Co-relation of SARS-CoV-2 related 30-d mortality with HRCT score and RT-PCR Ct value-based viral load in patients with solid malignancy
2022-06-17SatyaNarayanVineetTalwarVarunGoelKrushnaChaudharyAnuragSharmaPallaviRedhuSatyajeetSoniArpitJain
Satya Narayan, Vineet Talwar, Varun Goel, Krushna Chaudhary, Anurag Sharma, Pallavi Redhu, Satyajeet Soni, Arpit Jain
Satya Narayan, Vineet Talwar, Varun Goel, Krushna Chaudhary, Pallavi Redhu, Satyajeet Soni,Arpit Jain, Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi 110085, India
Anurag Sharma, Department of Research, Rajiv Gandhi Cancer Institute and Research Centre,New Delhi 110085, India
Abstract BACKGROUND Coronavirus disease 2019 (COVID-19) patients with malignancy are published worldwide but are lacking in data from India.AIM To characterize COVID-19 related mortality outcomes within 30 d of diagnosis with HRCT score and RT-PCR Ct value-based viral load in various solid malignancies.METHODS Patients included in this study were with an active or previous malignancy and with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection from the institute database. We collected data on demographic details, baseline clinical conditions, medications, cancer diagnosis, treatment and the COVID-19 disease course. The primary endpoint was the association between the mortality outcome and the potential prognostic variables, specially, HRCT score, RT-PCR Ct value-based viral load, etc. using logistic regression analyses treatment received in 30 d.RESULTS Out of 131 patients, 123 met inclusion criteria for our analysis. The median age was 57 years (interquartile range = 19-82) while 7 (5.7%) were aged 75 years or older. The most prevalent malignancies were of GUT origin 49 (39.8%), hepatopancreatobiliary (HPB) 40 (32.5%). 109 (88.6%) patients were on active anticancer treatment, 115 (93.5%) had active (measurable) cancer. At analysis on May 20, 2021, 26 (21.1%) patients had died. In logistic regression analysis, independent factors associated with an increased 30-d mortality were in patients with the symptomatic presentation. Chemotherapy in the last 4 wk, number of comorbidities (≥ 2 vs none: 3.43, 1.08-8.56). The univariate analysis showed that the risk of death was significantly associated with the HRCT score: for moderate (8-15) [odds ratio (OR): 3.44; 95% confidence interval (CI): 1.3-9.12; P = 0.0132], severe (> 15) (OR: 7.44; 95%CI: 1.58-35.1; P = 0.0112).CONCLUSION To the best of our knowledge, this is the first study from India reporting the association of HRCT score and RT-PCR Ct value-based 30-d mortality outcomes in SARS-CoV-2 infected cancer patients.
Key Words: SARS-CoV-2; COVID-19; Cancer; HRCT; Viral load
INTRODUCTION
After initially being identified in December 2019 in the Chinese city of Wuhan, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the associated sickness of coronavirus disease 2019 (COVID-19) has become a global pandemic[1]. The novel enveloped beta-coronavirus was immediately recognized as the infecting agent[2,3]. Coronaviruses are non-segmented enveloped positive-sense RNA viruses that belong to the Coronaviridae family[4]. This is the 3rdlarge-scale health crisis caused by beta-coronaviruses. The novel coronavirus pandemic (2019-nCoV) was designated a public health emergency of international concern by the World Health Organization (WHO) on January 30 and COVID19 was classified as a pandemic by the WHO on March 11, 2020[5]. As of May 31, 2021, there have been 171 million cases reported worldwide in 222 countries with 3.55 million deaths. In India alone, there are 28 million cases and 0.32 million fatalities. India represents an approximately 16.3% share of worldwide coronavirus cases and a 9.1% share of worldwide mortality[6]. Patients with a history of active malignancy may be at a higher risk of getting COVID-19 and having COVID-19-related problems according to various reports[7-9]. Initial reports, however, are limited by sample size, geographic region and the inability to generalize findings to the entire community of cancer patients. The impact of antineoplastic therapy and supportive care for cancer patients may impair their immune system. We conducted a retrospective study on cancer patients with COVID-19 infection comparing different demographic and clinical parameters with treatment-related mortality.
MATERIALS AND METHODS
Study design and settings
This is a single-center, retrospective study conducted at a tertiary cancer care hospital. Patients with active cancer presented to the hospital between April 2020 to April 2021 with a confirmed SARS-CoV-2 infection. The inclusion criteria were the patients with confirmed COVID-19 in a diagnosed case of solid malignancy.
The study has been approved by our Institutional Review Board (RGCIRC/Res/SCM/46 2021/95) and was conducted according to the Declaration of Helsinki.
Variables and outcomes
The primary endpoint was to measure mortality within 30 d of diagnosis of COVID-19 with HRCT score and RT-PCR Ct value-based viral load. Secondary endpoints were measuring mortality compared with demographic variables (i.e.age, sex, obesity, smoking status) and clinic variables such as HRCT scoring including baseline laboratory values for D dimer, C-reactive protein (CRP), number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status, requiring active treatment, recent surgery (including, but not limited to cancer surgeries, within 4 wk of COVID-19 diagnosis), type of malignancy, cancer status (remissionvsactive disease), with active further need as stablevsresponding to treatmentvsprogressing disease), anticancer therapy and COVID-19 treatment with azithromycin, hydroxychloroquine, ivermectin or in combinationvsvarious other treatment options used,i.e.Steroid alone or in combination with Remdesivir, Tocilizumab, Plasma therapy during infection. As it is a retrospective cohort study, selection bias occurred due to the unavailability of data for a few patients.
Statistical analysis
Descriptive statistics such as age and sex was used to show the baseline demographic information of the participants included in our analyses. All quantitative data are expressed as a mean ± SD. Categorical variables are expressed as numbers and their respective percentage. Univariate analysis was conducted to determine the risk factor of death in all the admitted patients by using Logistic regression. All data entries and statistical analyses will be performed by using SPSS®Version 23.0 software. All these statistics will be accompanied by 95% confidence intervals (CI). All the reported p-values will be twosided andP-values < 0.05 shall be considered to indicate statistical significance.
RESULTS
Out of 131 patients, 123 met the inclusion criteria for our analysis. The clinical features are shown in Table 1. The median age was 57 years (interquartile range [IQR] 19-82), 7 (5.7%) were aged ≥ 75 and 64 (52%) of the patients were female. The most common malignancies were of GUT origin 49 (39.8%) and hepato-pancreaticobiliary 40 (32.5%). 109 (88.6%) patients were receiving active anticancer therapy with 115 (93.5%) patients having active (measurable) cancer. At analysis on May 20, 2021, 26 (21.1%) of the patients had died.
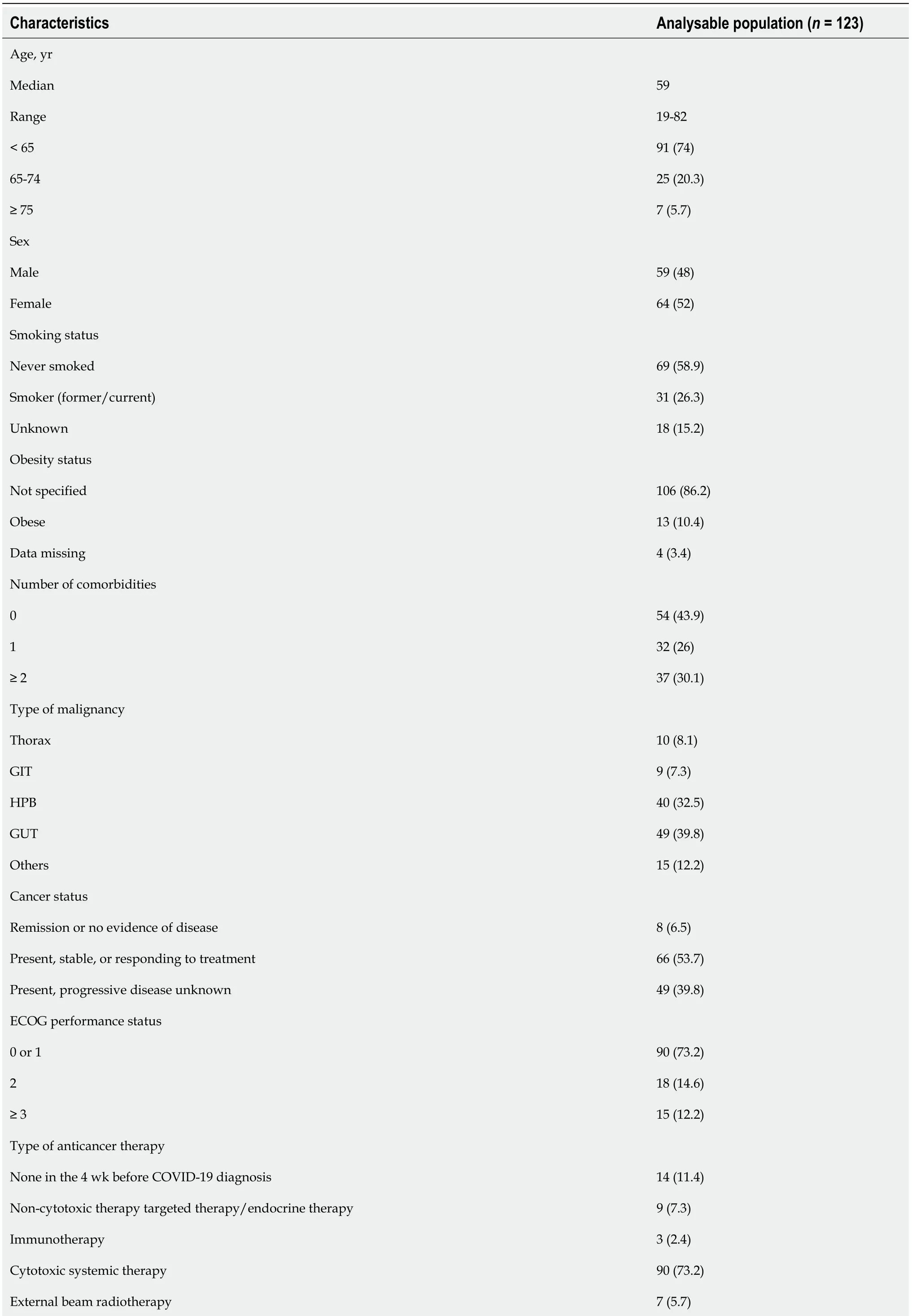
Table 1 Patient demographic, clinical, baseline laboratory parameter and tumour characteristics, n (%)

GIT: Gastrointestinal tract; HPB: Hepatopancreatobiliary; GUT: Genitourinary tract; COVID-19: Coronavirus disease 2019; ECOG: Eastern Cooperative Oncology Group.
Patients with mild or moderate disease were given symptomatic treatment, and most mild disease patients were treated with home-based care. Hydroxychloroquine, ivermectin and/or dexamethasone were administered in moderate disease cases. Corticosteroids, hydroxychloroquine, ivermectin, remdesivir, tocilizumab and convalescent plasma therapy were used to treat severe COVID-19-infected cancer patients. Assisted ventilation was given to 18 patients (6.45%) but all of these patients later experienced COVID-19-related complications such as pneumonitis and subsequent respiratory failure, septic shock, or sudden cardiac arrest and succumbed to their illness.
The univariate logistic regression analysis for mortality has been shown in Table 2. The risk of death was statistically significant with the presence of symptomatic presentation (odds ratio [OR] = 11.1,P= 0.0211), number of comorbidities ≥ 2vsnone (OR = 3.43,P= 0.0303), Eastern Cooperative Oncology Group performance status of 0/1 v/s ≥ 2 (OR = 3.88,P= 0.047). The odds of mortality were significantly higher in patients presenting with moderate OR = 3.44,P= 0.0132) and severe HRCT score (OR = 7.44,P= 0.0112) as compared to patients with mild HRCT score (Table 3). Similarly, patients with smoking habits were at a high risk of 30-d mortality (OR = 5.54,P< 0.001). Progressive disease was also found to be a significant risk factor for mortality with OR = 25.5) (Figure 1).
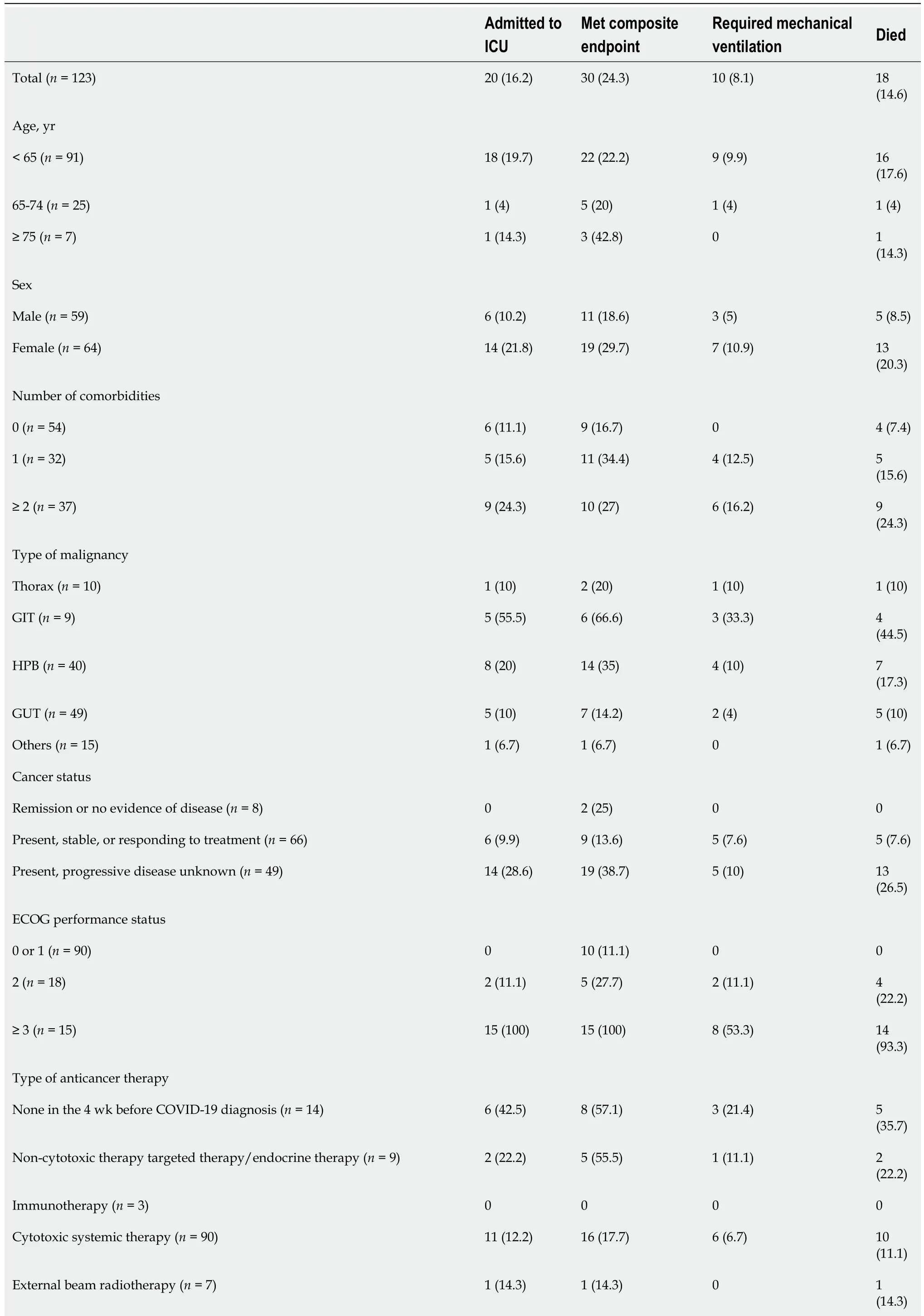
Table 2 Primary and Secondary outcomes by potential prognostic variables (n = 123), n (%)

GIT: Gastrointestinal tract; HPB: Hepatopancreatobiliary; GUT: Genitourinary tract; COVID-19: Coronavirus disease 2019; ECOG: Eastern Cooperative Oncology Group; ICU: Intensive care unit.
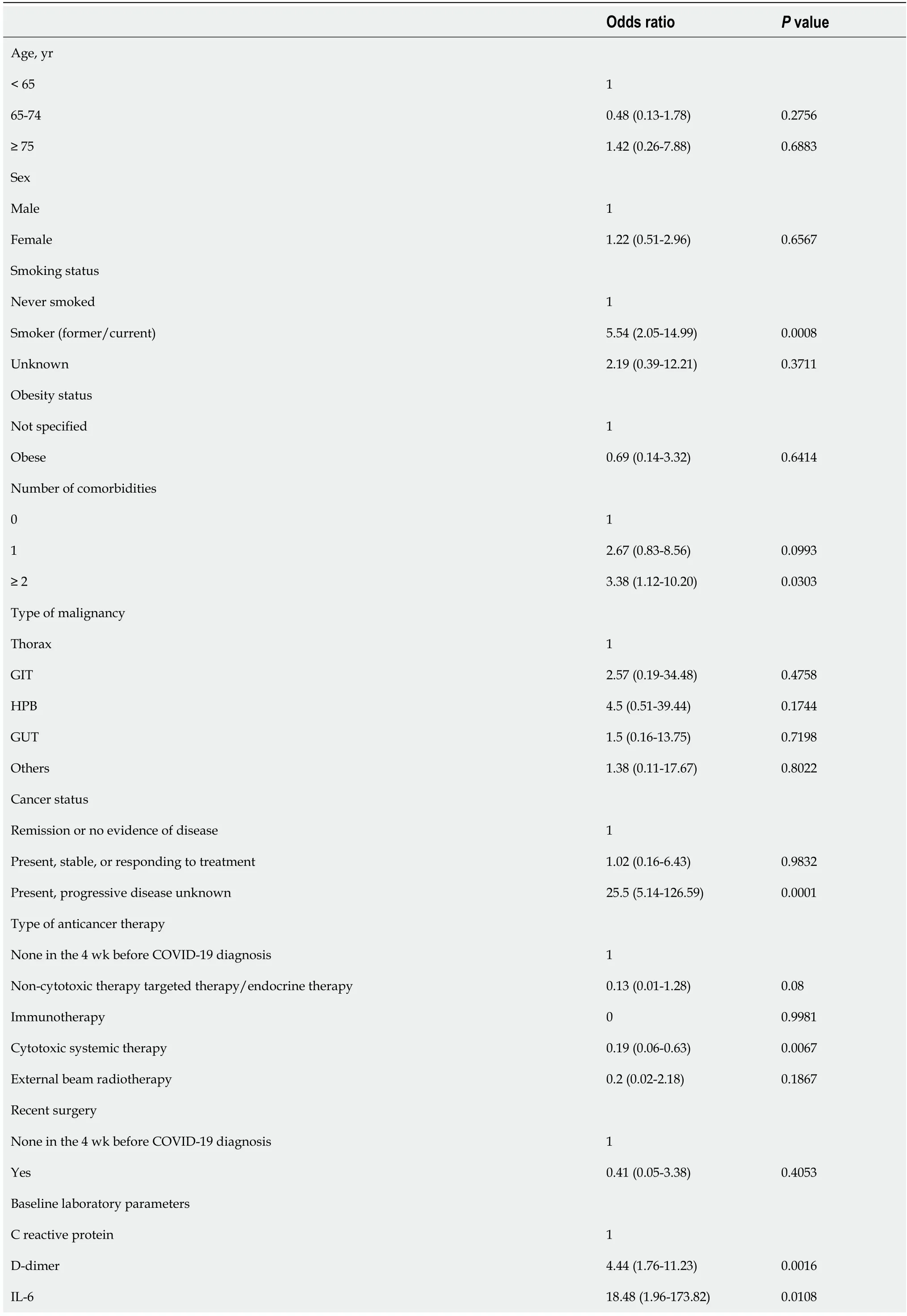
Table 3 Univariate regression models of potential variables associated with 30 d all-cause mortality (n = 123)

GIT: Gastrointestinal tract; HPB: Hepatopancreatobiliary; GUT: Genitourinary tract; COVID-19: Coronavirus disease 2019; ECOG: Eastern Cooperative Oncology Group; ICU: Intensive care unit.
No statistically significant association of 30-d mortality was found concerning age, sex, type of malignancy, type of anticancer therapy obesity status, recent surgery and active cancer (progressingvsremission). Also, no significant effect on mortality was noted for the patients with RT PCR based on different viral load levels.
DISCUSSION
Cancer patients are a particularly vulnerable group in the current COVID-19 pandemic. They are at a higher probability of severe illness and increased mortality once diagnosed with COVID-19. This article analyzes previously known cancer patients and COVID-19 prognostic factors provide information on clinical management and outcomes of cancer and COVID-19 patients.
In a few studies, men were found to have a higher mortality risk than women. In addition to sex disparities and smoking rates, this is due to the difference in immunological and endocrine systems between men and women which may result in differential responses to the SARS-CoV-2 infection. The present study has no similar difference related to the sex of the patient. In reports from Europe, the United States and China, non-malignant populations are consistent with COVID-19 outcome data reported for overall mortality and was associated with comorbidities such as obesity and advanced population age[10,11].
Moreover, case-fatality rates for patients with COVID-19 who had breast, thyroid, or cervical cancer were low in the previously published study. As reported, 62 (57%) of 109 women had one of these three types of cancers[12-14]. The United Kingdom Coronavirus Cancer Monitoring project (UKCCMP) with a database of 800 patients with the most common cancers were the gastrointestinal, respiratory, breast, male genital and hematological cancers. In our study, 43% of our patients had metastatic disease and 89 (72.3%) of these patients having the metastatic disease were in the GIT and HPB malignancy cohorts. Sixty-five patients had cancer treatment in the previous 4 wk for the UKCCMP while in our study 88.6% of patients had received some form of treatment in the last 4 wk. In the UKCCMP study and in our study ≥ 50% of the patients receiving treatment had received cytotoxic chemotherapy. 45 patients had a severe form of infection. The mortality rates were high at 28% (226 out of 800 in the UKCCMP) while in our present study 21.1% (26 out of 123). Disease fatality is the function of pathogen virulence, host tolerance and pathogen load[15]. Pathogenicity is often the consequence of an overactive immune or inflammatory response[15,16]. Cancer patients normally have a compromised immunity due to their existing cancer and associated treatment[17]. So, cancer patients may have persistent SARS-CoV-2 viral infection which cannot be cleared by their compromised immune system in a short time, but their COVID-19 disease is not severe and some of them may still recover from the COVID-19 disease. The majority of patients exhibited COVID-19-like symptoms and the overall rate of complications were higher. The patients who died had higher co-morbidities and were older than those who recovered. Patients who got chemotherapy within the last 4 wk of COVID-19 did not have a higher mortality rate than those who did not get chemotherapy. Patients who received non-chemotherapy treatments (radiation, hormone therapy, immunotherapy, and targeted therapy) did not have an increased risk of death[18]. COVID-19 has been associated to a greater fatality rate in cancer patients but cancer treatments have not been associated with an increased risk of mortality as found in this study.
The COVID-19 and Cancer Consortium (CCC19) published the results of 928 cancer patients from the United States, Canada and Spain who had COVID-19 infection. 654 patients with solid tumors, hematological malignancies diagnosed in 167 patients and 107 patients with multiple malignancies. In this study, 73.2% of the patients got cytotoxic chemotherapy in the previous 4 wk, whereas 160 patients received chemotherapy treatment and 206 patients received alternative forms of cancer therapy. The mortality rate was 13% within 30 d of COVID-19 diagnosis. Interestingly, 59% (n= 71) of the patients who died were never admitted to the intensive care unit (ICU); while in this study 30.8% (n= 8) died who were never admitted to the ICU. Outside the ICU, patients with active cancer have a higher death rate than those in remission. There was no association between 30-d all-cause mortality and noncytotoxic treatments, recent surgery and cytotoxic treatment[19].
Mehtaet al[20] have reported outcomes on 218 cancer patients with COVID-19. Seventy-five patients had solid tumors and 25% had hematological malignancies. The most common tumor types were genitourinary, breast and colorectal cancer, respectively. A total of 61 (28%) patients died. The mortality rate was 55% in patients with lung cancer and 67% with pancreatic cancer. Breast (14%) and genitourinary cancer (15%) were associated with a relatively lower mortality rate. Active chemotherapy and radiation therapy were not associated with increased mortality. Active disease (< 1 year) and metastatic disease were associated with higher numerical mortality values but without statistical significance[20].
Studies that report Ct values of RT-PCR to quantify SARS-CoV-2 RNA in clinical material is limited. Patients with severe disease had significantly higher viral loads and the viral load was higher during the early stages of the disease according to Zhenget al[21]. Karahasan Yagciet al[22] reported that higher viral load was linked with increased age, comorbidities, smoking status and recent chemotherapy. SARS-CoV-2 RNA had a median Ct value of 28.16 (IQR: 24.5–31.6) in hospitalized patients and 26.77 (IQR: 23.1-29.7) in outpatients in the study. The number of comorbidities were higher in hospitalized patients (P< 0.01). In COVID-19, Huanget al[23], in 2020, reported that elevated CRP was associated with higher composite poor outcome and disease severity.
The CRP levels available at the time of the PCR request were largely for hospitalized patients, hence a statistical comparison could not be established in this study. In a study of 76 patients, it was found that the Ct values of severe cases remained considerably lower for the first 12 d following commencement as compared to moderate instances[24].
Early in the disease course, Panet al[25] observed a lot of ground-glass opacity abnormalities followed by the development of crazy paving patterns on chest CT and finally increasing consolidation later on. According to study outcomes, chest CT has a high specificity but a low sensitivity, particularly in patients who appear within the first 4 d of the sickness. The clinical value of chest CT was observed to be limited in a review article, particularly for individuals who have no symptoms and are screened early in the disease progression[26]. The inverse relationship between viral load and chest CT TSS was the most striking finding. In hospitalized patients and outpatients with extensive lesions on CT, the viral load of nasopharyngeal samples was considerably lower. The severity of a CT scan was related to the patient's age and older patients having higher severity scores (P< 0.01). Hospitalization was related to the presence of any kind and number of comorbidities, but not to CT severity. Patients with obesity or other metabolic syndromes like diabetes mellitus still have a competent immunity which may be malfunctioning due to overnutrition. When these patients are infected by the SARS-CoV-2 virus, the infection may trigger hyper inflammation which makes a lot of collateral damage to all organs (those who were not infected by the virus) in the body. So, the COVID-19 disease can be very severe or even the quick demise of the patient, even if their competent immune system is able to clear the SARS-CoV-2 viruses effectively.
Even if the viral load of SARS CoV-2 in nasopharyngeal swab specimens is high in the early stages of COVID-19, it is not always related to changes in chest CT. The viral load of nasopharyngeal swab specimens decreases as SARS CoV-2 progresses but the viral load of lower respiratory tract samples increases and chest CT changes become more visible. It's thought that viral load is vital for recognizing early stages of Covid-19 infection and limiting transmission but CT can only help identify cases that require substantial medical care.
Patients who died had lower average Ct values across multiple time points during the disease course than those who recovered or were still hospitalized at the end of the study [recovered: median 37.43 (IQR 34.94-38.67); still hospitalized: median 36.97 (IQR 34.33-38.70); deceased: median 34.79 (IQR 24.46-37.65);P= 0.001] in a study of 308 patients from China. A study reported on the link between mortality and SARS-Cov-2 Ct values and found that lower Ct values were associated with a higher risk of death which is consistent with previous results regarding epidemic-causing coronaviruses[27,28]. C-reactive protein levels were shown to be adversely linked with Ct value in a study of 12 patients (r = -0.584;P= 0.03), but not in a study of 25 patients (P = 0.07)[29].
Several studies have reported the relationship between viral load as determined by Ct values and disease severity, and one of them (including 96 patients) found that higher viral loads were significantly related with more severe disease (Table 2)[30]. Mean viral loads were not significantly different between patients with pneumonia, severe pneumonia and those without pneumonia in a study by Shiet al[31]. Patients with severe pneumonia had a significantly higher viral load than those without pneumonia but severity outcomes were not statistically significant in this study. Shahet al[32] reported similar results in that there is no correlation between Ct values and severity of the disease.
CONCLUSION
With a CFR of 21.1%, this study reveals the significant rates of mortality in COVID-19 cancer patients. When comparing older cancer patients to younger cancer patients, mortality rates are not higher. Patients who had high baseline HRCT values at presentation and required ICU care had a higher mortality.
ARTICLE HIGHLIGHTS
Research background
The lack of correlation data between coronavirus disease 2019 (COVID-19) and Solid malignancy limits the understanding of the true mortality impact of the COVID-19 in the Indian settings.
Research motivation
Higher incidence of serious clinical events and intensive care unit (ICU) admissions in cancer patients.Analysis suggests patients have increased morbidity and mortality from recent cytotoxic chemotherapy.Patients with active untreated cancer, metastatic disease, progressive disease with multiple comorbidity and getting palliative treatment are at a higher risk of mortality.
Research objectives
The primary objectives of the study include COVID-19 related mortality outcomes within 30 d of diagnosis with HRCT score and RT-PCR Ct value-based viral load in various solid malignancies.
Research methods
This is a single-center, retrospective study conducted at a tertiary cancer care hospital including confirmed COVID-19 in a diagnosed case of solid malignancy. The primary endpoint was to measure mortality within 30 d of diagnosis of COVID-19 with HRCT score and RT-PCR Ct value-based viral load.
Research results
The risk of death was statistically significant with the presence of symptomatic presentation, number of comorbidities ≥ 2vsnone, Eastern Cooperative Oncology Group performance status of 0/1 v/s ≥ 2. The odds ratio of mortality were significantly higher in patients presented with moderate and severe HRCT scores as compared to patients with mild HRCT scores. No statistically significant association of 30-d mortality was found concerning age, sex, type of malignancy, type of anticancer therapy, obesity status, recent surgery and active cancer (progressingvsremission). Also, no significant effect on mortality was noted for the patients with RT PCR based on different viral load levels.
Research conclusions
Mortality rates are higher in patients with high baseline HRCT values at presentation and who need longer ICU stays. Mortality rates are not higher in older cancer patients as compared to younger counterparts with cancer. Mortality rates are not statistically significant in co-relation with high baseline RT-PCR based viral load values at presentation.
Research perspectives
If there are further COVID-19 outbreaks, the findings of these studies will be helpful for clinical practice to categorize the patients on the basis of various demographic and clinical parameters for prognostication of patients.
FOOTNOTES
Author contributions:Narayan S and Talwar V were involved in design of study and the acquisition of data; Goel V, Chaudhary K, Redhu P, Soni S, Jain A were involved in the drafting and revision of the manuscript; Narayan S and Sharma A were involved in the statistical calculation; All authors discussed and contributed to the final manuscript.
Institutional review board statement:The study has been approved by our Institutional Review Board (RGCIRC/Res/SCM/46 2021/95) and was conducted according to the Declaration of Helsinki.
Conflict-of-interest statement:Authors have no conflict of interest.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Satya Narayan 0000-0003-1957-1674; Vineet Talwar 0000-0001-9149-6969; Varun Goel 0000-0003-3590-3261; Krushna Chaudhary 0000-0003-4952-8888; Anurag Sharma 0000-0002-3482-0774; Pallavi Redhu 0000-0001-8829-9438;Satyajeet Soni 0000-0003-2564-2619; Arpit Jain 0000-0002-3916-4411.
Corresponding Author's Membership in Professional Societies:European Society for Medical Oncology.
S-Editor:Gong ZM
L-Editor:Filipodia
P-Editor:Gong ZM
杂志排行
World Journal of Clinical Oncology的其它文章
- Commentary: Evaluating potential glioma serum biomarkers, with future applications
- How to improve metastatic pancreatic ductal adenocarcinoma patients’ selection: Between clinical trials and the real-world
- Immune checkpoint inhibitors in head and neck squamous cell carcinoma: A systematic review of phase-3 clinical trials
- Assessing optimal Roux-en-Y reconstruction technique after total gastrectomy using the Postgastrectomy Syndrome Assessment Scale-45
- Modified binding pancreaticogastrostomy vs modified Blumgart pancreaticojejunostomy after laparoscopic pancreaticoduodenectomy for pancreatic or periampullary tumors
- Survival characteristics of fibrolamellar hepatocellular carcinoma: A Surveillance, Epidemiology, and End Results database study
