Immunosuppressive treatment and radiotherapy in kidney transplant patients: A systematic review
2022-06-01ValentinaLancellottaAndreaAvieroBrunoFiondaCalogeroCasIlariaEspositoFrancescoPreziosiAnnaAcamporaFabioMarazziGyrgyKovcsBarbaraAlicjaJereczekFossaAlessioGiuseppeMorgantiVincenzoValentiniMariaAntoniettaGambacortaJacopoRomagn
INTRODUCTION
Renal transplant patients have an increased risk of developing
cancers,
with an incidence up to four times higher than the general population[1-3]. Recipients of transplanted organs have variable risk of cancer development. In fact, the risk of developing malignancies depends on transplanted organ, exposure to lymphocyte-depleting antibody-based therapies, immune status of the donor/recipient, and type of immunosuppressive therapy[4,5]. Current immunosuppressive regimens involved in carcinogenesis after organ transplantation are based on a combination of T-cell depleting or inhibiting agents, such as calcineurin inhibitors, monoclonal and polyclonal antibodies, cell cycle inhibitors, antimetabolites, and corticosteroids[6]. As for other oncological settings, radiotherapy (RT) may play a significant role in the treatment of cancer in transplanted patients[7]. However, RT may also have adverse effects in these patients and in particular an increased immunosuppressive effect induced by anti-rejection drugs[8,9]. This effect depends on several factors such as total dose, treatment technique, dose/fractionation, and irradiated volume. Treatment techniques are external beam RT (EBRT) or interventional RT (IRT), also known as brachytherapy[10-12].
The very moment the fagot-maker and his wife reached home the lord of the manor11 sent them ten crowns, which he had owed them a long while, and which they never expected. This gave them new life, for the poor people were almost famished12. The fagot-maker sent his wife immediately to the butcher s. As it was a long while since they had eaten a bit, she bought thrice as much meat as would sup two people. When they had eaten, the woman said:
Despite the "fragility" of transplanted kidneys, RT seems to be feasible also in this patient population[13-22]. Moreover, modern and high-precision RT techniques can deliver the dose only to the macroscopic tumor while sparing immune cells in the surrounding tissues with consequent reduction of the suppressive effect on the immune system[23,24]. On the other hand, in kidney-transplanted patients, immunosuppressive regimens may counteracts the RT immunostimulatory effect. More generally, considering the immunosuppressive effect of RT due to bone marrow toxicity, and therefore the possible increased effect of anti-rejection drugs, a relevant problem in these patients concerns the need to modulate immunosuppressive therapy during and after RT. However, clear evidence regarding this topic is lacking in literature. Furthermore, guidelines on the management of immunosuppressive therapy in patients undergoing RT are also missing. Indeed, only a few studies have addressed this issue and literature reviews on this topic are missing. Based on this background, this systematic review aimed to define the need of immunosuppressive therapy modulation during RT.
MATERIALS AND METHODS
Development of clinical question
The clinical question was developed based on the Population, Intervention, Comparison, and Outcomes (PICO). The clinical question was: (P) In kidney transplant recipients with cancer undergoing RT, maintaining antimetabolites and/or calcineurin inhibitors and/or mammalian target of rapamycin (mTOR) inhibitors (I) is superior when compared to withdrawal of antimetabolites and/or calcineurin inhibitors and/or mTOR inhibitors (C), in relation to the outcomes (O) of benefit and harm (Table 1)? and reports the development of Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Recommendation.
Search strategy and selection of evidence
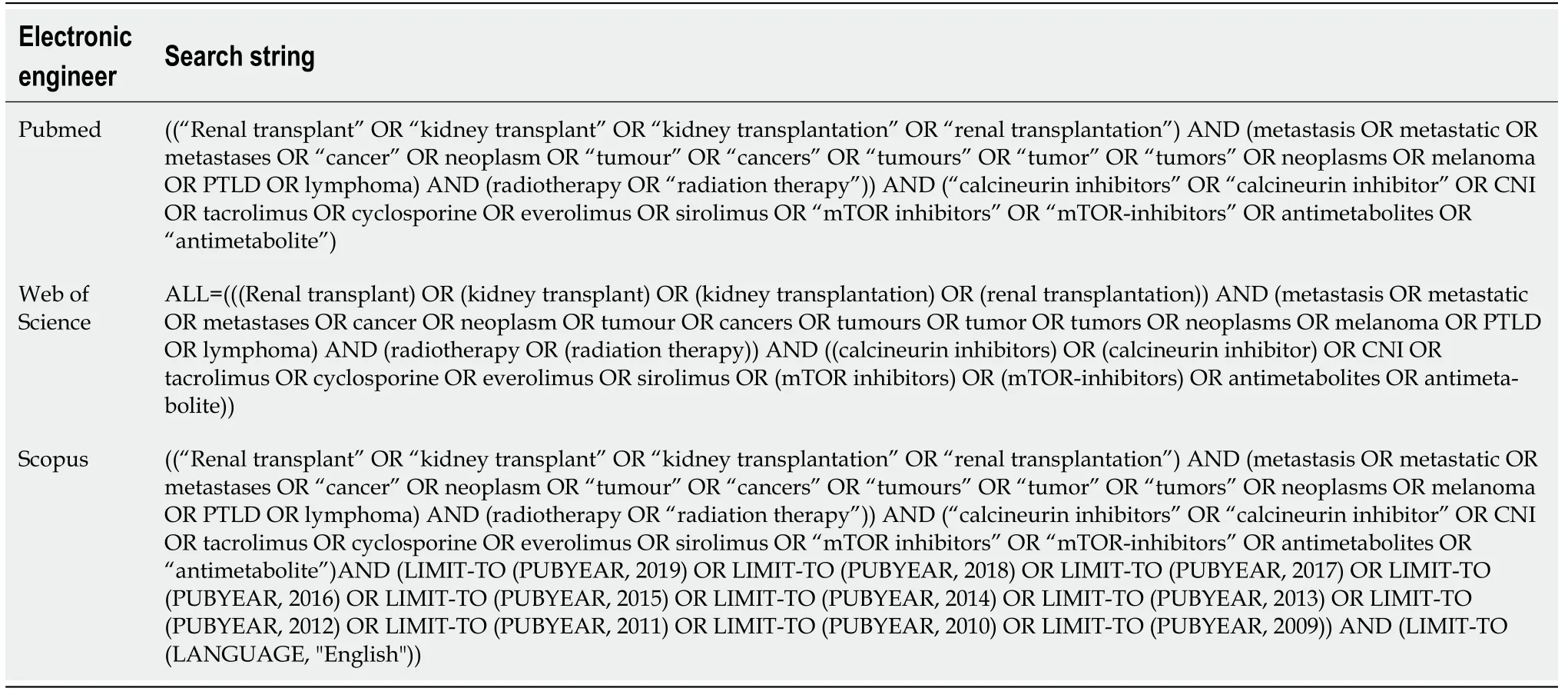
The systematic review was conducted in accordance with the PRISMA guidelines[25]. We performed a comprehensive literature search using PubMed, Scopus, and Web of Science (up to July 2019) using selected keywords linked through the Boolean operators "AND" and "OR" to build specific strings for each electronic search engine (Table 2 and Figure 1). ClinicalTrials.gov was searched for ongoing or recently completed trials, and PROSPERO was searched for ongoing or recently completed systematic reviews. Electronic search was supplemented by manually searching the references of included studies and review articles. The search was restricted to papers published in English. In order to avoid the missing of relevant studies, we chose this strategy burdened by high sensitivity and low specificity. Conference papers, surveys, letters, editorials, book chapters, case reports, and reviews were excluded. Time restriction (2010-July 2019) of the publication was considered. Studies were identified through a search process performed by three independent reviewers (LT, VL, AA), and uncertainty regarding eligibility was resolved by a multidisciplinary committee (JR-transplant surgeon, FM-radiation and medical oncology, FP-radiation oncologist, CC-radiation oncologist, IE-dermatologist). Eligible citations were retrieved for full-text review. An external expert committee defined the outcomes of benefit and harm (GK, BJF, AGM). A multidisciplinary master board (VV, MAG, LT, JR) coordinated the project and performed the final independent check and the definitive approval of the review. The GRADEpro Guideline Development Tool (McMaster University, 2015) was used to create summary of findings tables in Cochrane systematic reviews. The quality assessment showed high clinical and methodological heterogeneity and risks of bias in the included studies, making quantitative synthesis inappropriate. Therefore, meta-analysis outcomes were not reported.
Inclusion criteria
The present systematic review showed that in kidney transplant recipients developing cancer and undergoing RT, clear evidence on improved function of the graft and/or of patients survival after modulating or withdrawing immunosuppressive therapy, as opposed to continuing maintenance immunosuppression, is lacking; conversely, only few retrospective studies on small RT-treated cancer cohorts are available, mainly including PCa patients, without comparison between different immunosuppressive strategies[26,27]. RT appears to be a feasible therapeutic option also in this setting, with oncological outcomes not clearly different from the general patient population[28].
Exclusion criteria
Conference papers, surveys, letters, editorials, book chapters, and literature reviews.
Identification of Outcomes
The external expert committee identified the following outcomes of benefit: OS (defined as the time from baseline to death from any cause or last follow-up), graft survival (defined as time from transplant to graft failure), progression free survival (PFS, defined as time from baseline to clinical or radiological progression), and local control (LC, defined as time from baseline to cancer detected in the treated site at any time after initial treatment). The identified outcome of harm included acute and late toxicity. All these outcomes were considered as “critical” for the decision-making process.
Quality of evidence evaluation
Certainty of evidence for all selected outcomes was performed according to the GRADE approach, considering study limitations, imprecision, indirectness, inconsistency, and publication biases. Certainty level started at higher pre-specified level for randomized controlled trials, but levels of certainty could be downgraded if limitations in one of the above-mentioned domains were detected. Evidence was classified as having high, moderate, low, and very low level of certainty.
Based on the summary of evidence, the following judgments about the benefit-to-risk ratio between intervention and comparison were stated: Favorable, uncertain/favorable, uncertain, uncertain/ unfavorable, and unfavorable (both for intervention or comparison). The strength of the recommendation was considered as strong positive, conditional positive, uncertain, conditional negative, or strong negative.
Benefit/harm balance and clinical recommendation
Bill had advice for everyone - often whether it was sought or not - but he was so much one of nature s gentlemen that he was impossible to resist. You found yourself nodding sagely, and tracing the patterns of the end of his animated index finger, in receipt of the raised eyebrows, the thorough showmanship() of Bill Smith righting the wrongs of the world.
RESULTS
The flowchart of the study selection process is shown in Figure 1. The literature search resulted in 147 single citations. After literature screening, 21 records were identified for full-text evaluation. Out of these, 15 were excluded, and the reasons for exclusion are reported in Figure 1. Six full text papers were considered eligible and were included in the final analysis.
Cancer is the second most common cause of mortality and morbidity in kidney transplant recipients.Immunosuppression can influence the efficacy of cancer treatment and modification of the immunosuppressive regimen may restore anti-neoplastic immune responses improving oncologic prognosis.However, patients are usually reluctant to modify their immunosuppression, fearing rejection and potential graft loss.
Characteristics of the included studies
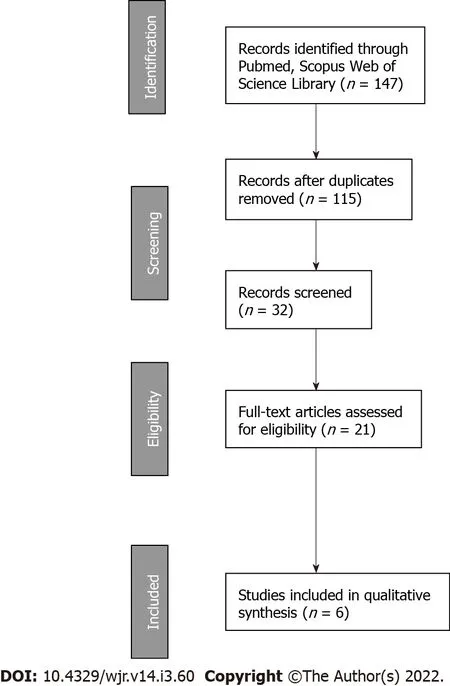
All studies were retrospective and included a total of 65 kidney transplant patients with subsequent cancer diagnosis. Regarding the type of cancer, five studies included prostate cancer (PCa) patients while one study reported on subjects with lymphoma. No direct comparisons between different treatment approaches in terms of immunosuppressive therapy modulation was performed. The main characteristics of included studies are shown in Table 3 (first author, objective, treatment features, and main results).
Literature review
Antunes
[13] analyzed the incidence of urologic malignancies in renal transplant recipients and reported on their treatment and outcomes. Twenty-nine PCa patients were included in the study with a mean age of 62.6 ± 6.1 years (range: 50-73 years). EBRT was performed in 5 patients. Although the authors did not find a statistically significant difference between type of immunosuppressive drugs and PCa development, they emphasized that 13 out of 29 patients (44.8%) received azathioprine. No statistically significant impact of duration or type of immunosuppression on
development of urologic malignancies or OS was recorded. No patient undergoing RT had allograft failure. Follow-up duration after PCa treatment ranged from 3 mo to 96 mo. One-, five-, and ten-year OS rates after PCa diagnosis were 86.2%, 86.2%, and 79.3%, respectively. Only 1 patient died of PCa. The remaining patients died of PCa-independent reasons (cardiac failure or infection)[13].
Binsaleh
[15] retrospectively analyzed treatment and outcome of 9 renal transplant patients with subsequent PCa. Median age at PCa diagnosis was 63.6 years. One patient was treated with androgen deprivation therapy alone, 4 patients with RT alone, and 4 patients with a combination of androgen deprivation therapy and EBRT (60-66 Gy). Immunosuppressive therapy was as follows: 4 patients were on cyclosporine, azathioprine, and steroids regimen; 3 patients received cyclosporine, mycophenolate, and steroids (then changed to a sirolimus-based therapy); 1 patient was on tacrolimus, azathioprine, and steroids regimen; 1 patient received tacrolimus, mycophenolate mofetil, and steroids. Three out of the 9 patients had their immunosuppressive regimen changed from cyclosporine, mycophenolate, and steroids to a sirolimus-based therapy, and 6 had “judicious reductions” in their calcineurin inhibitor dosages. Four transplanted kidneys showed renal failure, and 3 out of 4 of them were treated with RT: 1 patient was on tacrolimus, azathioprine, and steroids therapy and was treated with EBRT alone (60 Gy); 1 patient was on tacrolimus, mycophenolate mofetil, and steroids and was treated with androgen deprivation therapy plus EBRT (60 Gy); 1 patient was on cyclosporine, azathioprine, and steroids and was treated with androgen deprivation plus EBRT (60 Gy); finally, 1 patient was on cyclosporine, azathioprine, and steroids and was treated with androgen deprivation therapy alone. The authors concluded that a combination of RT with androgen deprivation therapy provides good control of the disease while preserving renal function. The comparative long-term follow-up of patients with reduced doses of calcineurin-inhibitor-based immunosuppression or sirolimus-based treatments is not known[15].
There is still no clear evidence that withdrawing anti-metabolites and/or calcineurin inhibitor and/or mammalian target of rapamycin inhibitors as opposed to continuing maintenance immunosuppression might improve patient survival in kidney transplant recipients with cancer undergoing radiotherapy. There are few retrospective studies on small cancer cohorts undergoing radiotherapy, especially prostate, without comparison of different immunosuppressive treatments. The radiation therapy can be performed with excellent oncological outcomes. No studies have compared different immunosuppressive treatment, and, when the immunosuppressive drugs are reported, patients’ survival seems to be correlated only with cancer stage or type. In addition, there are no data on the eventual effects of immunosuppressive drugs, especially mammalian target of rapamycin inhibitors, on the healing of radiotherapy-induced skin toxicity.
Pettenati
[20] published the results of their retrospective single center study. A control population of non-organ transplant and non-end-stage renal disease patients with PCa was used to compare tumor features and oncological outcome with 24 renal-transplanted patients (PCa incidence in all patients was 1.5%). Mean follow-up was 47 mo. PCa was mostly localized (
21, 87.5%) and treated with radical prostatectomy (
16, 76.2%), LDR-IRT (
3, 14.3%, 145 Gy), EBRT (
1, 4.7%), or active surveillance (
1, 4.7%). On the contrary, 3 patients had locally advanced PCa and were treated with EBRT combined with androgen deprivation therapy. Two patients were on a regimen of calcineurin inhibitors plus azathioprine plus steroids; 19 patients were on calcineurin inhibitors plus mycophenolate mofetil plus steroids; 2 patients were on mycophenolate mofetil plus mTOR inhibitors plus steroids. No graft failure due to PCa treatment was reported. Nineteen renal-transplant patients with localized PCa (90.5%) were free from biochemical recurrence at last follow-up. Considering the radical prostatectomy subset, no difference in PCa characteristics at diagnosis and biochemical recurrence rate was found between renal-transplant patients (
16) and control patients (
64). The authors concluded that localized PCa following renal transplantation was not associated with adverse features as compared to non-transplant patients. Standard treatments could be proposed to renal-transplanted patients with satisfying results both on oncological outcome and graft function[20].
Tasaki
[21] retrospectively analyzed safety and efficacy of IRT in 3 patients with PCa after renal transplantation. The clinical stage was cT1N0M0 in all patients. The median age at diagnosis was 65 years (range: 60-67 years). Immunosuppressive regimens were cyclosporine A plus mycophenolate mofetil plus methylprednisolone in 2 patients and tacrolimus plus mycophenolate mofetil plus methylprednisolone in 1 patient. The median time between transplantation and IRT was 7 years (range: 4-10 years). Two patients received low dose-rate IRT (dose, 145 Gy), and one patient was treated with high dose-rate IRT (dose, 19 Gy in 2 fractions) combined with external beam irradiation (EBRT, 39 Gy in 13 fractions). Median follow-up after IRT was 44 mo (range: 34-50 mo). No patient developed biochemical or clinical progression and no clinically significant RT-induced adverse events were reported. Two patients maintained a good graft function while one patient had a decline of graft function 2 years after IRT. The authors concluded that low dose-rate IRT and high dose-rate IRT of PCa seem feasible and safe in renal-transplanted recipient with oncological outcomes similar to those recorded in the general population[21].
Oh
[26] reported on biochemical disease-free survival, distant metastasis free, OS, and toxicity in 28 patients with renal transplant who were subsequently treated with definitive RT for PCa. The median age was 66 years, and median follow-up time was 30 mo. Twenty-four patients (86%) were treated with IRT (144 Gy), and 4 patients (14%) were treated with external-beam RT (78 Gy). Immunosuppressive regimens were cyclosporine (
8), mycophenolate mofetil (
13), azathioprine (
3), tacrolimus (
12), sirolimus (
9), and/or prednisone (
20). At last follow-up, 2 patients had died, 1 from metastatic PCa and 1 from other reasons. Three-year biochemical relapse-free survival, distant metastasis-free, and OS were 95.8%, 93.1%, and 93.8%, respectively. One patient developed grade 3 gastrointestinal late toxicity. The authors concluded that organ transplant recipient with PCa and treated with RT have excellent 3-year outcomes[26].
Little evidence is available on radiotherapy management of cancer in kidney transplant recipients; in certain instances (e.g., in case of pelvic cancer or cancer of the transplanted kidney) it is also unclear which could be the best loco-regional treatment option, among the full range of ablative devices/techniques, to be used as an alternative to nephron sparing surgery, currently the preferred option.
53. The wicked mother came to no good end: In The Seven Ravens, the wicked step-mother was taken before the judge, and put into a barrel filled with boiling oil and venomous snakes, and died an evil death. Return to place in story.
Data synthesis
No study showed that withdrawing antimetabolites and/or calcineurin inhibitor and/or mammalian target of rapamycin-inhibitors as opposed to continuing maintenance immunosuppression improves patient survival in kidney transplant recipients with cancer undergoing RT.
DISCUSSION
(1) Kidney transplant recipients with cancer undergoing RT; (2) Reporting patients overall survival (OS), progression free survival, graft survival, toxicity, and local control; (3) Published in English language as original articles; (4) Time restriction (2010-2019).
In fact, while no studies compared different immunosuppressive treatments, when immunosuppressive drugs were reported, patients’ survival seemed to be correlated only with cancer stage or type. Due to lack of evidence, it seems reasonable to entrust the clinical management of these patients to a multi-disciplinary team including nephrologists, cancer surgeons, medical and radiation oncologists, pathologists, and radiologists. In fact, discussion of clinical cases in a multidisciplinary expert team could allow a more homogeneous treatment approach and improvement of clinical outcomes. This evaluation needs to consider the clinical specificities beyond tumor burden, such as comorbidities, compliance to treatment, general performance status, and history of the disease to select the best approach for the individual patient following the principles of personalized medicine. Furthermore, for clinical and deontological reasons, it is also mandatory to discuss all possible implications with the patient to define the therapeutic strategy and obtain a detailed informed consent.
Moreover, due to the lack of available results from prospective trials, studies with this design should be promoted. However, considering the rarity of patients undergoing renal transplantation and requiring RT, and therefore the difficulty in carrying out prospective trials, an alternative aimed at generating evidence in this field could be to share retrospective data from different centers in order to create pooled analyses[29,30].
This study has several limitations. Only six studies were included in the analysis, totaling only 65 patients. Furthermore, all studies have been lacking in reporting important data such as details of RT, radiation-induced toxicity, a complete assessment of renal function, and the impact of RT on immune function. These limitations prevent clear conclusions from being drawn on the question of this review and, in particular, on the need to suspend or modulate immunosuppressive therapy in patients undergoing renal transplantation and subsequent RT.
CONCLUSION
There is no evidence that immunosuppressive therapy should be modulated in kidney transplant patients undergoing RT. Prospective studies or pooled analyses are needed to define the proper treatment for this very selected group of patients.
Well, what does she want, then? said the Flounder. Alas8, said the man, half scared, she wants to live in a great stone castle. Go to it, then, she is standing9 before the door, said the Flounder.
39.Married her: Marriage is the ultimate goal and reward in many romantic fairy tales. Despite the bridegroom s mercenary thoughts, we are intended to believe in a happily ever after for the couple.Return to place in story.
ARTICLE HIGHLIGHTS
Research background
In some ways the consequences have been quite dire22 and I no longer have contact with my mother. However, Dad s hug had a profound effect on me. It carried me along a path from childhood to adulthood23. At last I am my own woman and one who loves nothing better than a good old-fashioned hug.
Research motivation
To develop reference points for guiding the transplant professionals in the clinical decision-making process and to improve the management of kidney transplant recipients with cancer.
Research objectives
Velvet
[27] conducted a single center retrospective study on management and outcomes of central nervous system lymphomas in 6 kidney transplant patients. During the lymphoma treatment, immunosuppressive therapy was reduced in all patients. Mycophenolate mofetil and prednisolone without calcineurin inhibitor were prescribed to 5 out of 6 patients. Three out of six patients underwent RT: one patient was also treated with chemotherapy and four cycles of cytotoxic T lymphocytes (alive at last follow-up); one patient was also treated with craniotomy and rituximab (graft failure and then death for acute left ventricular failure); one patient was also treated with chemotherapy (unknown cause of death). RT total dose and technique were not reported and 6-mo OS was 66.6%. This study supports observational data suggesting that patients treated with mycophenolate mofetil and without calcineurin inhibitor may have increased risk of cancer after transplantation[27].
Research methods
The overall process included: (1) The formulation of one specific question based on the Population,Intervention, Comparison, and Outcomes methodology; (2) Systematic literature review and summary for experts for each question; and (3) Extracted data were narratively synthesized and, where possible,frequencies, percentages, and ranges were calculated.
Research results
Then the doors of the hall flew open, and there stood the whole Court round his wife, who was sitting on a high throne of gold and diamonds; she wore a great golden crown, and had a sceptre of gold and precious stones in her hand, and by her on either side stood six pages in a row, each one a head taller than the other
She would certainly have betrayed that this was not the kind of narcissus she wanted, but for the Fairy Melinette, who had been anxiously watching the inter25, and now thought it quite time to interfere26
Research conclusions
Although all the statements of the consensus are not methodologically evidence-based and their strength might therefore be questionable, they represent a starting point to orient transplant physicians in their everyday practice, and, above all, these statements clearly indicate the points that need to be addressed in the clinical research in this setting.
Research perspectives
Prospective studies or pooled analyses are needed to define the proper treatment for this very selected group of patients.
FOOTNOTES
Valentini V, Morganti AG, Tagliaferri L, and Romagnoli J contributed to scientific committee; Acampora A, Lancellotta V, and Tagliaferri L contributed to working group performing literature review and summary for experts; Romagnoli J, Marazzi F, Preziosi F, Casà C, and Esposito I contributed to resolve uncertainty regarding eligibility; Kovács G, Jereczek-Fossa A, and Gambacorta MA contributed to revise the manuscript.
the authors reported no potential competing interest.
Jeffrey Karp, an associate professor of medicine at Harvard Medical School and co-director of the Center for Regenerative Therapeutics at Brigham and Women s Hospital, is also a senior author of the paper. Lead author is Woo Kyung Cho, a postdoc in the Harvard-MIT Division of Health Sciences and Technology (HST).
The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Italy
Valentina Lancellotta 0000-0003-3507-7051; Andrea D'Aviero 0000-0002-9194-620X; Bruno Fionda 0000-0003-3368-1810; Calogero Casà 0000-0001-5785-0549; Ilaria Esposito 0000-0002-2047-6140; Francesco Preziosi 0000-0001-6780-6621; Anna Acampora 0000-0001-5363-403X; Fabio Marazzi 0000-0003-0977-0143; György Kovács 0000-0001-5149-9831; Barbara Alicja Jereczek-Fossa 0000-0001-8151-3673; Alessio Giuseppe Morganti 0000-0001-9793-3410; Vincenzo Valentini 0000-0003-4637-6487; Maria Antonietta Gambacorta 0000-0001-5455-8737; Jacopo Romagnoli 0000-0002-7153-0346; Luca Tagliaferri 0000-0003-2308-0982.
Wang LL
Filipodia
At this the fox was so angry that he dashed with all his force against the wall, and tried to knock it down. But it was too strong and well-built; and though the fox scraped and tore at the bricks with his paws he only hurt himself, and at last he had to give it up, and limp away with his fore-paws all bleeding and sore.
Wang LL
1 Agraharkar ML, Cinclair RD, Kuo YF, Daller JA, Shahinian VB. Risk of malignancy with long-term immunosuppression in renal transplant recipients.
2004; 66: 383-389 [PMID: 15200447 DOI: 10.1111/j.1523-1755.2004.00741.x]
2 Bosmans JL, Verpooten GA. Malignancy after kidney transplantation: still a challenge.
2007; 71: 1197-1199[DOI: 10.1038/sj.ki.5002306]
3 Engels EA. Epidemiologic perspectives on immunosuppressed populations and the immunosurveillance and immunocontainment of cancer.
2019; 19: 3223-3232 [PMID: 31206226 DOI: 10.1111/ajt.15495]
4 Acuna SA. Etiology of increased cancer incidence after solid organ transplantation.
2018; 32:218-224 [PMID: 30017342 DOI: 10.1016/j.trre.2018.07.001]
5 Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit.
2010; 10: 1889-1896 [PMID: 20659094 DOI:10.1111/j.1600-6143.2010.03181.x]
6 Gallo A, Miele M, Badami E, Conaldi PG. Molecular and cellular interplay in virus-induced tumors in solid organ recipients.
2019; 343: 103770 [PMID: 29523417 DOI: 10.1016/j.cellimm.2018.02.010]
7 Bosacki C, Vallard A, Jmour O, Ben Mrad M, Lahmamssi C, Bousarsar A. Radiotherapy and immune suppression: A short review.
2020; 107: 84-101 [DOI: 10.1016/j.bulcan.2019.09.010]
8 Schaue D. A Century of Radiation Therapy and Adaptive Immunity.
2017; 8: 431 [PMID: 28443099 DOI:10.3389/fimmu.2017.00431]
9 Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma.
2012; 366: 925-931 [DOI: 10.1056/nejmoa1112824]
10 Massaccesi M, Cusumano D, Boldrini L, Dinapoli N, Fionda B, Teodoli S, et al. A new frontier of image guidance: Organs at risk avoidance with MRI-guided respiratory-gated intensity modulated radiotherapy: Technical note and report of a case.
2019; 20: 194-198 [DOI: 10.1002/acm2.12575]
11 Lancellotta V, Cellini F, Fionda B, De Sanctis V, Vidali C, Fusco V, Barbera F, Gambacorta MA, Corvò R, Magrini SM,Tagliaferri L. The role of palliative interventional radiotherapy (brachytherapy) in esophageal cancer: An AIRO (Italian Association of Radiotherapy and Clinical Oncology) systematic review focused on dysphagia-free survival.
2020; 19: 104-110 [PMID: 31636025 DOI: 10.1016/j.brachy.2019.09.005]
12 Tagliaferri L, Fionda B, Bussu F, Parrilla C, Lancellotta V, Deodato F, Cammelli S, Boldrini L, Gambacorta MA,Morganti AG, Valentini V, Paludetti G, Peris K, Kovacs G. Interventional radiotherapy (brachytherapy) for squamous cell carcinoma of the nasal vestibule: a multidisciplinary systematic review.
2019; 29: 417-421 [PMID:31486400 DOI: 10.1684/ejd.2019.3599]
13 Antunes H, Tavares-da-Silva E, Oliveira R, Carvalho J, Parada B, Bastos C, Figueiredo A. De Novo Urologic Malignancies in Renal Transplant Recipients.
2018; 50: 1348-1354 [PMID: 29753463 DOI:10.1016/j.transproceed.2018.02.086]
14 Beydoun N, Bucci J, Malouf D. Iodine-125 prostate seed brachytherapy in renal transplant recipients: an analysis of oncological outcomes and toxicity profile.
2014; 6: 15-20 [PMID: 24790617 DOI:10.5114/jcb.2014.40769]
15 Binsaleh S. Diagnosis and treatment of prostate cancer in renal-transplant recipients.
2012; 44: 149-155[PMID: 21614508 DOI: 10.1007/s11255-011-9988-8]
16 Dahlke S, Schwarz A, Bruns F, Bremer M, Miemietz M, Christiansen H, Meyer A. Pelvic radiotherapy after renal transplantation.
2012; 32: 5083-5086 [PMID: 23155284]
17 Elkentaoui H, Robert G, Pasticier G, Bernhard JC, Couzi L, Merville P, Ravaud A, Ballanger P, Ferrière JM, Wallerand H.Therapeutic management of de novo urological malignancy in renal transplant recipients: the experience of the French Department of Urology and Kidney Transplantation from Bordeaux.
2010; 75: 126-132 [PMID: 19864001 DOI:10.1016/j.urology.2009.06.106]
18 Haroon UH, Davis NF, Mohan P, Little DM, Smyth G, Forde JC, Power RE. Incidence, Management, and Clinical Outcomes of Prostate Cancer in Kidney Transplant Recipients.
2019; 17: 298-303 [PMID: 30602361 DOI: 10.6002/ect.2018.0048]
19 Hevia V, Gómez V, Díez Nicolás V, Alvarez S, Gómez Del Cañizo C, Galeano C, Gomis A, García-Sagredo JM, Marcen R, Burgos FJ. Development of urologic de novo malignancies after renal transplantation.
2014; 46: 170-175 [PMID: 24507046 DOI: 10.1016/j.transproceed.2013.12.004]
20 Pettenati C, Jannot AS, Hurel S, Verkarre V, Kreis H, Housset M, Legendre C, Méjean A, Timsit MO. Prostate cancer characteristics and outcome in renal transplant recipients: results from a contemporary single center study.
2016; 30: 964-971 [PMID: 27251769 DOI: 10.1111/ctr.12773]
21 Tasaki M, Kasahara T, Kaidu M, Kawaguchi G, Hara N, Yamana K, Maruyama R, Takizawa I, Ishizaki F, Saito K,Nakagawa Y, Ikeda M, Umezu H, Nishiyama T, Aoyama H, Tomita Y. Low-Dose-Rate and High-Dose-Rate Brachytherapy for Localized Prostate Cancer in ABO-Incompatible Renal Transplant Recipients.
2019;51: 774-778 [PMID: 30979463 DOI: 10.1016/j.transproceed.2018.10.027]
22 Iizuka J, Hashimoto Y, Kondo T, Takagi T, Nozaki T, et al. Efficacy and Feasibility of Intensity-Modulated Radiation Therapy for Prostate Cancer in Renal Transplant Recipients.
2016; 48: 914-917
23 Fionda B, Massaccesi M, Tagliaferri L, Dinapoli N, Iezzi R, Boldrini L. Abscopal effect and interventional oncology: state of art and future perspectives.
2020; 24: 773-776 [PMID: 32016981 DOI:10.26355/eurrev_202001_20058]
24 Mazzola R, Jereczek-Fossa BA, Franceschini D, Tubin S, Filippi AR, Tolia M, Lancia A, Minniti G, Corradini S,Arcangeli S, Scorsetti M, Alongi F. Oligometastasis and local ablation in the era of systemic targeted and immunotherapy.
2020; 15: 92 [PMID: 32366258 DOI: 10.1186/s13014-020-01544-0]
25 Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement.
2009; 6: e1000097
26 Oh SC, Tariq MB, Reddy CA, Ciezki JP, Stephans KL, Tendulkar RD. Outcomes in Organ Transplant Recipients With Prostate Cancer Treated With Radiotherapy.
2019; 17: e162-e166 [PMID: 30446400 DOI:10.1016/j.clgc.2018.10.005]
27 Velvet AJJ, Bhutani S, Papachristos S, Dwivedi R, Picton M, Augustine T, Morton M. A single-center experience of posttransplant lymphomas involving the central nervous system with a review of current literature.
2019; 10: 437-448 [PMID: 30728897 DOI: 10.18632/oncotarget.26522]
28 Mazzola R, Cuccia F, Bertani A, Tubin S, Conaldi PG, Corradini S, Tolia M, Guba M, Alongi F. The role of radiotherapy in patients with solid tumours after solid organ transplantation: a systematic review.
2021; 22: e93-e104[PMID: 33662300 DOI: 10.1016/S1470-2045(20)30590-8]
29 Lancellotta V, Guinot JL, Fionda B, Rembielak A, Di Stefani A, Gentileschi S. SKIN-COBRA (Consortium for Brachytherapy data Analysis) ontology: The first step towards interdisciplinary standardized data collection for personalized oncology in skin cancer.
2020; 12: 105-110 [DOI: 10.5114/jcb.2020.94579]
30 Tagliaferri L, Budrukkar A, Lenkowicz J, Cambeiro M, Bussu F, Guinot JL, Hildebrandt G, Johansson B, Meyer JE,Niehoff P, Rovirosa A, Takácsi-Nagy Z, Boldrini L, Dinapoli N, Lanzotti V, Damiani A, Gatta R, Fionda B, Lancellotta V,Soror T, Monge RM, Valentini V, Kovács G. ENT COBRA ONTOLOGY: the covariates classification system proposed by the Head & Neck and Skin GEC-ESTRO Working Group for interdisciplinary standardized data collection in head and neck patient cohorts treated with interventional radiotherapy (brachytherapy).
2018; 10: 260-266[PMID: 30038647 DOI: 10.5114/jcb.2018.76982]
