in AA model mice[9]. Furthermore, hematopoiesis with PDS-C and its PND capsule is promoted
the intracellular signaling pathway, upregulating multiple transcription factors. The protein expression levels, phosphorylation status, and DNA binding activities were dramatically accentuated by PDS-C treatment in hematopoietic cells, promoting hematopoiesis and blood cells' formation[7,24]. However, PND capsule did not shorten the duration of neutrophil engraftment. Since the use of G-CSF on the sixth day after transplantation, the effect of PND capsule may be disturbed.
40. He asked her in all the languages he knew: Speaking several languages was an important skill and highly regarded in diplomats and royalty, as well as the higher classes, in times past. The importance of knowing more than one language is still important today, of course.Return to place in story.
In our study, PND capsule significantly reduced the post-transplantation recurrence rates and increased the 3-year RFS and PFS, perhaps due to its anti-cancer effect. Basic research on anti-tumor effect of PND capsule is limited. As previously reported, panaxadiol selectively inhibits cyclin Aassociated Cyclin-dependent kinase 2 activity by elevating the endogenous inhibitor proteins (CKIs) p21WAF1/CIP1 protein levels and arresting the cell cycle at the G1/S phase in human cancer cell lines[10,11]. The anti-tumor invasion and metastasis effects are related to the weakening of cell invasiveness[25]. Further
and
experiments are needed to explore the mechanism of anti-cancer effect of PND capsule. Yet, our data suggested that PND capsule does not affect the 3-year OS. The putative causes might be the insufficient follow-up time in the current study. Therefore, multicenter prospective studies with long follow-up times should be conducted to further investigate the potential influence of PND capsule on the survival of patients after haplo-HSCT.
Here the mirrors represent the stepsisters vanity and the family s wealth. The fact that the family owns mirrors large enough to give a full reflection of a person from head to toe shows that they have been extremely wealthy and thus powerful at least in the past if not Cinderella s present (Chevalier 1982).Return to place in story.
The cabby(,) has his point of view. It is more single-minded, perhaps, than that of a follower1 of any other calling. From the high, swaying seat of his hansom() he looks upon his fellowmen as nomadic2(,) particles, of no account except when possessed3 of migratory4(,) desires. He is Jehu, and you are goods in transit5. Be you President or vagabond() , to cabby you are only a fare. He takes you up, cracks his whip, joggles your vertebræe and sets you down.
He had to obey, and with groaning15 and pain cast off one skin after another, and for each skin the maiden threw off one of her shirts, until there lay on the floor seven lindorm skins and six snow-white shirts; the seventh she still had on
CONCLUSlON
Our data suggest that PND capsule could promote hematopoiesis reconstitution, improve the therapeutic efficacy of CM symptom scores, present anti-tumor effectiveness, and prolong the survival of AL patients with haplo-HSCT. Yet, larger multicenter randomized controlled studies are needed to confirm these findings.
ARTlCLE HlGHLlGHTS
Research background
PND capsule marginally reduced the rate of PGF and relapse. The 3-year relapse-free survival and progression-free survival were improved in the PND group than in the non-PND group. Also, the therapeutic efficacy of the PND group according to Chinese medical symptom scores was significantly better than that of the non-PND group.
Research motivation
The main problem in this study is whether PND capsule could promote hematopoiesis reconstitution following haplo-HSCT and whether it is related to the prognosis of AL patients with haplo-HSCT.
Research objectives
Objective to evaluate the role of PND capsule in AL patients with haplo-HSCT.
Research methods
We compared the therapeutic efficacy and the survival of AL patients with or without PND capsule during haplo-HSCT, using the chi-square test, Fisher’s exact test, and the Kaplan-Meier method.
How it could be that I moved her just one day, some neighbour ordered about the child to borrow things, wasn t that to bully1 the newcomer? Today, I lent you a candle, maybe tomorrow you would come here to brorrow onions, gingers2() and garlics, no, no! The hostess then replied, Ah, unfortunately, I just moved here, and don t prepare candles. With that, she got ready to close the door.
Research results
New Chinese patent medicine Pai-Neng-Da (PND) Capsule exerts dual effect in promoting hematopoiesis recovery and regulating immunity. However, the application of PND capsule in haplo-HSCT has not yet been reported. To the best of our knowledge, this is the first study that evaluates the efficacy of PND capsule in the treatment of acute leukemia (AL) patients following haplo-HSCT.
Research conclusions
PND capsule could promote hematopoiesis reconstitution, improve the therapeutic efficacy of Chinese medical symptom scores, present anti-tumor effectiveness, and prolong the survival of AL patients with haplo-HSCT.
Research perspectives
The pathogenesis of primary PGF following haplo-HSCT is yet unclear. The effective therapies for primary PGF need to be explored.
The correlation analysis related to risk factors of primary PGF could not be performed due to the small sample size. Notably, the primary PGF incidence (3.4%) caused by the use of PND capsule was lower than that reported in the current literature, ranging from 5% to 27%[4,5], which may be due to the following reasons: first, PND capsule has a role in improving hematopoietic recovery after transplantation, thereby reducing the rate of PGF. Second, the definition of primary PGF and the types of transplantation are different in the current and previous studies. Third, the number of our cases is limited. Thus, additional studies with more cases, unifying the definition of primary PGF and the types of transplantation, are required.
The king and queen marvelled18 greatly what this could mean; but just then the old woman who was spoken of in the beginning of the story was again brought in to the queen
FOOTNOTES
Yuan JJ wrote the first draft of the manuscript; Lu Y critically reviewed and revised the manuscript; Cao JJ and Pei RZ collected and analyzed the clinical data; Gao RL designed the study and confirmed the statistical methods for the research; all the authors read and approved the final manuscript.
The Zhejiang Provincial Science and Technology Program of Traditional Chinese Medicine, No. 2017ZA129 and No. 2018ZA112.
IN a certain far-away Tsardom not in this Empire, there lived a Tsar named Vyslav, who had three sons: the first Tsarevitch Dimitri, the second Tsarevitch Vasilii and the third Tsarevitch Ivan.
The study was reviewed and approved by the Ethics Committee of Affiliated People’s Hospital of Ningbo University Institutional Review Board (Approval No.2017022).
The authors declare that they have no conflict of interest.
No additional data are available.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Whilst I was fainting with terror he transported me here, and cried to me with his awful voice: There shall you remain, lonely and hideous84, despised even by the brutes85, till the end of your days, or till some one of his own free will asks you to be his wife
Jiao-Jiao Yuan 0000-0003-0149-6274; Ying Lu 0000-0002-8204-4917; Jun-Jie Cao 0000-0001-5542-2663; Ren-Zhi Pei 0000-0002-9006-8361; Rui-Lan Gao 0000-0002-7988-9775.
Xing YX
A
Xing YX
1 Lee CJ, Savani BN, Mohty M, Labopin M, Ruggeri A, Schmid C, Baron F, Esteve J, Gorin NC, Giebel S, Ciceri F, Nagler A. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation.
2017; 102: 1810-1822 [PMⅠD: 28883081 DOⅠ: 10.3324/haematol.2017.176107]
2 Salvatore D, Labopin M, Ruggeri A, Battipaglia G, Ghavamzadeh A, Ciceri F, Blaise D, Arcese W, Sociè G, Bourhis JH, Van Lint MT, Bruno B, Huynh A, Santarone S, Deconinck E, Mohty M, Nagler A. Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical
matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation.
2018; 103: 1317-1328 [PMⅠD: 29748438 DOⅠ: 10.3324/haematol.2018.189258]
3 Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A, Tischer J, Kröger N, Afanasyev B, Finke J, Elmaagacli A, Einsele H, Mohty M, Nagler A. Haploidentical
unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT.
2019; 104: 524-532 [PMⅠD: 30361416 DOⅠ: 10.3324/haematol.2017.187450]
4 Larocca A, Piaggio G, Podestà M, Pitto A, Bruno B, Di Grazia C, Gualandi F, Occhini D, Raiola AM, Dominietto A, Bregante S, Lamparelli T, Tedone E, Oneto R, Frassoni F, Van Lint MT, Pogliani E, Bacigalupo A. Boost of CD34+-selected peripheral blood cells without further conditioning in patients with poor graft function following allogeneic stem cell transplantation.
2006; 91: 935-940 [PMⅠD: 16818281]
5 Lee KH, Lee JH, Choi SJ, Kim S, Seol M, Lee YS, Kim WK, Lee JS. Failure of trilineage blood cell reconstitution after initial neutrophil engraftment in patients undergoing allogeneic hematopoietic cell transplantation - frequency and outcomes.
2004; 33: 729-734 [PMⅠD: 14755315 DOⅠ: 10.1038/sj.bmt.1704428]
6 Kong Y, Wang YT, Hu Y, Han W, Chang YJ, Zhang XH, Jiang ZF, Huang XJ. The bone marrow microenvironment is similarly impaired in allogeneic hematopoietic stem cell transplantation patients with early and late poor graft function.
2016; 51: 249-255 [PMⅠD: 26437066 DOⅠ: 10.1038/bmt.2015.229]
7 Gao RL, Chong BH. Research and development of the effective components of panaxdiol saponin as new Chinese patent medicine for treating hemocytopenia.
2012; 18: 897-902 [PMⅠD: 23238997 DOⅠ: 10.1007/s11655-012-1292-4]
8 Sun X, Zhao YN, Qian S, Gao RL, Yin LM, Wang LP, Chong BH, Zhang SZ. Ginseng-Derived Panaxadiol Saponins Promote Hematopoiesis Recovery in Cyclophosphamide-Ⅰnduced Myelosuppressive Mice: Potential Novel Treatment of Chemotherapy-Ⅰnduced Cytopenias.
2018; 24: 200-206 [PMⅠD: 28432529 DOⅠ: 10.1007/s11655-017-2754-8]
9 Zheng ZY, Yu XL, Dai TY, Yin LM, Zhao YN, Xu M, Zhuang HF, Chong BH, Gao RL. Panaxdiol Saponins Component Promotes Hematopoiesis and Modulates T Lymphocyte Dysregulation in Aplastic Anemia Model Mice.
2019; 25: 902-910 [PMⅠD: 31802424 DOⅠ: 10.1007/s11655-019-3049-z]
10 Jin YH, Choi J, Shin S, Lee KY, Park JH, Lee SK. Panaxadiol selectively inhibits cyclin A-associated Cdk2 activity by elevating p21WAF1/CⅠP1 protein levels in mammalian cells.
2003; 24: 1767-1772 [PMⅠD: 12819186 DOⅠ: 10.1093/carcin/bgg097]
11 Wang Z, Ding M, Lin Z, He C, Zhao Y. Esterified Derivatives of Panaxadiol and Their Ⅰnhibitory Effect on HL-60, THP-1, and PC-3 Cell Lines.
2019; 16: e1900188 [PMⅠD: 31298488 DOⅠ: 10.1002/cbdv.201900188]
12 Jiang ZY, Yu FQ, Gao RL, Kuang YM, Zhu Y, Chen YH, Li LJ, Ouyang GF, Hu J, Wu XL. Treatment of Chronic Aplastic Anemia with Chinese Patent Medicine Pai-Neng-Da capsule for Replacing Androgen Partially: A Clinical Multi-Center Study.
2021 Apr 10 [DOⅠ: 10.1007/s11655-021-3283-z]
13 Sun YQ, He GL, Chang YJ, Xu LP, Zhang XH, Han W, Chen H, Chen YH, Wang Y, Wang FR, Wang JZ, Liu KY, Huang XJ. The incidence, risk factors, and outcomes of primary poor graft function after unmanipulated haploidentical stem cell transplantation.
2015; 94: 1699-1705 [PMⅠD: 26152553 DOⅠ: 10.1007/s00277-015-2440-x]
14 Shi MM, Kong Y, Song Y, Sun YQ, Wang Y, Zhang XH, Xu LP, Liu KY, Huang XJ. Atorvastatin enhances endothelial cell function in posttransplant poor graft function.
2016; 128: 2988-2999 [PMⅠD: 27769957 DOⅠ: 10.1182/blood-2016-03-702803]
15 Zhao HY, Lyu ZS, Duan CW, Song Y, Han TT, Mo XD, Wang Y, Xu LP, Zhang XH, Huang XJ, Kong Y. An unbalanced monocyte macrophage polarization in the bone marrow microenvironment of patients with poor graft function after allogeneic haematopoietic stem cell transplantation.
2018; 182: 679-692 [PMⅠD: 29974948 DOⅠ: 10.1111/bjh.15452]
16 Song Y, Zhao HY, Lyu ZS, Cao XN, Shi MM, Wen Q, Tang FF, Wang Y, Xu LP, Zhang XH, Huang XJ, Kong Y. Dysfunctional Bone Marrow Mesenchymal Stem Cells in Patients with Poor Graft Function after Allogeneic Hematopoietic Stem Cell Transplantation.
2018; 24: 1981-1989 [PMⅠD: 29933074 DOⅠ: 10.1016/j.bbmt.2018.06.021]
17 Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, Pozzi S, Varaldo R, Lamparelli T, Bregante S, Van Lint MT, di Grazia C, Bacigalupo A. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation.
2014; 20: 1440-1443 [PMⅠD: 24862637 DOⅠ: 10.1016/j.bbmt.2014.05.016]
18 Chen J, Wang H, Zhou J, Feng S. Advances in the understanding of poor graft function following allogeneic hematopoietic stem-cell transplantation.
2020; 11: 2040620720948743 [PMⅠD: 32874483 DOⅠ: 10.1177/2040620720948743]
19 Xiao Y, Song J, Jiang Z, Li Y, Gao Y, Xu W, Lu Z, Wang Y, Xiao H. Risk-factor analysis of poor graft function after allogeneic hematopoietic stem cell transplantation.
2014; 11: 652-657 [PMⅠD: 24834012 DOⅠ: 10.7150/ijms.6337]
20 Wen WW, Sun X, Zhuang HF, Lin XJ, Zheng ZY, Gao RL, Yin LM. Effects of panaxadiol saponins component as a new Chinese patent medicine on proliferation, differentiation and corresponding gene expression profile of megakaryocytes.
2016; 22: 28-35 [PMⅠD: 25917792 DOⅠ: 10.1007/s11655-015-1970-3]
21 Zou Ch, Xiong NN, Jiang M, Gao WM, Zou JD, Liu F. Phase Ⅰ clinical study (human tolerance) on Painengda Capsules.
37: 2383-2386 [DOⅠ: 10.4268/cjcmm20151418]
22 Zhu Y, Kuang YM, Gao RL, Jiang ZY, Huang L, Tong YJ, Lou XG, Gao XF. Clinical observation of Painengda capsule in improving the quality of life in patients with chronic aplastic anemia.
2016; 26: 124-126 [DOⅠ: 10.1007/s11655-015-2158-6]
23 Wang YT, Kong Y, Song Y, Han W, Zhang YY, Zhang XH, Chang YJ, Jiang ZF, Huang XJ. Ⅰncreased Type 1 Ⅰmmune Response in the Bone Marrow Ⅰmmune Microenvironment of Patients with Poor Graft Function after Allogeneic Hematopoietic Stem Cell Transplantation.
2016; 22: 1376-1382 [PMⅠD: 27131864 DOⅠ: 10.1016/j.bbmt.2016.04.016]
24 Sun X, Gao RL, Lin XJ, Xu WH, Chen XH. Panax notoginseng saponins induced up-regulation, phosphorylation and binding activity of MEK, ERK, AKT, PⅠ-3K protein kinases and GATA transcription factors in hematopoietic cells.
2013; 19: 112-118 [PMⅠD: 23371459 DOⅠ: 10.1007/s11655-012-1306-4]
25 Nakhjavani M, Smith E, Townsend AR, Price TJ, Hardingham JE. Anti-Angiogenic Properties of Ginsenoside Rg3.
2020; 25 [PMⅠD: 33113992 DOⅠ: 10.3390/molecules25214905]
杂志排行
World Journal of Clinical Cases的其它文章
- Perfectionism and mental health problems: Limitations and directions for future research
- Ovarian growing teratoma syndrome with multiple metastases in the abdominal cavity and liver: A case report
- Development of plasma cell dyscrasias in a patient with chronic myeloid leukemia: A case report
- Suprasellar cistern tuberculoma presenting as unilateral ocular motility disorder and ptosis: A case report
- Rare pattern of Maisonneuve fracture: A case report
- PD-1 inhibitor in combination with fruquintinib therapy for initial unresectable colorectal cancer: A case report
