Outcome of percutaneous drainage for septic complications coexisted with COVID-19
2022-06-02MohamedDeifAhmadMounirSherifAboHedibahAhmedAbdelKhalekAliElmokadem
INTRODUCTION
Sepsis is defined as a life-threatening organ dysfunction that happens due to dysregulated host response to an infection[1]. In the bacterial type of sepsis, which is the most frequent etiology, early and rapid therapy by the appropriate antibiotic is essential to reduce the incidence of complications and mortality rates. Most patients infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) present no severe symptomatology, but almost 5% of patients show severe lung injury or even multiple organ dysfunction syndrome, with mortality at the ICUs between 8% and 38% depending on the country[2,3]. Patients admitted to ICU showed a dysregulated host response in the form of hyperinflammation, changes in the coagulation profile, and dysregulation in the immune response[4], similar to what happens in bacterial sepsis[5,6]. The body’s adaptive protection mechanism is formed by a moderate inflammatory response and immune suppression, and if any of them become excessive or uncontrolled, this protective compensation will transform into destructive and decompensated status, then sepsis develops[7-9]. Accordingly, most deaths in critically ill coronavirus disease 2019 (COVID-19) patients are caused by sepsis[10,11].
Hematological examinations for COVID-19 patients show elevated cytokines, C-reactive protein (CRP), abnormal liver and myocardial enzymes decreased lymphocytes, declined platelets, and increased D-dimmer[12]. These findings are similar to sepsis caused by bacterial infections. So, severe COVID-19 could be a sepsis-induced by viral infection causing severe systemic inflammatory response (so-called inflammatory storm)[13,14]. Inflammatory storms are not unique to COVID-19 but also happen in other respiratory viral infections that mimic COVID-19[15,16], such as influenza, SARS, avian influenza, swine flu, and MERS[17-19]. Additionally, specimen cultures in about 80% of COVID-19 patients with septic complications show no bacterial or fungal infection, and the viral infection seems to be the only cause for sepsis[20,21]. Accordingly, sepsis is expected to be responsible for worsening the clinical conditions of these critically ill COVID-19 patients. Our objective was to describe the clinical, radiological, and laboratory characteristics of a small cohort of patients infected by SARS-CoV-2 who underwent percutaneous drainage and their post-procedural outcomes. We hypothesized that septic complication associated with severe COVID-19 has a poor outcome despite adequate percutaneous drainage and antibiotic therapy.
The princess s body was lain the same day in a plain wooden chest, and set in the chapel16 of the castle, and on that night and every night after it, a sentinel was posted in the church, to keep watch over the chest
All, that is, except for a few irreplaceable bulbs. For years, Arnoldus had been trying to cultivate a black tulip, something no gardener had ever been able to do. He was now very close. By careful selection, he had created a dark-purple tulip. These few bulbs he guarded vigorously to prevent people from stealing them for food. He did not even give them to his family to eat, because they would make just one meager4() meal, and eating them would destroy his chance of restarting his business and restoring his village after the war.
MATERIALS AND METHODS
Patient selection
A local institutional review board approved this retrospective study, and waivers of consent of medical record review were received. COVID-19 patients who underwent image-guided percutaneous drainage for suspected septic complications were identified. Patient demographics and clinical and radiological reports were obtained through electronic medical records and picture archiving and communication system (PACS). The severity of the pulmonary parenchymal involvement and distribution of the pulmonary lesions secondary to COVID-19 was assessed by chest X-ray in 4 patients and chest CT in 7 patients. Flow chart of the study is shown in Figure 1.
Patients demographics
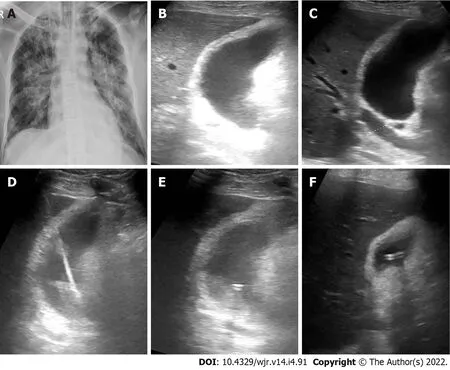
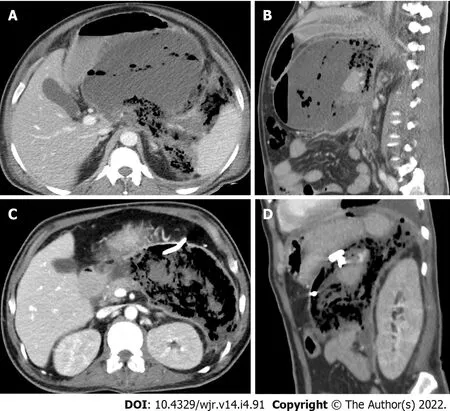
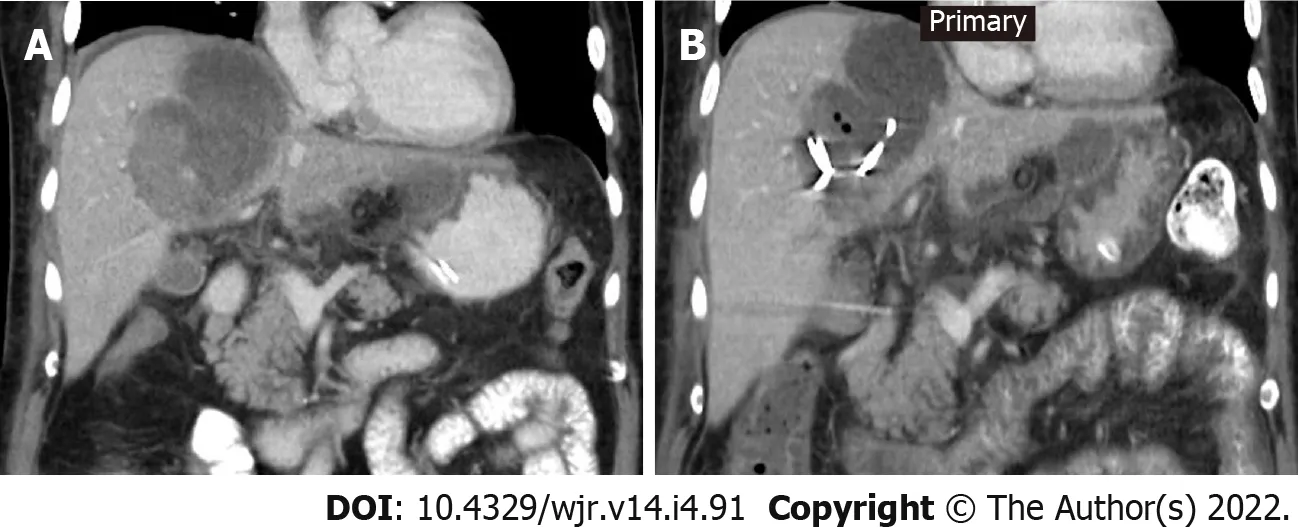
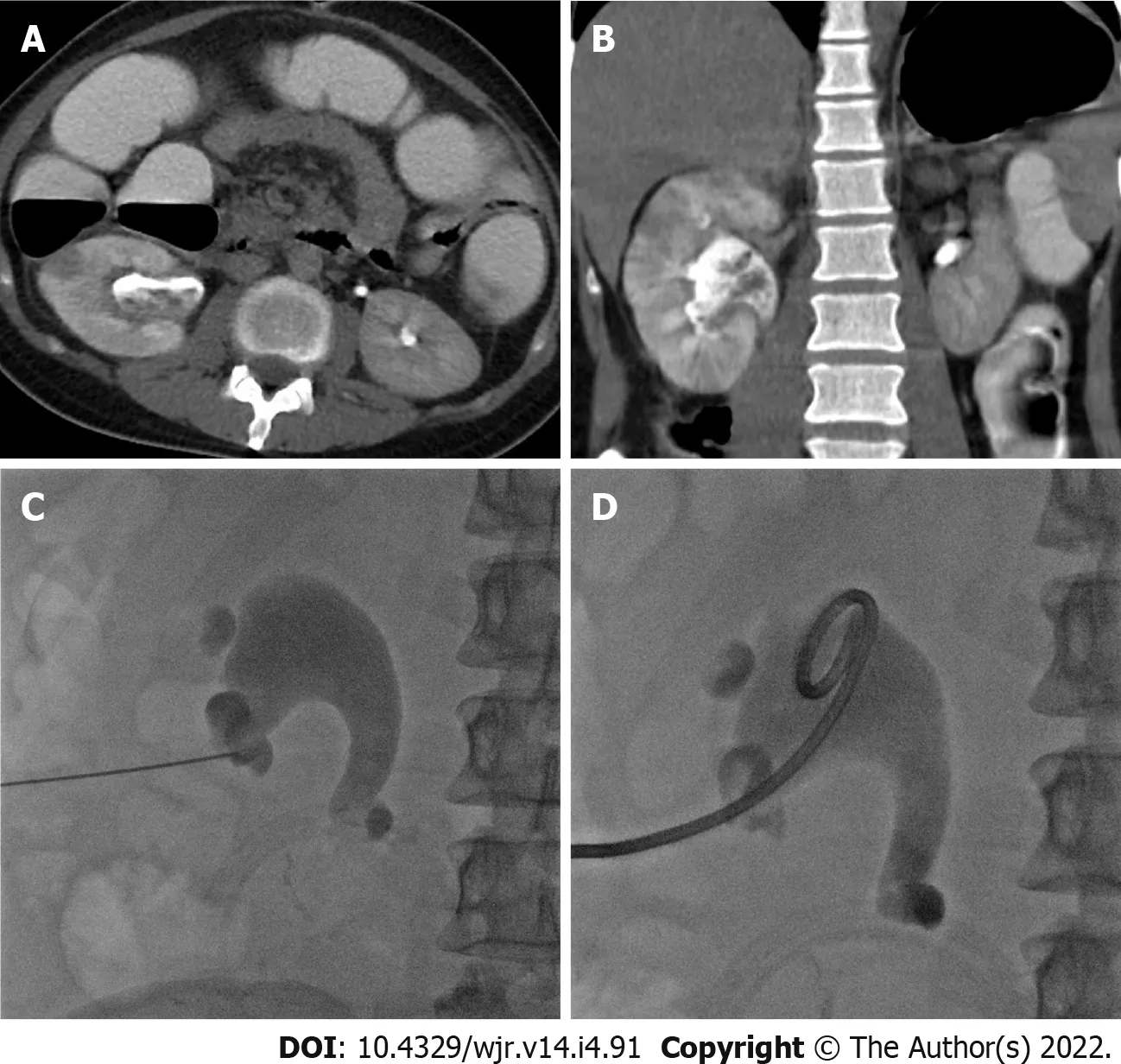
Eleven patients (10 males and 1 female) who were confirmed to have COVID-19 by RT-PCR test required drain placement for septic complications. The mean age ± SD of the patients was 48.5 ± 14 years (range 30-72 years). Three patients underwent cholecystostomy for acute acalculous cholecystitis (Figure 2). Percutaneous drainage was performed in seven patients; two peripancreatic collections (Figure 3); two infected bile leaks in hepatic donor and after resection of hepatic hemangioma; one recurrent hepatic abscess after eight days of surgical evacuation (Figure 4), one psoas abscess (Figure 5) and one lumbar abscess. One patient underwent percutaneous nephrostomy for acute pyelonephritis (Figure 6).
This retrospective study consisted of 11 patients who were confirmed to have COVID-19 by RT-PCR test and required drain placement for septic complications. The mean age ± SD of the patients was 48.5 ± 14 years (range 30-72 years). Three patients underwent cholecystostomy for acute acalculous cholecystitis.Percutaneous drainage was performed in seven patients; two peripancreatic collections; two infected leaks after hepatic resection; one recurrent hepatic abscess, one psoas abscess and one lumbar abscess.One patient underwent a percutaneous nephrostomy for acute pyelonephritis.

Study outcomes
The primary outcome measures were technical and clinical success. The technical success was achieved by completion of the procedure without procedural complications, while the definition of clinical success was the resolution of symptoms without the subsequent need for operative drainage or patient mortality secondary to related sepsis. Secondary outcomes included the amount of drained fluid, microbial analysis of drained fluid, the period of tube drainage, and changes in laboratory findings before and after drainage.
Percutaneous drainage procedures
Septic complications were diagnosed by ultrasonography, computed tomography, or magnetic resonance imaging. Two interventional radiologists at two institutions with 10 and 13 years of experience performed all percutaneous drainage procedures. All procedures were done after administration of local anesthesia. Percutaneous access into the collections, inflamed gall bladder, or kidney was achieved under sonographic guidance with an 18- or 21-gauge needle. Using the Seldinger technique and micro-puncture set, following serial dilatations, a drainage catheter was placed. The drainage catheters used ranged from 8-French to 10-French. In all cases, no immediate complications were noted.
His despair at being so late bewildered him so entirely57 that he appeared in his natural form and attempted to sprinkle some black liquid over the bride and bridegroom, which was intended to kill them, but the Fairy stretched out her wand and the liquid dropped on the Magician himself
Antibiotic therapy was started once the symptoms of septic complications presented on the patients. The antibiotics regimen was readjusted according to the drained fluid culture results. The drained fluid for each patient was analyzed regarding its character and maximum possible volume when the tubes were initially placed. Then a fluid sample was sent for bacterial culture and gram stain evaluation. Patients were observed for any major complications requiring surgical intervention till the last date of follow-up.
18.Red:Red is a color of passion and brilliance68. It demands attention, which the sisters are hoping for in their pursuit of the prince for marriage.Return to place in story.
Statistical analysis
Data were analyzed with SPSS
V. 21 (IBM Corp., New York, NY, United States; formerly SPSS Inc., Chicago, IL, United States). The normality of data was first tested with the Shapiro test. Qualitative data were described using numbers and percentages. Continuous variables were presented as mean ± SD for parametric data and median (range) for non-parametric data. Finally, the laboratory findings were compared with Wilcoxon test.
RESULTS
Fever and abdominal pain were the most common presenting symptoms, and acute kidney injury (AKI) was the most frequent comorbidity. Technical success was achieved in 100% of patients, while clinical success was achieved only in 4 of 11 patients (36.4%). Despite percutaneous drainage, one patient (9%) needed exploratory laparotomy five days after drainage that revealed perforated sigmoid colon, which was managed by resection followed by patient improvement and discharge after 18 d. Six other patients (54.5%) died within a month after proper percutaneous drainage and adequate antibiotic coverage, all of them were admitted to ICU and put under mechanical ventilation. The cause of death was overlapped between COVID-19 related respiratory failure and sepsis. One patient needed cystogasterostomy for peripancreatic collection after 21 d of tube insertion. Two patients (18.2%) had two drainage procedures to drain multiple fluid collections. Two patients (18.2%) had recurrent fluid collections and repeated percutaneous drainage procedures. The average volume of the drained fluid immediately after tube insertion was 85 mL. The average duration of drainage was 16 d. Follow-up scans showed a reduction of the retained content and associated inflammatory changes after tube insertion in all patients. Patient demographics, comorbidities, and outcomes are listed in Table 1.
All of a sudden, there, at the back of the store, in gleaming silver, full of lifejackets, paddles and fishing stuff, sat the exact canoe of my husband s picture. I gasped7 and blinked three times. Yup. It was still there. The Supremo Numero-Uno blah, blah. My heart beat wildly. I elbowed my way through the crowds, scrambled8 over junk in the aisles and darned near fell into the canoe looking for the price tag.There it was - a little tattered9, with the manufacturer s suggested retail10 price at $6,750 plus tax crossed out and a handwritten TO CLEAR $750 AS IS. NO RETURNS. Must be a mistake. $6000 off? Salesman. I had to talk to a salesman.I spotted11 a young fellow with a Hi. I m Mathew tag trying to hide out from the mob of bargain hunters. I clutched his sleeve. Mathew. Tell me about this El Supremo canoe. What s wrong with it? Why is it only $750?
The nature of drained fluid was reported in all cases. The fluid was reported as “dark green” or “pus” in cholecystostomy cases, “serosanguinous” and “infected bile” in complicated hepatic resection cases, “brownish” in the peripancreatic collection, “clotted blood” in the hepatic abscess, and “pus” in the other collections. After all procedures, samples from drained fluid samples were sent for microbial analysis. Peripheral blood culture was performed for 9 out of 11 patients. In three cases (27.3%), fluid culture results were negative for bacterial growth; however, in one of them, the peripheral blood culture was positive for
pneumonia. Eight cases (72.7%) were found to have positive fluid culture, with Escherichia coli being the most common isolated pathogen followed by
pneumonia.
Then the boy would try his strength with the rest, but he threw the quoit so far that it went beyond what had ever been thrown before, and fell in the crowd, striking a man so that he died
Only three patients had imaging features of severe pulmonary parenchymal disease attributed to COVID-19 at drainage tome, nevertheless three other patients were admitted to ICU and put under ventilator due to progression of respiratory symptoms. The parenchymal lesions were ground-glass opacities and consolidations with the basal and peripheral predominant distribution. In addition, pleural effusion was reported in three patients. The median time between confirmed diagnosis of COVID-19 by RT-PCR test and drainage of septic complications (time to drainage) was 8 d (range 0 d to 48 d). Table 2 shows data of drainage procedure, drained fluid, outcome, and chest imaging.
Deif MA and Elmokadem AH designed the research study; Deif MA and Mounir AM performed the research; Elmokadem AH, Abo-Hedibah SA and Abdel Khalek AM analyzed the data and wrote the manuscript; all authors have read and approved the final manuscript.

DISCUSSION
Prospective, larger multicentric study is needed to validate our results.
This study included different types of septic complications as acute acalculous cholecystitis, acute pancreatitis, post-operative infection, abscesses in different locations, and acute pyelonephritis. Several reports described acute acalculous cholecystitis in COVID-19 patients[25-30] and raised the possibility of underlying dysregulated immune response or presence of viral RNA within the gall bladder wall as a culprit factor[28-30]. Percutaneous cholecystostomy for COVID-19 patients is recommended by multisociety position statement in case of surgical contraindication and after the failure of conservative therapy with antibiotics[31]. It is generally a preferred non-surgical procedure due to its relative safety, simplicity of execution, and reduced costs. Mattone
[25] reported clinical failure of percutaneous cholecystostomy after 3-d from tube insertion; the patient was shifted to surgery that revealed gangrenous cholecystitis. In this study, clinical success was reported only in one of three patients had cholecystostomy drainage of acute cholecystitis. Contrary to this result, cholecystostomy improved the clinical status of patients presented by acute acalculous cholecystitis co-existed with COVID-19[26,27]; however, the period of hospitalization was prolonged (25-67 d) compared to the mean hospitalization period in non-COVID-19 patients (10.5 d)[32].
COVID-19 associated pancreatic injury and acute pancreatitis are thought to be a result of direct cytopathic effect mediated by local viral replication or indirect mechanism related to either a systemic response to a harmful immune response or respiratory failure induced by the SARS-CoV-2[33]. COVID-19 patients with acute pancreatitis are more likely to experience admission to the ICU, peripancreatic fluid collections, pancreatic necrosis, persistent organ failure, prolonged hospital stay, and higher than usually reported 30-d mortality[34]. We encountered two cases of pancreatitis in the current study, one of them died 28 days after drainage.
In a meta-analysis performed by Abate
[35], twenty-three articles with 2947 participants were included. The meta-analysis showed a very high global rate of postoperative mortality among COVID-19 patients of 20%. Percutaneous drainage was performed for two patients after complicated hepatic resection for hemangioma and liver donor, only the second patient survived and was discharged 18 days after drainage. The good outcome in this patient is attributed to the non-inflammatory nature of the drained fluid, lower inflammatory marker and less severity of COVID-19 as compared to the other patient.
Hepatic abscesses have been described in association with COVID-19[36,37]. While García Virosta
[36] reported clinically successful percutaneous drainage for hepatic abscess and patient discharge after ten days from tube insertion, Elliot
[37] reported a rapidly progressive severe acute respiratory distress syndrome, which was complicated by multiorgan failure and severe sepsis that ended by death after percutaneous drainage of hepatic abscess in a patient with COVID-19. One patient in this study presented with a large lumbar region abscess secondary to sigmoid colon perforation as proved by laparotomy. Bowel perforation secondary to COVID-19 has been attributed to microcirculation thrombosis[38] or direct insult to the colonic cells by the SARS-CoV-2 itself[39].
There is scanty literature on the association between COVID-19 and acute pyelonephritis. van 't Hof
[40] described an unusual course of acute pyelonephritis in a young female with persistent fever and multiple blood clotting and hemorrhagic events one week after recovery from COVID-19. Similar to our results, pyelonephritis was managed successfully by percutaneous nephrostomy. More frequently, AKI is encountered among critically ill patients with COVID-19, affecting approximately 20%-40% of patients admitted to the hospital and particularly to the ICU[41]. AKI was the most frequent comorbidity (5/11) in this study. A significantly higher in-hospital death rate for patients with kidney abnormalities and AKI was reported by a study consisting of 701 SARS-CoV-2 positive patients[42].
We performed percutaneous drainage for septic complications of COVID-19 and wanted to report our experience.
COVID-19 requires a multidisciplinary approach to treatment with interventional radiology procedures that have contributed to worldwide patient care. In a study consisting of 92 patients who underwent 124 interventional procedures[43] [abscess drainage (12), percutaneous cholecystostomy (8), and nephrostomy tube (4)], the mortality rate in this study was 16.3 % (15/92). However, there was no specific data as regards clinical, laboratory, and radiological data of the included patients or correlation between specific IR procedures and mortality. In this study the poor outcome was related to the combined burden of severe COVID-19 pneumonia, presence of other co-morbidities and extent of sepsis.
This study has several limitations. First, our study cohort is small. Second, this study was retrospective in nature. Third, our results were not compared to a negative SARS-CoV-2 group with matched age, complication, and comorbidities; this may have overestimated the poor outcome of percutaneous drainage in this study group.
CONCLUSION
The current study demonstrates relatively poor clinical outcomes for patients having suspected septic complications associated with COVID-19 despite technically successful tube drainage and adequate antibiotic therapy. This study emphasizes the need for a large-scale comparative study on the relationship between septic complications, COVID-19, and comorbidities that might lead to poor clinical outcomes and clarifies the necessary precautions for percutaneous drainage in such patients.
ARTICLE HIGHLIGHTS
Research background
The resulting tissue hypoxia and increased inflammation secondary to severe coronavirus disease 2019(COVID-19) combined with viral load, and other baseline risk factors contribute to an increased risk of severe sepsis or co-existed septic condition exaggeration.
Research motivation
14.When a star falls, a soul ascends to God: A folklore40 superstition41. A corollary superstition states that a shooting star represents a soul escaping purgatory42.
Research objectives
To describe the clinical, radiological, and laboratory characteristics of a small cohort of patients infected by severe acute respiratory syndrome coronavirus 2 who underwent percutaneous drainage for septic complications and their post-procedural outcomes.
Research methods
Mike, seated beside me, shook his head sadly, I wish just one of them could have won, he said. They have a lot of potential, but losing like this could take the heart right out of them.
Research results
Technical success was achieved in 100% of patients, while clinical success was achieved in 4 out of 11 patients (36.3%). Six patients (54.5%) died despite proper percutaneous drainage and adequate antibiotic coverage. One patient (9%) needed operative intervention. Two patients (18.2%) had two drainage procedures to drain multiple fluid collections. Two patients (18.2%) had repeat drainage procedures due to recurrent fluid collections. The average volume of the drained fluid immediately after tube insertion was 85 mL. Follow-up scans show a reduction of the retained content and associated inflammatory changes after tube insertion in all patients. There was no significant statistical difference (P = 0.6 and 0.4)between the mean of WBCs and neutrophils count before drainage and seven days after drainage. The lymphocyte count shows significant increased seven days after drainage (P = 0.03).
In very ancient times there lived a King, whose power lay not only in the vast extent of his dominions1, but also in the magic secrets of which he was master
Research conclusions
In this study, patients having septic complications associated with COVID-19 showed relatively poor clinical outcomes despite technically successful percutaneous drainage.
He had his pocket full of money, and treated them, and drank with them and boasted and made game of the good-for-nothings who were afraid to stand on guard, because they were frightened that the dead princess would eat them
Research perspectives
This study presents the clinical, radiological, and laboratory data for patients who underwent percutaneous drainage to manage septic complications associated with COVID-19 infection. The main finding is that patients with suspected septic complications associated with COVID-19 show relatively poor outcomes with 36.4% clinical success of percutaneous drainage despite 100% technical success. This finding was confirmed by the insignificant difference between the inflammatory markers before and after tube drainage insertion. Severe sepsis related to COVID-19 viral infection may be related to a decrease in mitochondrial efficiency and dysfunction of the respiratory chain[22,23]. In addition, autopsies have confirmed hyperinflammatory state with organ fibrosis, especially in high metabolic cells with high mitochondrial volume such as pneumocytes, endothelial cells, hepatocytes, and renal cells[24]. The resulting tissue hypoxia and increased inflammation, viral load, and other baseline risk factors contribute to an increased risk of severe sepsis or co-existed septic condition exaggeration.
FOOTNOTES
The mean WBCs and neutrophil counts show reduction 1 d and 7 d after drainage however there was no significant statistical difference (
= 0.6) between the mean of WBCs count before drainage (15.4 × 10
/L) and seven days after drainage (12.1 × 10
/L) and between the mean count of neutrophil (
= 0.4) before drainage (82.8 × 10
/L) and seven days after drainage (70.9 × 10
/L). The lymphocyte count shows significant increased seven days after drainage (
= 0.03). Five patients had AKI manifested by elevation of the serum creatinine and urea levels. Total bilirubin level was elevated in eight patients and showed no significant reduction after drainage (
= 0.2). The CRP values were not significantly different (
= 0.06) before (182.0 mg/dL) and one week after tube insertion (133.0 mg/dL). Other inflammatory markers as D-dimer, procalcitonin and LDH were elevated in all patients before drainage and showed variable degree of non-statistically reduction and increase after drainage. The laboratory findings are listed in Table 3.
The study was reviewed and approved by the Mansoura university Institutional Review Board (R.21.12-1545).
A local institutional review board approved this retrospective study, and waivers of consent of medical record review were received.
All authors declare no conflict of interest.
Technical appendix, statistical code, and dataset available from the corresponding author at mokadem83@yahoo.com.
8. Alone in the wood: Julius Heusher states that the woods represent the loss of security and previous values (Heuscher 1974).Return to place in story.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Egypt
Mohamed A Deif 0000-0002-8486-2622; Ahmad M Mounir 0000-0002-3322-7960; Sherif A Abo-Hedibah 0000-0002-1863-9828; Ahmed M Abdel Khalek 0000-0002-7751-7660; Ali H Elmokadem 0000-0001-5119-9548.
Gao CC
A
Gao CC
1 Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD,Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL,Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).
2016; 315: 801-810 [PMID: 26903338 DOI: 10.1001/jama.2016.0287]
2 Carter C, Notter J. COVID-19 disease: a critical care perspective.
2020; 1: 100003
3 Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature.
2020; 24: 285 [PMID: 32498689 DOI: 10.1186/s13054-020-03006-1]
4 Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK.COVID-19: consider cytokine storm syndromes and immunosuppression.
2020; 395: 1033-1034 [PMID: 32192578 DOI: 10.1016/S0140-6736(20)30628-0]
5 Ding R, Meng Y, Ma X. The Central Role of the Inflammatory Response in Understanding the Heterogeneity of Sepsis-3.
2018; 2018: 5086516 [PMID: 29977913 DOI: 10.1155/2018/5086516]
6 Nedeva C, Menassa J, Puthalakath H. Sepsis: Inflammation Is a Necessary Evil.
2019; 7: 108 [PMID:31281814 DOI: 10.3389/fcell.2019.00108]
7 Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care.
2012; 72: 1491-1501 [PMID: 22695412 DOI: 10.1097/TA.0b013e318256e000]
8 Rosenthal MD, Kamel AY, Rosenthal CM, Brakenridge S, Croft CA, Moore FA. Chronic Critical Illness: Application of What We Know.
2018; 33: 39-45 [PMID: 29323761 DOI: 10.1002/ncp.10024]
9 Liu Y, Mao B, Liang S, Yang JW, Lu HW, Chai YH, Wang L, Zhang L, Li QH, Zhao L, He Y, Gu XL, Ji XB, Li L, Jie ZJ,Li Q, Li XY, Lu HZ, Zhang WH, Song YL, Qu JM, Xu JF; Shanghai Clinical Treatment Experts Group for COVID-19.Association between age and clinical characteristics and outcomes of COVID-19.
2020; 55 [PMID: 32312864 DOI: 10.1183/13993003.01112-2020]
10 Thomas-Rüddel D, Winning J, Dickmann P, Ouart D, Kortgen A, Janssens U, Bauer M. [Coronavirus disease 2019(COVID-19): update for anesthesiologists and intensivists March 2020].
2020; 69: 225-235 [PMID: 32189015 DOI: 10.1007/s00101-020-00758-x]
11 Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B,Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M,Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19).
2020; 46: 854-887 [PMID:32222812 DOI: 10.1007/s00134-020-06022-5]
12 Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY,Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P,Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China.
2020; 382: 1708-1720[PMID: 32109013 DOI: 10.1056/NEJMoa2002032]
13 Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X,Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.
2020; 395: 497-506 [PMID: 31986264 DOI:10.1016/S0140-6736(20)30183-5]
14 Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19)Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention.
2020; 323: 1239-1242 [PMID: 32091533 DOI: 10.1001/jama.2020.2648]
15 Elmokadem AH, Batouty NM, Bayoumi D, Gadelhak BN, Abdel-Wahab RM, Zaky M, Abo-Hedibah SA, Ehab A, El-Morsy A. Mimickers of novel coronavirus disease 2019 (COVID-19) on chest CT: spectrum of CT and clinical features.
2021; 12: 12 [PMID: 33533965 DOI: 10.1186/s13244-020-00956-6]
16 Elmokadem AH, Bayoumi D, Abo-Hedibah SA, El-Morsy A. Diagnostic performance of chest CT in differentiating COVID-19 from other causes of ground-glass opacities.
2021; 52: 1-10 [DOI: 10.1186/s43055-020-00398-6]
17 Penn R, David-Sanchez RY, Long J, Barclay W. Aberrant RNA replication products of highly pathogenic avian influenza viruses and its impact in the mammalian associated cytokine storm.
2019; 1 [DOI:10.1099/acmi.ac2019.po0457]
18 Spencer JV, Religa P, Lehmann MH. Editorial: Cytokine-Mediated Organ Dysfunction and Tissue Damage Induced by Viruses.
2020; 11: 2 [PMID: 32038654 DOI: 10.3389/fimmu.2020.00002]
19 Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS,Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure.
2011; 306: 2594-2605 [PMID: 22187279 DOI: 10.1001/jama.2011.1829]
20 Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S,Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan,China: a retrospective cohort study.
2020; 395: 1054-1062 [PMID: 32171076 DOI:10.1016/S0140-6736(20)30566-3]
21 Abo-Hedibah SA, Tharwat N, Elmokadem AH. Is chest X-ray severity scoring for COVID-19 pneumonia reliable?
2021; 86: e432-e439 [PMID: 34429790 DOI: 10.5114/pjr.2021.108172]
22 Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock.
2002; 360: 219-223 [PMID:12133657 DOI: 10.1016/S0140-6736(02)09459-X]
23 Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure.
2014; 5: 66-72 [PMID:24185508 DOI: 10.4161/viru.26907]
24 Shenoy S. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality.
2020; 69: 1077-1085 [PMID: 32767095 DOI: 10.1007/s00011-020-01389-z]
25 Mattone E, Sofia M, Schembari E, Palumbo V, Bonaccorso R, Randazzo V, La Greca G, Iacobello C, Russello D, Latteri S. Acute acalculous cholecystitis on a COVID-19 patient: a case report.
2020; 58: 73-75 [PMID:32895611 DOI: 10.1016/j.amsu.2020.08.027]
26 Ying M, Lu B, Pan J, Lu G, Zhou S, Wang D, Li L, Shen J, Shu J; From the COVID-19 Investigating and Research Team.COVID-19 with acute cholecystitis: a case report.
2020; 20: 437 [PMID: 32571224 DOI:10.1186/s12879-020-05164-7]
27 Wahid N, Bhardwaj T, Borinsky C, Tavakkoli M, Wan D, Wong T. Acute Acalculous Cholecystitis During Severe COVID-19 Hospitalizations.
2020; 115: S794 [DOI: 10.14309/01.ajg.0000708300.51603.f0]
28 Alhassan SM, Iqbal P, Fikrey L, Mohamed Ibrahim MI, Qamar MS, Chaponda M, Munir W. Post COVID 19 acute acalculous cholecystitis raising the possibility of underlying dysregulated immune response, a case report.
2020; 60: 434-437 [PMID: 33224493 DOI: 10.1016/j.amsu.2020.11.031]
29 Bruni A, Garofalo E, Zuccalà V, Currò G, Torti C, Navarra G, De Sarro G, Navalesi P, Longhini F, Ammendola M.Histopathological findings in a COVID-19 patient affected by ischemic gangrenous cholecystitis.
2020; 15: 43 [PMID: 32615987 DOI: 10.1186/s13017-020-00320-5]
30 Balaphas A, Gkoufa K, Meyer J, Peloso A, Bornand A, McKee TA, Toso C, Popeskou SG. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall.
2020; 73: 1566-1568[PMID: 32890595 DOI: 10.1016/j.jhep.2020.08.020]
31 Campanile FC, Podda M, Arezzo A, Botteri E, Sartori A, Guerrieri M, Cassinotti E, Muttillo I, Pisano M, Brachet Contul R, D'Ambrosio G, Cuccurullo D, Bergamini C, Allaix ME, Caracino V, Petz WL, Milone M, Silecchia G, Anania G,Agrusa A, Di Saverio S, Casarano S, Cicala C, Narilli P, Federici S, Carlini M, Paganini A, Bianchi PP, Salaj A, Mazzari A, Meniconi RL, Puzziello A, Terrosu G, De Simone B, Coccolini F, Catena F, Agresta F. Acute cholecystitis during COVID-19 pandemic: a multisocietary position statement.
2020; 15: 38 [PMID: 32513287 DOI:10.1186/s13017-020-00317-0]
32 Popowicz A, Lundell L, Gerber P, Gustafsson U, Pieniowski E, Sinabulya H, Sjödahl K, Tsekrekos A, Sandblom G.Cholecystostomy as Bridge to Surgery and as Definitive Treatment or Acute Cholecystectomy in Patients with Acute Cholecystitis.
2016; 2016: 3672416 [PMID: 26839538 DOI: 10.1155/2016/3672416]
33 Wang F, Wang H, Fan J, Zhang Y, Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia.
2020; 159: 367-370 [PMID: 32247022 DOI: 10.1053/j.gastro.2020.03.055]
34 Pandanaboyana S, Moir J, Leeds JS, Oppong K, Kanwar A, Marzouk A, Belgaumkar A, Gupta A, Siriwardena AK,Haque AR, Awan A, Balakrishnan A, Rawashdeh A, Ivanov B, Parmar C, M Halloran C, Caruana C, Borg CM, Gomez D,Damaskos D, Karavias D, Finch G, Ebied H, K Pine J, R A Skipworth J, Milburn J, Latif J, Ratnam Apollos J, El Kafsi J,Windsor JA, Roberts K, Wang K, Ravi K, V Coats M, Hollyman M, Phillips M, Okocha M, Sj Wilson M, A Ameer N,Kumar N, Shah N, Lapolla P, Magee C, Al-Sarireh B, Lunevicius R, Benhmida R, Singhal R, Balachandra S, Demirli Atıcı S, Jaunoo S, Dwerryhouse S, Boyce T, Charalampakis V, Kanakala V, Abbas Z, Nayar M; COVID PAN collaborative group. SARS-CoV-2 infection in acute pancreatitis increases disease severity and 30-day mortality: COVID PAN collaborative study.
2021; 70: 1061-1069 [PMID: 33547182 DOI: 10.1136/gutjnl-2020-323364]
35 Abate SM, Mantefardo B, Basu B. Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis.
2020; 14: 37 [PMID: 33062056 DOI: 10.1186/s13037-020-00262-6]
36 García Virosta M, Ortega I, Ferrero E, Picardo AL. Diagnostic Delay During the COVID-19 Pandemic: Liver Abscess Secondary to Acute Lithiasic Cholecystitis.
2020; 98: 409 [PMID: 32408994 DOI:10.1016/j.ciresp.2020.04.010]
37 Elliott R, Ohene Baah N, Grossman VA, Sharma AK. COVID-19 Related Mortality During Management of a Hepatic Abscess.
2020; 39: 271-274 [PMID: 32982611 DOI: 10.1016/j.jradnu.2020.09.001]
38 Nahas SC, Meira-JÚnior JD, Sobrado LF, Sorbello M, Segatelli V, Abdala E, Ribeiro-JÚnior U, Cecconello I. Intestinal perforation caused by COVID-19.
2020; 33: e1515 [PMID: 33237160 DOI:10.1590/0102-672020190001e1515]
39 De Nardi P, Parolini DC, Ripa M, Racca S, Rosati R. Bowel perforation in a Covid-19 patient: case report.
2020; 35: 1797-1800 [PMID: 32458395 DOI: 10.1007/s00384-020-03627-6]
40 van 't Hof LJ, Pellikaan L, Soonawala D, Roshani H. An Unusual Presentation of Pyelonephritis: Is It COVID-19 Related?
2021; 1-6 [PMID: 33937632 DOI: 10.1007/s42399-021-00909-0]
41 Revzin MV, Raza S, Srivastava NC, Warshawsky R, D'Agostino C, Malhotra A, Bader AS, Patel RD, Chen K, Kyriakakos C, Pellerito JS. Multisystem Imaging Manifestations of COVID-19, Part 2: From Cardiac Complications to Pediatric Manifestations.
2020; 40: 1866-1892 [PMID: 33136488 DOI: 10.1148/rg.2020200195]
42 Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with inhospital death of patients with COVID-19.
2020; 97: 829-838 [PMID: 32247631 DOI:10.1016/j.kint.2020.03.005]
43 Lee KS, Talenfeld AD, Browne WF, Holzwanger DJ, Harnain C, Kesselman A, Pua BB. Role of interventional radiology in the treatment of COVID-19 patients: Early experience from an epicenter.
2021; 71: 143-146 [PMID:33259979 DOI: 10.1016/j.clinimag.2020.10.048]
