Role of cannabinoids and the endocannabinoid system in modulation of diabetic cardiomyopathy
2022-05-19MonaElAzabAhmedWakielYossefNafeaMahmoudYoussef
INTRODUCTION
Diabetes mellitus is one of the most common chronic disorders worldwide, and it continues to increase in number and significance. The total number of individuals with diabetes worldwide is 463 million, with a prevalence rate of 9.3% according to the International Diabetes Federation[1]. It is estimated that the prevalence of diabetes on a global scale could reach 578 million by 2030 and 700 million by 2045. In 2017, diabetes-related mortality accounted for 4 million people worldwide and the total healthcare expenditure reached 727 billion United States Dollars[2].
Diabetes mellitus is a complex metabolic condition that is characterized by hyperglycemia resulting from a lack of absolute or relative insulin[3]. It is linked to insulin resistance in many instances. Type 1 diabetes is caused by an autoimmune destruction of insulin-secreting cells in the pancreas, and type 2 diabetes is caused by insufficient compensatory insulin production in the presence of peripheral insulin resistance. Ninety percent of diabetes cases are of the latter type[4].
Both microvascular (retinopathy, nephropathy, and neuropathy)[5-9] and macrovascular (cardiovascular disease) problems are linked to diabetes[6-10]. Despite substantial advances in antidiabetic therapy, diabetic complications, which are most commonly recognized in the long-term, are consistently harmful to a large extent, necessitating multi-factorial risk reduction measures beyond glycemic control[11]. Diabetes-related morbidity and mortality are primarily caused by cardiovascular problems[12]. Indeed, 50% of diabetic patients die of a cardiovascular disease[13]. Endothelial dysfunction, coronary artery disease, and myocardial left ventricular dysfunction (which leads to heart failure) are all well-known cardiovascular problems[14]. Diabetic patients have a 2-4 times higher risk of heart failure than non-diabetic patients, according to clinical research[15,16].
One first-year student from Guangdong who received 500 yuan as a donation, said, I don t want others to know I am a poor student. If I have to do that, I would rather not accept the donation.
Benson, who is also 46, and devises grade school curriculums, said she worried her husband would judge her if she continued to eat meat, “but we talked it out and he is not proselytizing36
Diabetic cardiomyopathy is a deficiency in ventricular contractile function that occurs in diabetic individuals regardless of the presence of coronary artery disease or other cardiovascular disorders. It is a complicated diabetes-related condition marked by severe alterations in the heart's physiology, anatomy, and mechanical performance[17]. Diabetic cardiomyopathy is a complicated and poorly understood process. To explain the structural and functional alterations associated with diabetic cardiomyopathy, several pathogenic processes have been explored and suggested[18]. Increased oxidative/nitrative stress[19-21], accumulation of advanced glycation end products (AGEs)[22], activation of various pro-inflammatory and cell death signaling pathways[23], and changes in the composition of extracellular matrix with elevated cardiac fibrosis[24] are some of the proposed pathological mechanisms. Unfortunately, despite the growing body of information concerning diabetic cardiomyopathy over the last few decades, therapeutic choices remain inadequate. Other treatments for diabetic cardiomyopathy's multi-factorial pathogenic pathways have yet to be developed.
When the Emperor heard from the Prince how he had gained possession of his fair prize, he at once recognized that he had been helped by some magic art, and on the spot gave up all claim to the beautiful mermaid
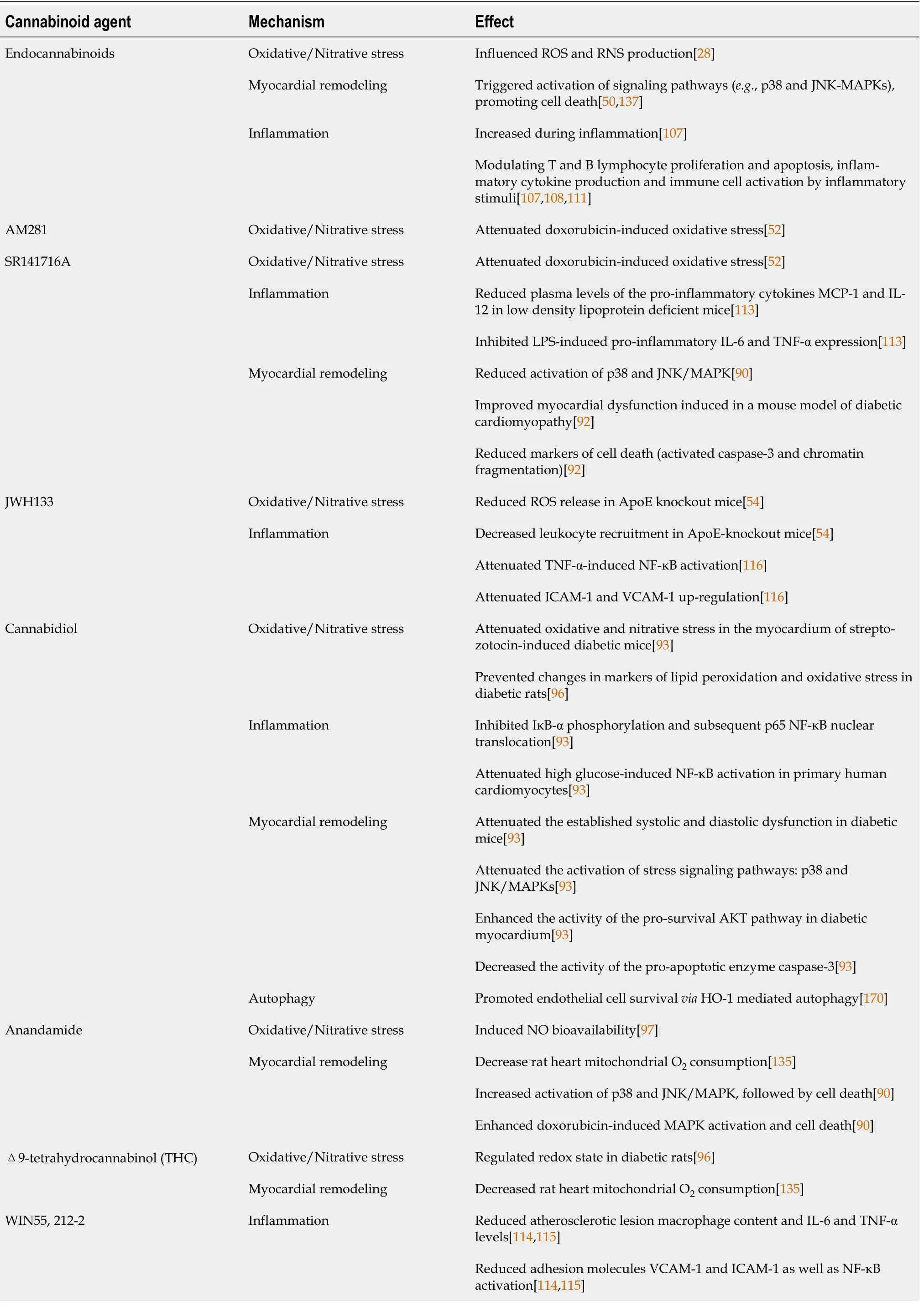
The endocannabinoid system is an endogenous lipid signaling system that consists of: (1) Two main receptors identified as cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2); (2) Endogenous ligands for these two receptors known as endocannabinoids; and (3) Proteins that control endocannabinoid tissue levels (anabolic and catabolic enzymes)[25]. The endocannabinoid system has become a novel therapeutic target in a range of cardiovascular illnesses in the last decade, including atherosclerosis, myocardial infarction, and heart failure[26]. Furthermore, the significance of the endocannabinoid system in the development of diabetes and associated consequences has been suggested in various pre-clinical and clinical research[27-29]. The possible mechanisms through which cannabinoids and the endocannabinoid system could modulate the pathogenesis of diabetic cardiomyopathy are highlighted in this review (Table 1), an approach that could pave the way for the use of this system as an effective tool in the management of these harmful diabetic complications.
DIABETIC CARDIOMYOPATHY: A DISTINCT COMPLEX DISORDER
Cardiomyopathies are a group of diseases characterized by myocardial dysfunction that is not induced by common causes, such as coronary artery disease, valvular dysfunction, or hypertension. Cardiomyopathies are divided into four categories depending on hemodynamic characteristics: Dilated, hypertrophic, restrictive, and obliterative cardiomyopathy[30]. Dilated cardiomyopathy is characterized by ventricular dilatation and systolic dysfunction, which commonly affects both ventricles. The most common symptom of hypertrophic cardiomyopathy is significant ventricular hypertrophy. Restrictive cardiomyopathy is characterized by inflexible and poorly distensible myocardium, resulting in poor compliance. Endo-myocardial fibrosis is a symptom of obliterative cardiomyopathy. The endocardium's severe fibrosis encroaches on and reduces the size of the ventricular cavities[31]. Diabetic cardiomyopathy can be classified as either dilated or hypertrophied cardiomyopathy[32].
Rubler
[33] coined the name diabetic cardiomyopathy in 1972 after observing a specific type of cardiomyopathy in diabetic patients who did not have other cardiovascular issues such as coronary artery disease, valvular or congenital heart disease, or hypertension. Diabetic cardiomyopathy is defined by a series of cardiac alterations, including interstitial fibrosis, myocardial hypertrophy, and microcirculatory abnormalities, that arise due to diabetes mellitus. These circulatory issues impair heart function, eventually leading to cardiac failure[4]. Heart failure lowers an individual's quality of life and makes diabetes control more difficult. As a result, early diagnosis and treatment of these patients are regarded as top priorities[34].
Inflammation is a complex nonspecific response of vascular tissues to harmful stimuli such as pathogens, damaged cells, or irritants, and it involves several functional and molecular mediators, such as the recruitment and activation of leukocytes such as mast cells, neutrophils, and monocytes/ macrophages. On an acute basis, inflammation is usually good since it represents the organism's defensive attempt to eliminate damaging stimuli and begin the healing process. Inflammation, on the other hand, might have negative consequences if it continues for a long period[98]. The increased expression of many inflammatory proteins is regulated at the level of gene transcription through the activation of pro-inflammatory transcription factors which play a critical role in amplifying and perpetuating the inflammatory process[99].
But the fox said, My dear daughter, your joy is in vain, because, let me tell you, this blood is of no earthly use to you unless you add some of mine to it, and with these words he took to his heels

THE ENDOCANNABINOID SYSTEM
The discovery of the endogenous signaling system now recognized as the endocannabinoid system began with the chemical detection of 9-tetrahydrocannabinol (THC), the main psychoactive component of
[42]. THC's psychotropic and immunomodulatory effects are due to the ability to bind to and activate specific receptors, including the CB1 receptor, which is one of the most abundant Gprotein-coupled receptors in the central nervous system[43], and the CB2 receptor, which is abundantly expressed in several immune cells and tissues[44].
The existence of endogenous substances (the endocannabinoids) capable of binding to and activating CB1 and CB2 receptors was suggested. Anandamide (N-arachidonoyl ethanolamine)[45] and 2-arachidonoyl glycerol (2-AG)[46] are the two most well-studied examples of these compounds. The endocannabinoid system is made up of cannabinoid receptors, endocannabinoids, and proteins that catalyze endocannabinoid biosynthesis (N-acyl-phosphatidylethanolamine phospholipase-D for anandamide and diacylglycerol lipases for 2-AG), transport, and inactivation [fatty acid amide hydrolase (FAAH) for anandamide and monoacyl glycerol lipase for 2-AG][47]. Signaling
CB1 and CB2 receptors is complex, involving inhibition (and activation in some cases) of adenyl cyclase activity, activation of various MAPKs [
, p38- and p44/42-MAPKs, c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK)], protein kinases A and C (PKA and PKC), and modulation of various calcium and potassium channels[48].
Since its discovery about two decades ago, the endocannabinoid system has gained considerable importance as a fundamental signaling system implicated in almost all physiological and pathological processes in animals[49]. In a wide range of pathological conditions, including mood and anxiety disorders, movement disorders, neuropathic pain, multiple sclerosis, cancer, glaucoma, osteoporosis, reproductive disorders, immune dysfunction, cardiovascular and metabolic disorders, there is growing evidence that the endocannabinoid system plays pivotal roles and holds tremendous therapeutic options[50,51].
Besides the primary distribution of CB1 receptors in the CNS and CB2 receptors in immune cells, as these are responsible for the psychoactive and immunomodulatory effects of cannabinoids, both receptors have been found to be expressed in cardiovascular system cells such as cardiomyocytes, fibroblasts, endothelial and vascular smooth muscle cells, and infiltrating immune cells[26]. CB1 receptors activation by endocannabinoids or synthetic ligands has complex depressive effects in the cardiovascular system and has been linked to the development of pathophysiological alterations and compromised cardiovascular function in various forms of shock[51] and heart failure[52]. In addition, several studies indicated that stimulation of CB1 receptors in the cells of the cardiovascular system is associated with activation of stress signaling pathways promoting cell death, ROS production, and induction of inflammatory cascades[26,52]. On the other hand, an increased CB2 receptor expression has been reported in the cardiovascular system under pathophysiological conditions such as inflammatory stimulation or tissue injury, which likely reflects a protective response to limit these effects[53]. A great body of evidence suggest a protective role of CB2 receptors in experimental models of cardiovascular disorders including mouse models of atherosclerosis[54], restenosis[55] and myocardial ischemia/ reperfusion injury[56].
Then he gave his good wishes for the journey and his blessing80, and the prince kissed his Bet, said good-bye, and, with thanks to the Causer of Causes, took the road
Different expression patterns of CB1 and CB2 receptors together with other components of the endocannabinoid system such as synthesizing and degrading enzymes have been reported in islet cells of humans, rats and mice[57-59]. There are controversial results regarding the role of CB1 receptors in insulin secretion with studies showing an increased insulin secretion in islet cells by activation of CB1 receptors[57,58], and others showing decreased insulin secretion[60]. In addition, activation of CB2 receptors in islet cells has also been shown to either stimulate[61] or attenuate insulin secretion[57]. Several studies have found that the endocannabinoid system plays a significant role in the etiology of diabetes. Serum levels of anandamide and 2-AG have been found to be greater in type 2 diabetics than in healthy individuals[27]. Furthermore, in these diabetic patients, subcutaneous tissue levels of anandamide were found to be elevated, indicating endocannabinoid system overactivity[28].
Mukhopadhyay
[52] similarly found that pharmacological blockage of CB1 receptors with AM281 or SR141716A reduced doxorubicin-induced oxidative/nitrative stress and related cell death. In comparison to their wild-type counterparts, mice lacking the FAAH gene showed a significant increase in acute and chronic doxorubicin-induced cardiac oxidative and nitrative stress, as well as impaired antioxidant defense and tissue injury[91]. Furthermore, anandamide increased the sensitivity of inflammatory cells isolated from FAAH mutant mice to ROS generation. These findings imply that, in pathological situations involving oxidative/nitrative stress (such as doxorubicin-induced myocardial injury), FAAH plays an important role in regulating endocannabinoid-induced cardiac cell injury, which is mediated in part by CB1 receptor activation because these effects may be attenuated by selective CB1 antagonists[91].
The majority of diabetic complications are linked to abnormalities in the vascular system[67]. Hyperglycemia has been related to a number of critical processes, including oxidative/nitrative damage, AGE buildup, and inflammatory system stimulation[68]. Endothelial dysfunction occurs in arteries, which contributes to the development of numerous diabetes problems. Indeed, cannabinoids and the endocannabinoid system represent an outstanding therapeutic approach to manage these deleterious complications. Interestingly, this notion is supported by a great body of evidence implicating the endocannabinoid system in the pathogenesis of nearly all diabetic complications including nephropathy, retinopathy, and neuropathy, in addition to cardiovascular complications, mainly through modulation of the aforementioned mechanisms[29]. Still, the role of the endocannabinoid system in diabetic cardiomyopathy; the distinct diabetic complication, has not been fully investigated in detail.
POSSIBLE MECHANISMS THROUGH WHICH CANNABINOIDS AND THE ENDOCANNABINOID SYSTEM COULD MODULATE DIABETIC CARDIOMYOPATHY
Oxidative/Nitrative stress
Nearly 95% of oxygen consumed by tissues is used in metabolic processes to produce adenosine triphosphate (ATP), and approximately 5% of oxygen consumed is transformed into superoxide (O
) radical, the principal oxygen free radical produced by mitochondria[69]. The antioxidant enzymes superoxide dismutase (SOD1, SOD2, and SOD3) quickly convert superoxide to hydrogen peroxide (H2O2) within the cell[70]. Antioxidant enzymes such as catalase, glutathione peroxidase, and other peroxidases generally convert excess H2O2 to harmless water[71]. Although H2O2 is not a free radical, it can undergo the Fenton reaction with reduced transition metals [
, ferrous ion (Fe
)] or with superoxide in the presence of metal ions (usually iron or copper) to produce the highly reactive hydroxyl radical (OH), which is a far more damaging molecule to the cell[72]. Superoxide radicals can quickly react with nitric oxide (NO) to produce cytotoxic peroxynitrite anions (ONOO-) in addition to producing H2O2[73]. Superoxide and NO are less reactive than peroxynitrite, which might combine with carbon dioxide to generate nitrotyrosine, that triggers protein degradation and lipid oxidation[74].
Resveratrol, an autophagy inducer, was discovered to have a cardio-protective impact in cardiomyocytes exposed to hyperglycemia
the AMPK-mTOR-p70S6K signaling pathway[168]. AMPK-mTOR signaling contributed to the cardio-protective effect in STZ-induced diabetic mice by increasing autophagy[161,169]. According to these findings, Wu
[167] found that administering HU308 to selectively activate CB2 enhanced AMPK phosphorylation while lowering mTOR and p70S6K phosphorylation, initiating the AMPK-mTOR-p70S6K signaling cascade in murine primary ventricular cardiomyocytes. Furthermore, using compound C, an AMPK inhibitor, significantly reduced the cardioprotective effect of HU308, showing that AMPK-mTOR-p70S6K signaling-induced autophagy was essential in CB2-mediated cardiac protection in dilated cardiomyopathy[167]. However, because the mechanisms behind CB2-mediated autophagy activation are complex, more research is required. Figure 4 summarizes the effect of cannabinoid receptors on AMPK/mTORC1/NLRP3 signaling.
Besides mitochondria, other cellular sources of reactive oxygen and nitrogen species (RNS) exist. NADPH oxidase, for example, promotes the enzymatic conversion of oxygen to superoxide anion. Several critical cytosolic proteins (p44phox, p67phox, p40phox, and Rac2) must be translocated to the cellular membrane for NADPH oxidase activation[75]. Other sources of ROS and RNS, in addition to NADPH oxidase, include nitric oxide synthase (NOS), which stimulates NO synthesis[76], and peroxisomes, that are known to create H2O2 primarily through fatty acid oxidation[77] and phagocytic cell activation[78].
The oxidative stress pathway has emerged as a common thread connecting all major diabetic cardiomyopathy pathophysiological mechanisms[79]. These pathways are the result of a single hyperglycemia-induced process: The overproduction of superoxide by the mitochondrial electron transport chain[80]. Formation of AGE products, auto-oxidation of glucose, activation of PKC, and NADPH oxidase are some of the other sources of ROS in diabetes[81]. Once oxidative stress develops, it results in a vicious self-sustaining cycle of generating more free radicals and causing more stress as a result of the activation of multiple stress-induced pathways and due to its ability to cause damaging effects to multiple components within the cell[82].
Through a variety of mechanisms, ROS induce cellular damage in the diabetic myocardium. Increased ROS directly damage cellular proteins and DNA[83]. In addition, ROS activate matrix metalloproteinases, which modify the extracellular matrix architecture and cause fibrosis[84], as well as regulating signal transduction pathways that cause cardiomyocyte hypertrophy[85] and apoptosis, which results in the loss of contractile tissue[86]. In a similar manner, peroxynitrite induces vasoconstriction, enhanced leukocyte adherence, platelet activation, oxidation, pro-thrombotic state, impaired coagulation, and vascular inflammation, among other pro-atherosclerotic pathogenic processes[87]. In type 1 diabetic mice, selective suppression of mitochondrial ROS was demonstrated to prevent diabetic cardiac abnormalities, confirming the importance of mitochondrial ROS role in developing cardiac abnormalities[88]. Moreover, Rac1 increases mitochondrial ROS generation
NADPH oxidase activation and plays an important role in cardiomyocyte death and cardiac failure in streptozotocininduced diabetes in mice[89].
Previous studies have shown that the endocannabinoid system can influence ROS and RNS production, implying that modulating the endocannabinoid system and administering exogenous cannabinoids with antioxidant properties could be beneficial in the treatment of diabetes-related cardiovascular complications, such as diabetic cardiomyopathy[29].
It has been shown that genetic deletion of CB1 receptors attenuated the rise in markers of oxidative [4-hydroxy-trans-2-nonenal (4-HNE)] and nitrative (nitrotyrosine) stress in the myocardium of mice treated with acute or chronic doses of the potent, cardio-toxicant, anticancer drug doxorubicin[90]. In addition, doxorubicin treatment led to decreased myocardial content of the components of the antioxidant defense system: Glutathione, glutathione peroxidase, and SOD. These changes were significantly reduced in the myocardium of CB1 knockout mice[90]. Consistent with the data obtained from rodents, activation of CB1 receptors by anandamide or the potent agonist HU210, with or without doxorubicin, induced ROS production in human primary cardiomyocytes (HCM). The previous deleterious effect was attenuated by the use of CB1 antagonists: SR141716A or AM281[90].
A clinical trial was conducted in obese patients with type 2 diabetes inadequately controlled by either metformin or sulfonylureas using the CB1 antagonist rimonabant (SR141716A). Rimonabant treatment caused a reduction in weight, hemoglobin A1c levels, fasting blood glucose, high-density lipoprotein cholesterol and triglycerides, as well as improvement in systolic blood pressure[62]. In the type 2 diabetic patients naive to anti-diabetic treatment, rimonabant showed similar results with improved glycemic control and metabolic profile[63]. Another study demonstrated that the treatment of type 2 diabetic patients on standard insulin treatment with rimonabant also improved glycemic control and the metabolic profile[64]. The psychoactive cannabinoid THC was shown to attenuate the severity of autoimmune responses in an experimental model of autoimmune diabetes in addition to lowering blood glucose level and preserving pancreatic insulin content[65]. Unfortunately, the psychoactive effects of THC hampered this therapeutic approach. The non-psychoactive cannabidiol (CBD) reduced the incidence of diabetes in a mouse model of type 1 diabetes, an effect that involved immunosuppressive and anti-inflammatory effects[66].
The role of the endocannabinoid system in oxidative stress control has also been proven in atherosclerosis models such as the apolipoprotein E (ApoE) deficient animal model. In ApoE and CB2 double knockout mice, the release of superoxide radical was increased two-fold in intact aortic segments compared to ApoE knockout mice. The selective CB2 agonist JWH-133 reduced ROS release in ApoE knockout mice to comparable levels to those in wild-type animals[54].
And then there I was, standing7 at the podium. I didn t tell anyone what was planned in case I chickened out. While the minister told me when to come up during the service, Shirley, who was giving the eulogy8 asked, But what if someone stands up before Jennifer? I shot back, Well, now - they ll just have to wait, won t they? She laughed, You are just like your mother. I smiled and thanked her for the compliment.
The first evidence of a direct link between the endocannabinoid system and the pathogenesis of diabetic cardiomyopathy came from the interesting study conducted by Rajesh and co-workers in 2011. This research group demonstrated an increased expression of CB1 receptors and anandamide levels in the myocardium of streptozotocin-induced diabetic mice compared to their non-diabetic counterparts[92]. Streptozotocin-induced diabetic cardiomyopathy was characterized by a profound accumulation of markers of oxidative and nitrative stress in the myocardium, an effect that was ameliorated by genetic deletion of CB1 receptors. In addition, genetic deletion of CB1 mitigated the expression of the p40
NADPH oxidase active subunit in myocardial tissue of diabetic mice[92].
Her voice was old, with a hunger that was not of the stomach. They left then, holding their bundles of papers against the wind. They hadn t said thank you. They didn t need to. They had done more than that. Plain blue pottery8 cups and saucers. But they matched.
Earlier, the same research group demonstrated a protective effect of CBD in diabetic cardiomyopathy[92]. CBD is the most common non-psychotropic cannabinoid in
, and it has been approved for the treatment of inflammation, pain, and spasms associated with multiple sclerosis in humans[93]. CBD exerts several actions that are independent of the CB1 and CB2 receptors[94]. In this study, CBD therapy was found to reduce oxidative and nitrative stress in the myocardium of streptozotocin-induced diabetic mice. Additionally, CBD was found to reduce ROS production as well as the expression of active ROS-generating NADPH oxidase isoforms p22phox, p67phox, and gp91phox. It also increased glutathione levels and SOD activity and reduced nitrotyrosine production. These protective effects of CBD against oxidative/nitrative stress were also demonstrated
in human primary cardiomyocytes[95].
In a study published in 2017, Vella
[96] found that giving cannabinoids to diabetic rats reversed changes in lipid peroxidation and oxidative stress markers, as well as blocking maladaptive alterations in the structure and function of the heart and blood vessels. Similar findings were previously published by Rajesh's group, who reported that administering CBD to diabetic C57BL/6J mice for 11 wk reduced the formation of lipid peroxides, protein carbonyls, and ROS in the heart[95]. Furthermore, the binding site of anandamide has been linked to NO release[97], implying a possible mechanism by which cannabinoids could increase NO bioavailability. THC treatment of STZ-induced diabetic rats resulted in a controlled redox state that granted improvements in end organ function of the myocardium and vasculature[96]. This was demonstrated by preservation of myocardial pump function, cardiac electrophysiology, noradrenergic-mediated contraction, and endothelial-dependent relaxation of resistance arteries. These findings suggested that cannabinoid receptor activation in an experimental type I diabetes animal might be a potential pharmacological target for diabetic cardiomyopathy management[96] (Table 2).
Inflammation
Insulin resistance and hyperglycemia are significant drivers in diabetic patients, activating a variety of adaptive and maladaptive responses that ultimately affect cardiac function[35]. To explain the complicated structural and functional abnormalities associated with diabetic cardiomyopathy, several pathogenic processes have been examined and proposed (Figure 1). These systems work in concert and may even enhance one another[36]. Hyperglycemia increases oxidative stress by accelerating glucose oxidation and mitochondrial production of reactive oxygen species (ROS), which induce DNA damage and promote apoptosis[37]. AGEs build up in tissues, including the myocardium, and have been linked to structural abnormalities in diabetic hearts[22]. Activation of numerous pro-inflammatory and stress signaling pathways, such as mitogen activated protein kinases (MAPKs), also stimulates apoptotic pathways and cell death, and promote myocardial cell death[23,38]. Finally, there is increased collagen formation in the myocardium that leads to fibrosis and reduced contractile function of the heart[24,39].
Activation of the transcription factor nuclear factor-kappa B (NF-κB), which binds to DNA and activates gene transcription, appears to play a pivotal role in the regulation of inducible enzymes such as inducible nitric oxide synthase (iNOS), inflammatory cytokines such as tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β) and IL-6, prostaglandins, cell adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), in addition to other substances that are initiators or enhancers of the inflammatory process[100,101]. The aforementioned inflammatory mediators bind to specific target receptors on the cells and may increase vascular permeability, promote inflammatory cell chemotaxis, stimulate smooth muscle contraction, increase direct enzymatic activity, induce pain, and/or mediate oxidative damage[102].
Numerous investigations have indicated that inflammatory processes play a critical role in the development of diabetes macro- and microvascular complications[29,103]. Cardiac inflammation is a common and early symptom of diabetes, and it plays a key role in the progression of heart failure in diabetic cardiomyopathy[104]. Furthermore, various research on the heart of diabetic or diabetic hypertensive rats has shown that NF-κB plays a major role in the development of diabetic cardiomyopathy[105,106].
Cannabinoid receptor expression in immune cells can be influenced by various inflammatory factors and other triggers activating these cells[107]. Inflammatory stimuli may potentially boost the synthesis of endocannabinoids in immune cells (
, macrophages, monocytes, and dendritic cells) by activating multiple biosynthetic pathways and/or decreasing the expression of metabolic enzymes that degrade them[107,108]. THC and other natural or synthetic cannabinoids have been studied for their immunomodulatory effects in mice and/or rats
, as well as in cultured human immune cells. Overall, cannabinoid ligands exhibit suppressive effects on B-lymphocytes, T-lymphocytes, natural killer cells, and macrophages[109,110], which are most likely due to both CB1 and CB2 receptordependent and -independent mechanisms. Other studies have revealed that endocannabinoids can influence immune functions by modulating T and B lymphocyte proliferation and apoptosis, inflammatory cytokine production and immune cells activation in response to inflammatory stimuli, macrophage-mediated killing of sensitized cells, chemotaxis, and inflammatory cell migration[107,110,111]. Furthermore, cannabinoids may influence the expression of iNOS and the formation of ROS in immune cells, which play significant roles in the defense against invading pathogens and in modulation of the inflammatory response[21]. The involvement of cannabinoid receptors in inflammation is shown in Figure 3.
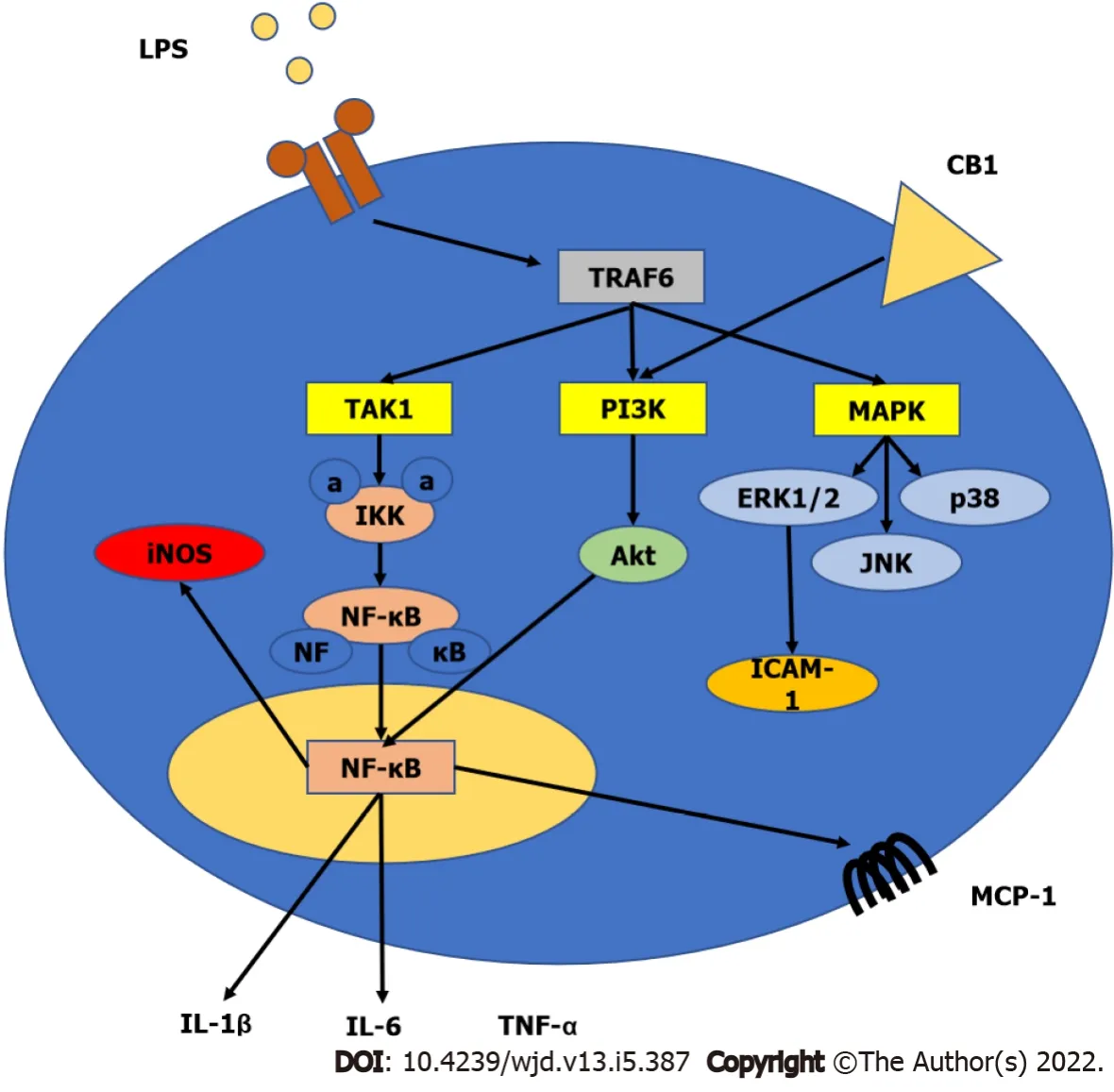
Han
[112] demonstrated that CB1 receptors promote pro-inflammatory responses of macrophages through ROS production, and subsequent synthesis of TNF-α and monocyte chemoattractant protein-1 (MCP-1). This effect was negatively regulated by CB2 and was attenuated by CB1 blockade. In a mouse model of atherosclerosis, the CB1 antagonist SR141716A (rimonabant) was able to reduce plasma levels of the pro-inflammatory cytokines MCP-1 and IL-12 in low density lipoprotein deficient mice fed with a high fat diet[113]. In addition, rimonabant inhibited lipopolysaccharide (LPS)-induced pro-inflammatory IL-6 and TNF-α expression in mouse peritoneal macrophages
. Importantly, this effect was still observed when cells from CB1-knockout mice were used, suggesting a CB1-independent antiinflammatory effect of rimonabant[113]. In another model of atherosclerosis, Hoyer and co-workers demonstrated a severe vascular leukocyte infiltration in ApoE and CB2 double knockout mice which was more intense than that observed in ApoE-knockout mice[54]. Interestingly, treatment with the selective CB2 agonist JWH-133 decreased leukocyte recruitment in ApoE-knockout mice compared to their wild-type counterparts[54]. In 2010, Zhao
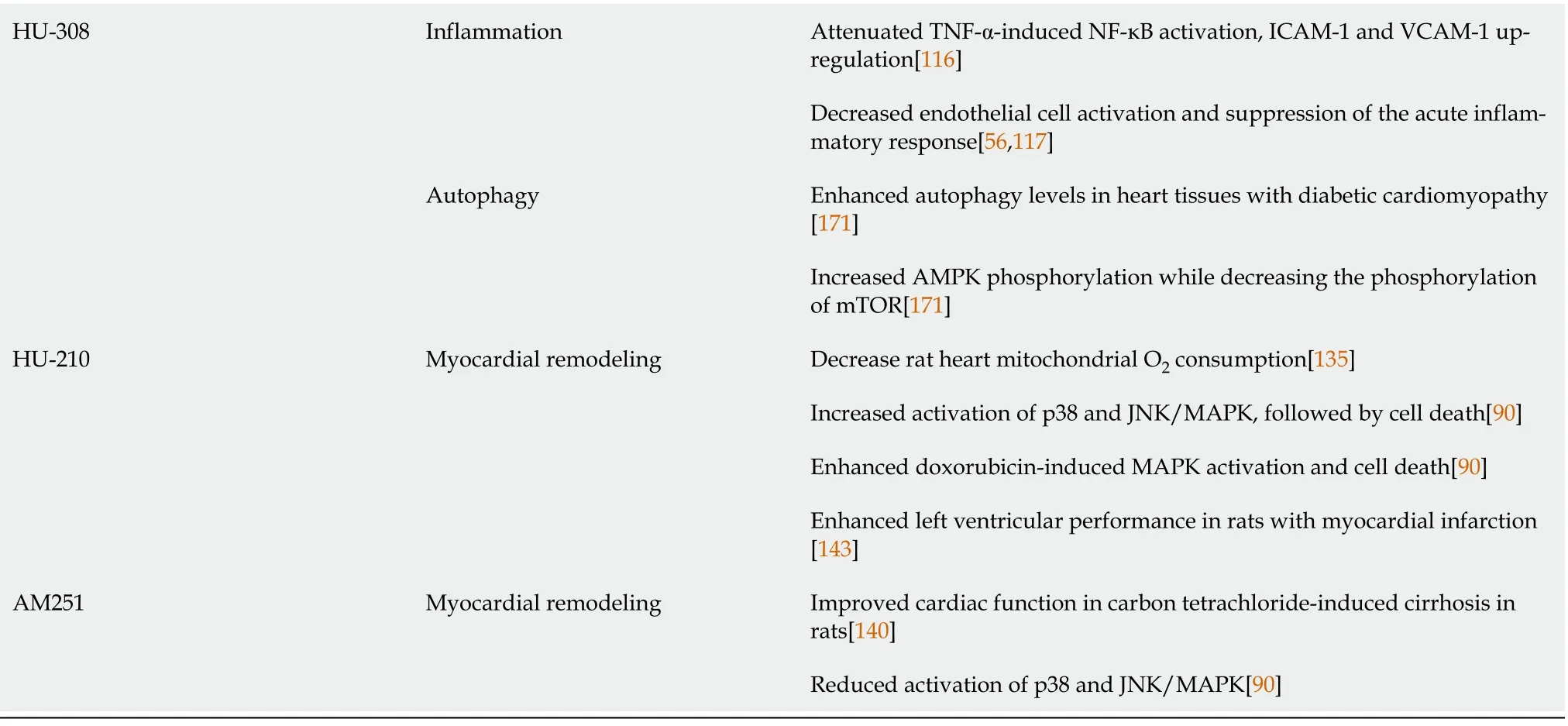
[114,115] showed that treatment with the synthetic cannabinoid WIN55,212-2 reduced atherosclerotic lesion macrophage content and mRNA levels of inflammatory markers IL-6 and TNF-α; adhesion molecules VCAM-1 and ICAM-1 as well as NF-κB activation in ApoE-deficient mice fed on high-cholesterol diets. In human coronary artery endothelial cells, activation of CB2 receptors with the selective agonists HU-308 or JWH-133 attenuated the TNF-αinduced NF-κB activation, ICAM-1 and VCAM-1 up-regulation, MCP-1 release, as well as transendothelial migration and adhesion of monocytes, which are hallmarks of the development of atherosclerosis[116].
The beneficial effects of CB2 receptor activation by selective synthetic ligands, such as JWH-133 and HU-308, was largely attributed to decreased endothelial cell activation and suppression of the acute inflammatory response in animal models of myocardial ischemia/reperfusion injury, which are characterized by a rapid increase in cytokines and chemokines in addition to an enhanced influx of leukocytes into the vulnerable region. Attenuated expression of adhesion molecules, chemokine secretion, leukocyte chemotaxis, adherence to endothelium, stimulation of trans-endothelial migration, and linked oxidative/nitrative stress associated with reperfusion damage were all beneficial effects of CB2 receptor activation[56,117].
In a mouse model of streptozotocin-induced diabetic cardiomyopathy, which is characterized by upregulation of the expression of various inflammatory cytokines in the myocardium, genetic deletion, or pharmacological blockade of CB1 receptors resulted in attenuation of the expression of: Inflammatory cytokines such as TNF-α and IL-1β, adhesion molecules such as ICAM-1 and VCAM-1, iNOS, and cyclooxygenase 2 (COX2)[92]. In another study using the same model of diabetic cardiomyopathy, it was shown that there was a marked phosphorylation of the inhibitor of NF-κB (IκB-α) in the cytosol of diabetic hearts, leading to the release of the active p65 subunit of NF-κB, which subsequently translocated to the nucleus to induce the expression of inflammatory and apoptotic genes[95]. Treatment with CBD, the non-psychoactive cannabinoid, inhibited the IκB-α phosphorylation and subsequent p65 NF-κB nuclear translocation. The CBD treatment also inhibited the NF-κB-dependent mRNA and/or protein expression of adhesion molecules (ICAM-1 and VCAM-1), the pro-inflammatory cytokine TNF-α, and iNOS in the diabetic myocardial tissues. Cannabidiol (CBD) was also able to attenuate high glucose-induced NF-κB activation in primary human cardiomyocytes[95] (Table 2).
Accumulation of AGEs
An important consequence of high glucose-induced cellular injury is the formation of AGEs. AGEs are a heterogeneous group of compounds formed by the non-enzymatic glycation reaction of glucose and other glycating compounds with proteins and, to a lesser extent, lipids, and DNA[118]. In addition, AGEs can easily make covalent cross-linkages (adducts) with macromolecules like proteins and, in this way, can change the structure and function of these proteins[119]. In diabetic patients, the rate of formation of AGEs is increased. Thus, over time, even modest hyperglycemic excursions can result in significant adduct accumulation in long-lived macromolecules[120]. It is now well established that AGEs interact with cell surface receptors and binding proteins to evoke varied downstream responses. These include the pro-inflammatory responses that could play a critical role in the pathogenesis of diabetic complications including cardiomyopathy[121]. The receptor for AGEs (RAGE) is the most established and the best characterized AGE binding protein[122]. The RAGE is a trans-membrane receptor that belongs to the immunoglobulin super-family and is constitutively expressed in a range of tissues including neurons, endothelium, smooth muscle, epithelium, and inflammatory cells[123].
There are two basic methods by which AGEs might alter myocardial function. AGEs, for starters, can form covalent adducts with proteins including collagen, laminin, and elastin[118,124]. As shown in the myocardium of an animal model of type 2 diabetes, this can inhibit collagen degradation, resulting in collagen buildup and fibrosis, producing increased myocardial stiffness, and reduced ventricular relaxation[19]. Second, soluble extracellular AGEs can bind to RAGE, causing up-regulation of transforming growth factor-β (TGF-β) and NADPH oxidase, resulting in the generation of substantial quantities of cytoplasmic and extracellular superoxide, which can then interact with NO to produce RNS[118]. Furthermore, when the RAGE receptor is active, it promotes the transcription factor NF-κB and associated genes by elevating intracellular free radical levels and by triggering multiple other signaling pathways[125].
In the literature, little is known about the interaction of the endocannabinoid system and the AGE and/or RAGE. In a mouse model of streptozotocin-induced diabetic cardiomyopathy, genetic deletion of CB1 receptors attenuated accumulation of AGEs and the expression of RAGE in the myocardium of diabetic mice[92] (Table 2).
Then the wolf reminded him that he had twice got him out of prison, and that if he would only trust in him, and do exactly as he told him, he would certainly succeed in this last undertaking26
Myocardial remodeling
Diabetes has a multifactorial nature, therefore there are changes at the cellular and molecular levels that predispose the heart to pathological, structural, and functional remodeling[4]. Diabetic cardiomyopathy is characterized by an unusually increased left ventricular mass and myocardial fibrosis. Left ventricular hypertrophy has been associated with hyperinsulinemia, insulin resistance, increased non-esterified fatty acids, and activation of the renin-angiotensin-aldosterone system[82]. Chronic cardiac remodeling and structural alterations are promoted by a continual cycle of increased ROS production[126,127]. Diastolic dysfunction is defined as an elevation in ventricular wall stiffness and prolonged diastolic relaxation time, and it is common in the early stages of cardiomyopathy[128].
Increased triglyceride buildup and decreased calcium absorption have been linked to diastolic dysfunction[128]. The progression of systolic dysfunction is marked by dilated cardiac remodeling, which leads to heart failure[129]. Cardiomyocyte mortality is accompanied by fibroblast replacement, which leads to interstitial fibrosis driven predominantly by TGF-β[130]. Cardiomyocyte death is mediated by activation of various stress signaling pathways and consequent apoptosis. The deleterious effect of accumulating free fatty acids on mitochondrial biogenesis eventually leads to mitochondrial apoptosis and lowered ATP generation, which is insufficient to meet cardiac demands, resulting in impaired cardiac contractility and lowered ejection fraction[128]. Myocardial dysfunction is caused by impaired endothelial function linked with insulin resistance[13].
Autophagy, an essential metabolic process, is a self-degradative and recycling procedure dependent on lysosomes. It targets dysfunctional organelles and long-lived proteins[144,145]. This occurs through the biogenesis of double-membrane vesicles containing cytoplasmic components destined for lysosomal degradation, these vesicles are known as autophagosomes[146]. Autophagosome biogenesis entails nucleation, expansion, and closure of the phagophore (a cup-shaped membrane) thereby sequestering cytoplasmic cargo. This is followed by fusion with endolysosomal compartments to facilitate degradation of the sequestered material[146].
Doxorubicin treatment is linked to increased anandamide levels in the myocardium, but not to alterations in CB1 or CB2 receptor expression[52]. Doxorubicin triggered apoptosis in a cardiac cell line (H9c2) that was reduced by CB1 receptor blockage, but the result was not sensitive to a CB2 blocker or CB1 and CB2 receptor agonists[52]. Similarly, studies on cardiac function suggest that endocannabinoids have a role in cirrhosis-related cardiac dysfunction[136]. AM251, which blocks CB1 receptors, enhanced cardiac function in rats with carbon tetrachloride-induced cirrhosis, and anandamide levels were shown to be elevated in the hearts of cirrhotic rats compared to controls[137]. In contrast, agingassociated cardiac dysfunction is reduced in FAAH-null mice, which could be interpreted as showing a need for increased endocannabinoid activity in the heart[135].
Mukhopadhyay
[90] demonstrated that genetic deletion of CB1 receptors attenuated cardiac dysfunction induced by doxorubicin in mice. In this study, doxorubicin-induced activation of stress signaling pathways (p-38 and JNK/MAPKs) with subsequent apoptosis was attenuated in CB1 knockout mice. In addition, these findings were supported
in human primary cardiomyocytes as the activation of CB1 receptors by anandamide or HU210 resulted in increased activation of p38 and JNK/MAPK, followed by cell death, which are effects that were attenuated by both selective CB1 antagonists (SR141716A or AM281) and MAPK inhibitors[90]. Furthermore, doxorubicin-induced MAPK activation and cell death in human cardiomyocytes were significantly enhanced when doxorubicin was co-administered with anandamide or HU210, an effect which could also be attenuated by both CB1 antagonists and MAPK inhibitors[90]. Another aspect of doxorubicin-induced cardiotoxicity is the induction of myocardial fibrosis, an effect that was attenuated by genetic deletion of CB1 indicating its role in this model of cardiotoxicity[90]. In another study using the same model of doxorubicin-induced cardiotoxicity in mice, it has been shown that FAAH knockout mice exhibited significantly increased doxorubicin-induced cardiac dysfunction and myocardial cell death compared to their wild-type counterparts. The effects of doxorubicin in FAAH knockouts were attenuated by CB1 receptor antagonists[91].
Monday morning I let him out for a run while the children got ready for school. He didn t come back. As evening came and German didn t appear, we were all disappointed. We were convinced that he had gone home or been found by his owners, and that we would never see him again. We were wrong. The next Friday evening, German was back on our doorstep. Again we took him in, and again he stayed until Monday morning, when our housekeeper arrived.
Acute myocardial infarction causes cardiomyocyte necrosis, which triggers repair mechanisms that result in scarring[138]. This post-infarction cardiac remodeling process involves adaptive changes in the ventricular shape, size, and function, which can lead to contractile dysfunction and heart failure[138]. In ischemic cardiomyocyte death, fibrosis, and cardiac dysfunction, Defer and coworkers showed considerable evidence for the protective impact of CB2 receptors[139]. CB2-knockout mouse hearts displayed larger infarcts and more persistent cell loss 3 d after ischemia, as well as accelerated damage and apoptosis in the non-ischemic remote myocardium compared to wild-type mice[139]. Cardiomyocytes and fibroblasts lacking CB2 were more vulnerable to oxidative stress-induced cell death
. Longterm effects of cardiac remodeling in CB2-knockout hearts involved marked fibrosis, accelerated cardiomyocyte hypertrophy, dilated cardiomyopathy, and cardiac dysfunction, as reported 4 wk postinfarction[139]. On other hand, wild-type post-ischemic hearts acquired mild fibrosis and cardiomyocyte hypertrophy while maintaining cardiac function[139]. Wagner and colleagues revealed another investigation where the administration of the CB1 antagonist AM251 for 12 wk after an experimentally induced infarction exacerbated the decline in left ventricular function, but administration of the non-selective cannabinoid agonist HU-210 improved left ventricular performance[140].
A previous study conducted by Liao and co-workers demonstrated that CB1 deficiency contributed to the exacerbation of chronic cardiac remodeling induced by pressure overload in mice, revealing a new role of CB1 in the pathophysiology of congestive heart failure[141]. Genetic deletion of CB1 was found to worsen left ventricular hemodynamics and exacerbate cardiac hypertrophy compared to wild-type mice. Furthermore, it was found that CB1 deficiency led to enhanced activation of the epidermal growth factor receptor, p38, and ERK/MAPKs, which contributed to the exacerbation of cardiac hypertrophy[141].
In patients with chronic heart failure, clinical data revealed an increase in cardiac CB2 expression as well as increased levels of the endocannabinoids, anandamide, and 2-AG[142]. Additionally, in these patients, cannabinoid receptor expression was also found to be slightly downregulated[142]. It was believed that CB2 up-regulation could have a negative inotropic effect due to lower cyclic adenosine monophosphate (cAMP) levels, which could lead to ventricular weakness. CB2 receptors, on the other hand, may mediate positive inotropic effects
cAMP-independent processes, hence serving as a compensation strategy to sustain heart function[53]. Furthermore, as demonstrated in rats, CB2 upregulation could be a protective response to counteract structural alterations caused by chronic heart failure[139]. Recently, it has been shown that in biopsies collected from the hypertrophic myocardium of patients with aortic stenosis, there were elevated concentrations of anandamide, higher expression of its degrading enzyme FAAH, and of CB2 receptors[143].
Then a gust of wind came and blew Curdken s hat away, so that he had to fly over hill and dale after it, and the girl in the meantime quietly combed and plaited her hair: all this the old King observed, and returned to the palace without anyone having noticed him
Rajesh
[92] indicated that myocardial dysfunction induced in a mouse model of diabetic cardiomyopathy was improved in CB1-knockout mice or in diabetic mice treated with CB1 antagonists (SR141716A or AM281). This was demonstrated by improved indices of left ventricular systolic and diastolic dysfunction, ejection fraction, contractility, and ventricular stiffness. In the same study, there was attenuated activity of MAPKs and reduced markers of cell death (activated caspase-3 and chromatin fragmentation) in the myocardium of diabetic CB1-knockout mice and in diabetic wild-type mice treated with the CB1 antagonist (SR141716A). Diabetic mice developed myocardial fibrosis as a structural consequence of diabetic cardiomyopathy, and this was characterized by increased accumulation of collagen and enhanced expression of markers of fibrosis such as TGF-β and fibronectin. Interestingly, these changes were attenuated by genetic deletion or pharmacological blockade of CB1 receptors[92]. In another study using the same model, chronic treatment of diabetic mice with the nonpsychoactive CBD attenuated the established systolic and diastolic dysfunction in diabetic mice[95]. In addition, CBD treatment attenuated the activation of stress signaling pathways: p38 and JNK/MAPKs. It also enhanced the activity of the pro-survival AKT pathway in diabetic myocardium. Another beneficial effect of CBD treatment in this model was its ability to decrease the activity of the proapoptotic enzyme caspase-3 and to reduce the rate of cell death in diabetic myocardium. Finally, CBD treatment protected diabetic myocardium from the deleterious process of fibrosis by decreasing myocardial collagen content and attenuating the expression of fibrosis markers: TGF-β, fibronectin, and the enzyme matrix metalloproteinase[95] (Table 2).
Autophagy
Cannabinoid receptors are believed to have the ability to control apoptosis since they may signal through both pro- and anti-apoptotic pathways. Due to the lipophilic nature of their structures, they may be able to operate intracellularly without the help of a membrane transporter[131]. A number of cannabinoid drugs, including HU-210, THC, and anandamide, have been demonstrated to reduce cardiac mitochondrial O2 consumption in rats[132], as well as the role of mitochondria in marijuanainduced cell death[133]. Stimulation of CB1 receptors by endocannabinoids has also been linked to the activation of signaling pathways (
, p38 and JNK-MAPKs) and cell death in various clinical circumstances[51,134]. It is reasonable to conclude, based on earlier results and observations of reduced cardiac apoptosis in FAAH-null animals, that endocannabinoids have strong potential for the regulation of apoptosis, and hence remodeling, in the heart[135].
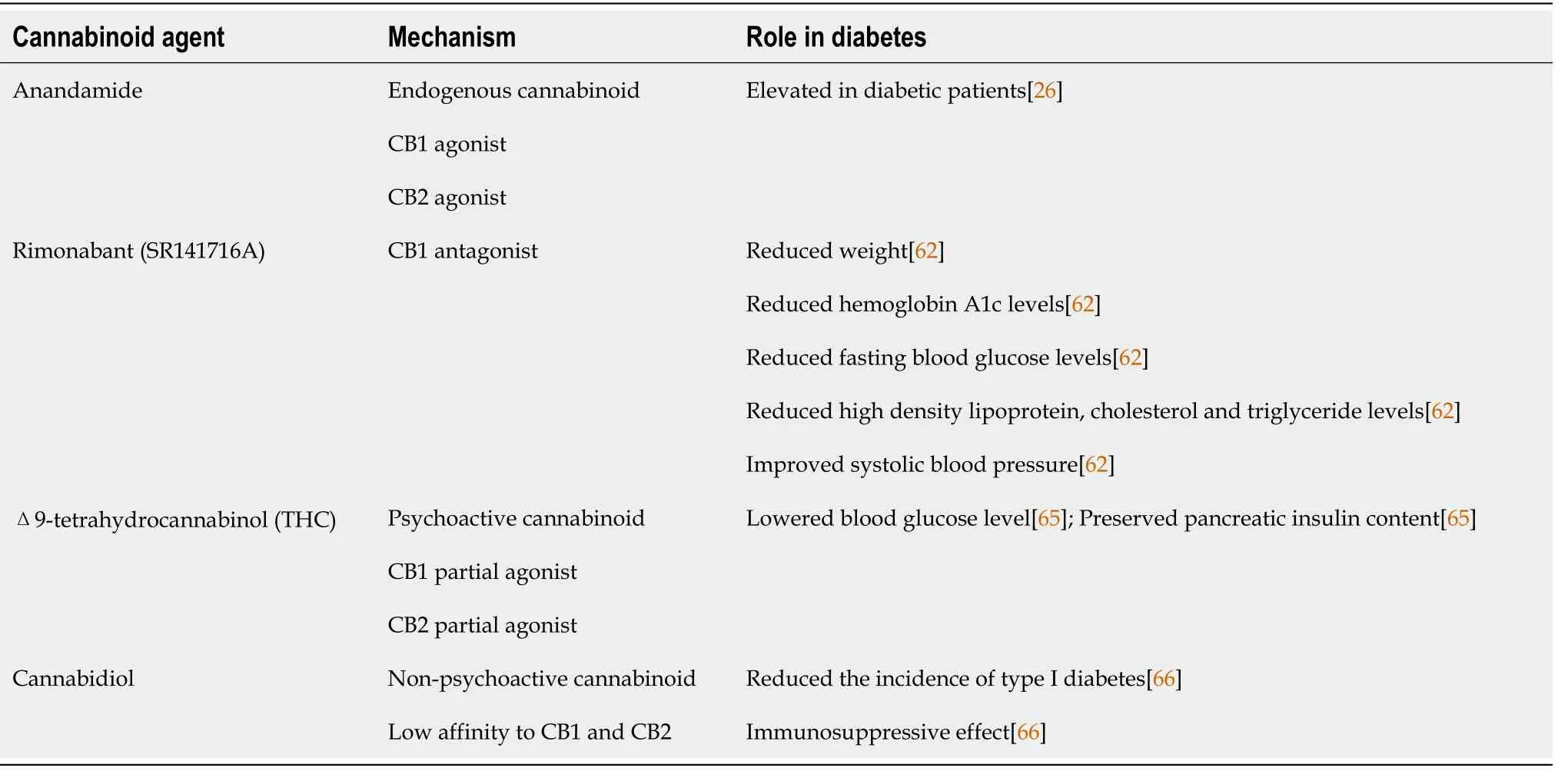
Diabetic cardiomyopathy is divided into three stages (Figure 2): Early-stage, middle-stage, and latestage[4]. In the early stage, the heart develops hypertrophy and has diastolic dysfunction with normal ejection fraction, and it is asymptomatic[40]. Increased left ventricular size, wall thickness, and mass, as well as diastolic dysfunction and a modest decline in systolic performance, characterize the intermediate stage. Insulin resistance, AGE formation, elevated renin-angiotensin-aldosterone system levels, apoptosis, necrosis, and fibrosis are all associated with this stage[41]. As the disease progresses from the medium to late stage, it becomes more severe, impairing both systolic and diastolic functioning[13].
Autophagy is performed by genes called autophagy-related (ATG) genes[147]. The discovery of ATG genes in yeast in the 1990s allowed researchers to identify how autophagy works[148]. Today, 36 ATG proteins have been identified as being particularly significant for autophagy, with 18 of them belonging to the basic machinery[149]. Through the Unc-51-like kinases, ULK1 and ULK2 (mammalian homologues of ATG1), two protein kinases (mTOR and AMPK) control autophagy in mammals[150]. The ULK kinases are dephosphorylated and activated when autophagy is induced. Beclin-1 (mammalian ortholog of ATG6), which is part of a protein complex, is phosphorylated and activated by the ULK[151]. The active ULK and Beclin-1 complexes translocate to the phagophore, the site of autophagosome initiation, where they both help to stimulate downstream autophagy components[152].
Now the grandparents... I closed my eyes, dreading10 the hopelessness of my situation. I had no grandparent to stand proudly for me. I finally opened my eyes, and there they were, Job and Molly, standing11 proudly with all the other grandparents. Job looked over at me, his eyes beaming like diamonds.
Autophagosome production requires two ubiquitin-like conjugation mechanisms[153]. The first one covalently binds the ubiquitin-like protein ATG12 to ATG5. The conjugate protein subsequently attaches to ATG16L1, forming an E3-like complex that is part of the second ubiquitin-like conjugation system[154]. This complex binds and activates ATG3, which covalently binds to the mammalian homologues of the ubiquitin-like yeast protein LC3 to the lipid phosphatidylethanolamine (PE) on autophagosome surfaces[155]. Lipidated LC3 aids autophagosome closure[156] and facilitates the docking of particular cargos and adaptor proteins such as Sequestosome-1/p62[157]. The autophagosome then unites with a lysosome to produce an autolysosome. The autolysosome's contents are then destroyed, and their constituents are liberated from the vesicle[158].
Autophagy has a role in the control of cardiovascular disorders such as myocardial infarction and atherosclerosis[159]. Increased autophagy levels have been shown to protect against diabetic cardiomyopathy[160,161]. As a result, pharmacological activation of autophagy might be a promising therapeutic strategy for diabetic cardiomyopathy.
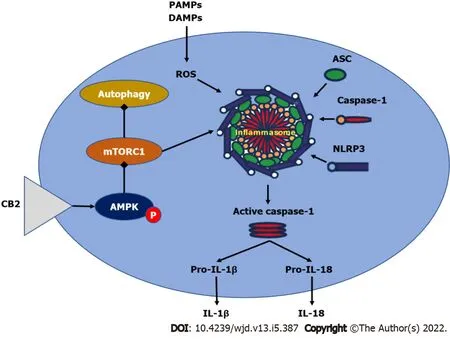
Autophagy also plays an essential role in the functioning of a variety of receptors. In the case of cannabinoid receptors, autophagy has been related to the protective effects of CB2 in a variety of disorders[162-164], suggesting the relevance of autophagy in disease treatment. Autophagy was previously shown to contribute to the alleviative effects mediated by CB2 activation in inflammatory disorders such as multiple sclerosis, alcoholic liver disease, and inflammatory bowel disease[162-164]. Activating CB2 improved inflammatory bowel disease in mouse models by inhibiting the NLRP3 inflammasome and triggering autophagy in murine macrophages, according to Ke and colleagues[163]. In mouse multiple sclerosis models, a similar relationship between CB2 and autophagy was discovered[164].
In the case of autophagy in diabetic cardiomyopathy, it was shown that increasing autophagy levels contributed to improving the condition. Several treatments have been demonstrated to be beneficial in reducing the etiology and development of diabetic cardiac myopathy by utilizing enhanced autophagy levels[160,161,165,166]. CB2 activation
autophagy induction provided protection against diabetic cardiomyopathy, according to a recent study by Wu and coworkers[167]. They used HU308 to selectively activate CB2, resulting in a substantial increase in autophagy levels in diabetic cardiomyopathy heart tissues
and hyperglycemia-challenged cardiomyocytes
. Furthermore, inhibiting autophagy with bafilomycin A1 reduced the cardioprotective effect of HU308 in both
and
models. Wu
[167] concluded that CB2-induced autophagy was involved in the CB2-mediated cardio-protective effect.
That was it. That was all he said. See what happens? We are going to get up in the air, and see what happens? Couldn t we have another plan, one that s been worked out just a little better?
On the third31 morning after they had left their father s house they set about their wandering again, but only got deeper and deeper into the wood, and now they felt that if help did not come to them soon they must perish
Cannabidiol (CBD), has recently gained increased interest for therapeutic use. Indeed, CBD has been shown to suppress a high glucose-induced inflammatory response and barrier disruption of endothelial cells[170] and to attenuate myocardial dysfunction, cardiac fibrosis, oxidative/nitrative stress, inflammation, cell death, and interrelated signaling pathways in a mouse model of type I diabetic cardiomyopathy[95]. The critical role of HO-1 has been evident in the regulation of autophagy, with survivalenhancing effects in various cell types, including endothelial cells[171-173]. Moreover, HO-1 showed positive
effects in animal models of atherosclerosis and restenosis[174]. Böckmann and Hinz have recently proved that CBD promoted endothelial cell survival
HO-1 mediated autophagy[170] (Table 2).
CONCLUSION
Diabetes-induced cardiomyopathy is a deleterious complication of the cardiovascular system characterized by structural and functional changes in the myocardium that ultimately lead to cardiac failure. The mechanisms underlying the development of diabetic cardiomyopathy are complex and involve several pathogenic pathways. A great body of evidence supported a special role of oxidative/nitrative stress and inflammation in the pathogenesis of diabetic cardiomyopathy. The endocannabinoid system has been implicated in the development of several pathological conditions including cardiovascular disorders. Several mechanisms have been proposed as targets by which cannabinoids and the endocannabinoid system could modulate cardiovascular disorders and recent evidence suggested the involvement of this system in the pathogenesis of diabetic cardiomyopathy. Indeed, the manipulation of the endocannabinoid system could represent a promising therapeutic approach for diabetic cardiomyopathy, and several mechanisms have been proposed for this role including its effects on oxidative/nitrative stress, inflammatory pathways, and autophagy together with possible effects on cardiac remodeling. However, more research is needed to define the exact mechanisms of the intervention of the different components of this system in diabetic cardiomyopathy.
The authors would like to thank Dr. Moshira Ibrahim; Professor of Pharmacology at Al-Azhar University (Girls), for her sincere guidance and supervision of AEW during writing the initial draft.
El-Azab MF was responsible for conceptualization and supervision; El-Azab MF and Wakiel AE were responsible for original draft preparation; El-Azab MF, Nafea YK and Youssef ME were responsible for review of the literature and visualization; all authors contributed to the final draft of the manuscript, have read and agreed to the published version of the manuscript.
Authors declare no conflict of interests for this article.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Egypt
Mona F El-Azab 0000-0003-4982-445X; Ahmed E Wakiel 0000-0001-7152-3552; Yossef K Nafea 0000-0001-8551-9482; Mahmoud E Youssef 0000-0001-8887-6346.
Fan JR
Webster JR
Fan JR
1 Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K,Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9
edition.
2019; 157: 107843 [PMID: 31518657 DOI: 10.1016/j.diabres.2019.107843]
2 Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults.
2011; 94: 322-332 [PMID:22100977 DOI: 10.1016/j.diabres.2011.10.040]
3 Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA. Diabetic Cardiomyopathy: Mechanisms and Therapeutic Targets.
2010; 7: e135-e143 [PMID: 21274425 DOI: 10.1016/j.ddmec.2010.08.001]
4 Chavali V, Tyagi SC, Mishra PK. Predictors and prevention of diabetic cardiomyopathy.
2013; 6: 151-160 [PMID: 23610527 DOI: 10.2147/DMSO.S30968]
5 Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis.
2007; 9: 767-780 [PMID: 17924861 DOI: 10.1111/j.1463-1326.2006.00655.x]
6 Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes.
2012; 3: 170 [PMID: 23267348 DOI: 10.3389/fendo.2012.00170]
7 El-Azab MF, Hazem RM, Moustafa YM. Role of simvastatin and/or antioxidant vitamins in therapeutic angiogenesis in experimental diabetic hindlimb ischemia: effects on capillary density, angiogenesis markers, and oxidative stress.
2012; 690: 31-41 [PMID: 22705060 DOI: 10.1016/j.ejphar.2012.06.002]
8 Mysona BA, Al-Gayyar MM, Matragoon S, Abdelsaid MA, El-Azab MF, Saragovi HU, El-Remessy AB. Erratum to:Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats.
2015; 58: 644 [PMID: 25527000 DOI: 10.1007/s00125-014-3476-5]
9 Eissa LD, Ghobashy WA, El-Azab MF. Inhibition of thioredoxin-interacting protein and inflammasome assembly using verapamil mitigates diabetic retinopathy and pancreatic injury.
2021; 901: 174061 [PMID: 33766618 DOI: 10.1016/j.ejphar.2021.174061]
10 Youssef ME, Abdelrazek HM, Moustafa YM. Cardioprotective role of GTS-21 by attenuating the TLR4/NF-κB pathway in streptozotocin-induced diabetic cardiomyopathy in rats.
2021; 394: 11-31[PMID: 32776158 DOI: 10.1007/s00210-020-01957-4]
11 American Diabetes Association. Standards of medical care in diabetes--2013.
2013; 36 Suppl 1: S11-S66[PMID: 23264422 DOI: 10.2337/dc13-S011]
12 Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, Standl E, Beulens JW. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications.
2019; 26: 25-32[PMID: 31722562 DOI: 10.1177/2047487319878371]
13 Goyal BR, Mehta AA. Diabetic cardiomyopathy: pathophysiological mechanisms and cardiac dysfuntion.
2013; 32: 571-590 [PMID: 23174745 DOI: 10.1177/0960327112450885]
14 Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy.
2009; 2: 454-466 [PMID: 19726805 DOI: 10.1242/dmm.001941]
15 Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, Davis BR, Holmes DR Jr. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial.
2004; 109: 476-480 [PMID:14732749 DOI: 10.1161/01.CIR.0000109693.64957.20]
16 Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study.
1979; 241: 2035-2038[PMID: 430798]
17 van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and newonset heart failure in patients with stable coronary artery disease: data from the heart and soul study.
2010;33: 2084-2089 [PMID: 20805280 DOI: 10.2337/dc10-0286]
18 Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects.
2010; 11: 31-39 [PMID:20180026 DOI: 10.1007/s11154-010-9131-7]
19 Duerr GD, Heinemann JC, Kley J, Eichhorn L, Frede S, Weisheit C, Wehner S, Bindila L, Lutz B, Zimmer A, Dewald O.Myocardial maladaptation to pressure overload in CB2 receptor-deficient mice.
2019; 133: 86-98[PMID: 31181227 DOI: 10.1016/j.yjmcc.2019.06.003]
20 Li X, Xu Z, Li S, Rozanski GJ. Redox regulation of Ito remodeling in diabetic rat heart.
2005; 288: H1417-H1424 [PMID: 15539426 DOI: 10.1152/ajpheart.00559.2004]
21 Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease.
2007; 87: 315-424[PMID: 17237348 DOI: 10.1152/physrev.00029.2006]
22 Murarka S, Movahed MR. Diabetic cardiomyopathy.
2010; 16: 971-979 [PMID: 21111987 DOI:10.1016/j.cardfail.2010.07.249]
23 Westermann D, Rutschow S, Van Linthout S, Linderer A, Bücker-Gärtner C, Sobirey M, Riad A, Pauschinger M,Schultheiss HP, Tschöpe C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus.
2006; 49: 2507-2513 [PMID: 16937126 DOI: 10.1007/s00125-006-0385-2]
24 Fowlkes V, Clark J, Fix C, Law BA, Morales MO, Qiao X, Ako-Asare K, Goldsmith JG, Carver W, Murray DB,Goldsmith EC. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts.
2013; 92: 669-676[PMID: 23333820 DOI: 10.1016/j.lfs.2013.01.003]
25 Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation.
2009; 60: 77-84 [PMID: 19559360 DOI: 10.1016/j.phrs.2009.02.010]
26 Pacher P, Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease.
2009; 31: 63-77 [PMID: 19357846 DOI: 10.1007/s00281-009-0145-8]
27 Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R,Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia.
2006; 91: 3171-3180 [PMID: 16684820 DOI: 10.1210/jc.2005-2679]
28 Annuzzi G, Piscitelli F, Di Marino L, Patti L, Giacco R, Costabile G, Bozzetto L, Riccardi G, Verde R, Petrosino S,Rivellese AA, Di Marzo V. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients.
2010; 9: 43 [PMID: 20426869 DOI:10.1186/1476-511X-9-43]
29 Horváth B, Mukhopadhyay P, Haskó G, Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications.
2012; 180: 432-442 [PMID: 22155112 DOI:10.1016/j.ajpath.2011.11.003]
30 Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ,Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases.
2008; 29: 270-276 [PMID: 17916581 DOI: 10.1093/eurheartj/ehm342]
31 Merlo M, Cannatà A, Gobbo M, Stolfo D, Elliott PM, Sinagra G. Evolving concepts in dilated cardiomyopathy.
2018; 20: 228-239 [PMID: 29271570 DOI: 10.1002/ejhf.1103]
32 Dyntar D. Diabetic cardiomyopathy: effects of fatty acids and glucose on adult rat cardiac cells.
2003 [DOI:10.3929/ethz-a-005895335]
33 Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis.
1972; 30: 595-602 [PMID: 4263660]
34 Pappachan JM, Varughese GI, Sriraman R, Arunagirinathan G. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management.
2013; 4: 177-189 [PMID: 24147202 DOI: 10.4239/wjd.v4.i5.177]
35 Bertero E, Maack C. Metabolic remodelling in heart failure.
2018; 15: 457-470 [PMID: 29915254 DOI:10.1038/s41569-018-0044-6]
36 Dei Cas A, Spigoni V, Ridolfi V, Metra M. Diabetes and chronic heart failure: from diabetic cardiomyopathy to therapeutic approach.
2013; 13: 38-50 [PMID: 23369136 DOI:10.2174/1871530311313010006]
37 Giacco F, Brownlee M. Oxidative stress and diabetic complications.
2010; 107: 1058-1070 [PMID: 21030723 DOI: 10.1161/CIRCRESAHA.110.223545]
38 Calle MC, Fernandez ML. Inflammation and type 2 diabetes.
2012; 38: 183-191 [PMID: 22252015 DOI:10.1016/j.diabet.2011.11.006]
39 Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications.
2004; 25: 543-567 [PMID: 15294881 DOI: 10.1210/er.2003-0012]
40 Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic,normotensive patients with diabetes mellitus.
2004; 93: 870-875 [PMID: 15050491 DOI:10.1016/j.amjcard.2003.12.026]
41 Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features.
2013; 18: 149-166 [PMID: 22453289 DOI: 10.1007/s10741-012-9313-3]
42 Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish.
1964; 86: 1646-1647
43 Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain.
1988; 34: 605-613 [PMID: 2848184]
44 Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids.
1993;365: 61-65 [PMID: 7689702 DOI: 10.1038/365061a0]
45 Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A,Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor.
1992; 258:1946-1949 [PMID: 1470919]
46 Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR,Compton DR. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors.
1995; 50: 83-90 [PMID: 7605349]
47 Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation.
2004; 3: 771-784 [PMID: 15340387 DOI: 10.1038/nrd1495]
48 Howlett AC. Cannabinoid receptor signaling.
2005; 53-79 [PMID: 16596771]
49 Montecucco F, Di Marzo V. At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction.
2012; 33: 331-340 [PMID: 22503477 DOI: 10.1016/j.tips.2012.03.002]
50 Mackie K. Cannabinoid receptors as therapeutic targets.
2006; 46: 101-122 [PMID:16402900 DOI: 10.1146/annurev.pharmtox.46.120604.141254]
51 Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy.
2006; 58: 389-462 [PMID: 16968947 DOI: 10.1124/pr.58.3.2]
52 Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity.
2007; 50: 528-536 [PMID: 17678736 DOI: 10.1016/j.jacc.2007.03.057]
53 Steffens S, Pacher P. Targeting cannabinoid receptor CB(2) in cardiovascular disorders: promises and controversies.
2012; 167: 313-323 [PMID: 22612332 DOI: 10.1111/j.1476-5381.2012.02042.x]
54 Hoyer FF, Steinmetz M, Zimmer S, Becker A, Lütjohann D, Buchalla R, Zimmer A, Nickenig G. Atheroprotection
cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo.
2011; 51: 1007-1014[PMID: 21884703 DOI: 10.1016/j.yjmcc.2011.08.008]
55 Tallant EA, Howlett A, Grabenauer M, Thomas BF, Gallagher PE. The CB2 Cannabinoid Receptor Mediates the Anti-Proliferative Actions of Angiotensin-(1-7) in Vascular Smooth Muscle Cells.
2013
56 Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion.
2009; 46: 612-620[PMID: 19162037 DOI: 10.1016/j.yjmcc.2008.12.014]
57 Bermúdez-Silva FJ, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz AL, Fuentes E, Juan-Pico P, Castro MJ,Milman G, Mechoulam R, Nadal A, Rodríguez de Fonseca F. Presence of functional cannabinoid receptors in human endocrine pancreas.
2008; 51: 476-487 [PMID: 18092149 DOI: 10.1007/s00125-007-0890-y]
58 Li C, Bowe JE, Jones PM, Persaud SJ. Expression and function of cannabinoid receptors in mouse islets.
2010; 2:293-302 [PMID: 21099327]
59 Vilches-Flores A, Delgado-Buenrostro NL, Navarrete-Vázquez G, Villalobos-Molina R. CB1 cannabinoid receptor expression is regulated by glucose and feeding in rat pancreatic islets.
2010; 163: 81-87 [PMID: 20451564 DOI: 10.1016/j.regpep.2010.04.013]
60 Nakata M, Yada T. Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet beta-cells
CB1 receptors.
2008; 145: 49-53 [PMID: 17884194 DOI: 10.1016/j.regpep.2007.08.009]
61 Li C, Jones PM, Persaud SJ. Cannabinoid receptors are coupled to stimulation of insulin secretion from mouse MIN6 beta-cells.
2010; 26: 187-196 [PMID: 20798502 DOI: 10.1159/000320527]
62 Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF; RIO-Diabetes Study Group. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study.
2006; 368: 1660-1672 [PMID: 17098084 DOI: 10.1016/S0140-6736(06)69571-8]
63 Rosenstock J, Hollander P, Chevalier S, Iranmanesh A; SERENADE Study Group. SERENADE: the Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes.
2008; 31: 2169-2176 [PMID: 18678611 DOI: 10.2337/dc08-0386]
64 Hollander PA, Amod A, Litwak LE, Chaudhari U; ARPEGGIO Study Group. Effect of rimonabant on glycemic control in insulin-treated type 2 diabetes: the ARPEGGIO trial.
2010; 33: 605-607 [PMID: 20009090 DOI:10.2337/dc09-0455]
65 Li X, Kaminski NE, Fischer LJ. Examination of the immunosuppressive effect of delta9-tetrahydrocannabinol in streptozotocin-induced autoimmune diabetes.
2001; 1: 699-712 [PMID: 11357882]
66 Weiss L, Zeira M, Reich S, Slavin S, Raz I, Mechoulam R, Gallily R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice.
2008; 54: 244-249 [PMID: 17714746 DOI: 10.1016/j.neuropharm.2007.06.029]
67 Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting.
2008;88: 1322-1335 [PMID: 18801863 DOI: 10.2522/ptj.20080008]
68 Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, Pacher P. Interplay of oxidative, nitrosative/nitrative stress,inflammation, cell death and autophagy in diabetic cardiomyopathy.
2015; 1852: 232-242 [PMID:24997452 DOI: 10.1016/j.bbadis.2014.06.030]
69 Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms.
2013; 1: 244-257 [PMID: 24024158 DOI: 10.1016/j.redox.2013.01.014]
70 Culotta VC. Superoxide dismutase, oxidative stress, and cell metabolism.
2000; 36: 117-132 [PMID:10842749]
71 Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases.
1999; 32: 595-603 [PMID: 10638941]
72 Webster NR, Nunn JF. Molecular structure of free radicals and their importance in biological reactions.
1988; 60: 98-108
73 Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite:implications for endothelial injury from nitric oxide and superoxide.
1990; 87: 1620-1624[PMID: 2154753]
74 Huie RE, Padmaja S. The reaction of no with superoxide.
1993; 18: 195-199 [PMID: 8396550]
75 Babior BM. NADPH oxidase.
2004; 16: 42-47 [PMID: 14734109]
76 White KA, Marletta MA. Nitric oxide synthase is a cytochrome P-450 type hemoprotein.
1992; 31: 6627-6631 [PMID: 1379068]
77 Dröge W. Free radicals in the physiological control of cell function.
2002; 82: 47-95 [PMID: 11773609 DOI:10.1152/physrev.00018.2001]
78 Broeke RT, Leusink-Muis T, Hilberdink R, Van Ark I, van den Worm E, Villain M, De Clerck F, Blalock JE, Nijkamp FP, Folkerts G. Specific modulation of calmodulin activity induces a dramatic production of superoxide by alveolar macrophages.
2004; 84: 29-40 [PMID: 14631377 DOI: 10.1038/sj.labinvest.3700002]
79 Zhang X, Chen C. A new insight of mechanisms, diagnosis and treatment of diabetic cardiomyopathy.
2012;41: 398-409 [PMID: 22322947 DOI: 10.1007/s12020-012-9623-1]
80 Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications.
2000; 77: S26-S30 [PMID: 10997687]
81 Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP,Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage.
2000; 404: 787-790 [PMID: 10783895 DOI: 10.1038/35008121]
82 Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes.
2013; 92: 601-608 [PMID: 23147391 DOI: 10.1016/j.lfs.2012.10.028]
83 Halliwell B. Biochemistry of oxidative stress.
2007; 35: 1147-1150 [PMID: 17956298 DOI:10.1042/BST0351147]
84 King MK, Coker ML, Goldberg A, McElmurray JH 3rd, Gunasinghe HR, Mukherjee R, Zile MR, O'Neill TP, Spinale FG. Selective matrix metalloproteinase inhibition with developing heart failure: effects on left ventricular function and structure.
2003; 92: 177-185 [PMID: 12574145]
85 Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy.
2001; 89: 279-286 [PMID: 11485979]
86 Takano H, Zou Y, Hasegawa H, Akazawa H, Nagai T, Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases.
2003; 5: 789-794 [PMID: 14588152 DOI: 10.1089/152308603770380098]
87 Imrie H, Abbas A, Kearney M. Insulin resistance, lipotoxicity and endothelial dysfunction.
2010;1801: 320-326 [PMID: 19818873 DOI: 10.1016/j.bbalip.2009.09.025]
88 Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, Lu Y, Le Tissier S, Peng T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy.
2016; 90: 12-23[PMID: 26577173 DOI: 10.1016/j.freeradbiomed.2015.11.013]
89 Kowluru RA, Kowluru A, Veluthakal R, Mohammad G, Syed I, Santos JM, Mishra M. TIAM1-RAC1 signalling axismediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy.
2014; 57: 1047-1056 [PMID: 24554007 DOI: 10.1007/s00125-014-3194-z]
90 Mukhopadhyay P, Rajesh M, Bátkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Haskó G, Pacher P.CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes.
2010; 85: 773-784 [PMID: 19942623 DOI:10.1093/cvr/cvp369]
91 Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S, Patel V, Tanchian G, Gao RY, Cravatt BF,Haskó G, Pacher P. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury.
2011; 50: 179-195 [PMID: 21070851 DOI: 10.1016/j.freeradbiomed.2010.11.002]
92 Rajesh M, Bátkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horváth B, Holovac E, Cinar R, Liaudet L, Mackie K,Haskó G, Pacher P. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy.
2012; 61: 716-727 [PMID: 22315315 DOI: 10.2337/db11-0477]
93 Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain.
2006; 7: 607-615 [PMID: 16553576 DOI: 10.1517/14656566.7.5.607]
94 Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb.
2009; 30: 515-527 [PMID: 19729208 DOI:10.1016/j.tips.2009.07.006]
95 Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B,Becker L, Haskó G, Liaudet L, Wink DA, Veves A, Mechoulam R, Pacher P. Cannabidiol attenuates cardiac dysfunction,oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy.
2010; 56: 2115-2125 [PMID: 21144973 DOI: 10.1016/j.jacc.2010.07.033]
96 Vella RK, Jackson DJ, Fenning AS. Δ
-Tetrahydrocannabinol Prevents Cardiovascular Dysfunction in STZ-Diabetic Wistar-Kyoto Rats.
2017; 2017: 7974149 [PMID: 29181404 DOI: 10.1155/2017/7974149]
97 Dresner LS, Wang SP, West MW, Ponomarenko IN, Mueller CM, Wait RB. Nitric oxide inhibition simulates the enhancement of alpha 1 agonist-induced vasoconstriction in diabetes.
1997; 70: 119-123 [PMID: 9245559 DOI: 10.1006/jsre.1997.5106]
98 Iwalewa EO, McGaw LJ, Naidoo V, Eloff JN. Inflammation: the foundation of diseases and disorders. A review of phytomedicines of South African origin used to treat pain and inflammatory conditions.
2007; 6
99 Esch T, Stefano G. Proinflammation: a common denominator or initiator of different pathophysiological disease processes.
2002; 8: HY1-HY9 [PMID: 12011758]
100 Buyken AE, Flood V, Empson M, Rochtchina E, Barclay AW, Brand-Miller J, Mitchell P. Carbohydrate nutrition and inflammatory disease mortality in older adults.
2010; 92: 634-643 [PMID: 20573797 DOI:10.3945/ajcn.2010.29390]
101 Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors.
1999; 18: 6853-6866 [PMID:10602461 DOI: 10.1038/sj.onc.1203239]
102 Coleman JW. Nitric oxide: a regulator of mast cell activation and mast cell-mediated inflammation.
2002; 129: 4-10 [PMID: 12100016]
103 King GL. The role of inflammatory cytokines in diabetes and its complications.
2008; 79: 1527-1534[PMID: 18673007 DOI: 10.1902/jop.2008.080246]
104 Lorenzo O, Picatoste B, Ares-Carrasco S, Ramírez E, Egido J, Tuñón J. Potential role of nuclear factor κB in diabetic cardiomyopathy.
2011; 2011: 652097 [PMID: 21772665 DOI: 10.1155/2011/652097]
105 Chen S, Khan ZA, Cukiernik M, Chakrabarti S. Differential activation of NF-kappa B and AP-1 in increased fibronectin synthesis in target organs of diabetic complications.
2003; 284: E1089-E1097 [PMID:12582013 DOI: 10.1152/ajpendo.00540.2002]
106 Al-Maghrebi M, Benter IF, Diz DI. Endogenous angiotensin-(1-7) reduces cardiac ischemia-induced dysfunction in diabetic hypertensive rats.
2009; 59: 263-268 [PMID: 19166939 DOI: 10.1016/j.phrs.2008.12.008]
107 Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics.
2005; 5: 400-411 [PMID:15864274 DOI: 10.1038/nri1602]
108 Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease.
2007; 18: 129-140 [PMID: 17353660 DOI: 10.1097/MOL.0b013e32803dbdec]
109 Cabral GA, Staab A. Effects on the immune system.
2005; 385-423 [PMID: 16596782]
110 Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation.
2003; 74: 486-496 [PMID: 12960289 DOI: 10.1189/jlb.0303101]
111 Cencioni MT, Chiurchiù V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly
CB2 receptors.
2010; 5:e8688 [PMID: 20098669 DOI: 10.1371/journal.pone.0008688]
112 Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, Kang SS, Ahn YK, Park CS, Kim JJ. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages.
2009; 84: 378-386 [PMID: 19596672 DOI: 10.1093/cvr/cvp240]
113 Dol-Gleizes F, Paumelle R, Visentin V, Marés AM, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P, Bono F.Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice.
2009; 29: 12-18 [PMID: 18845788 DOI: 10.1161/ATVBAHA.108.168757]
114 Zhao Y, Liu Y, Zhang W, Xue J, Wu YZ, Xu W, Liang X, Chen T, Kishimoto C, Yuan Z. WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice.
2010; 649: 285-292 [PMID: 20868672 DOI: 10.1016/j.ejphar.2010.09.027]
115 Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, Zhang W, Shen Y, Xu W, Liang X, Chen T. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules.
2010; 55: 292-298 [PMID: 20075743 DOI: 10.1097/FJC.0b013e3181d2644d]
116 Hashiesh HM, Sharma C, Goyal SN, Jha NK, Ojha S. Pharmacological Properties, Therapeutic Potential and Molecular Mechanisms of JWH133, a CB2 Receptor-Selective Agonist.
2021; 12: 702675 [PMID: 34393784 DOI:10.3389/fphar.2021.702675]
117 Pacher P, Haskó G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning.
2008; 153: 252-262 [PMID: 18026124 DOI: 10.1038/sj.bjp.0707582]
118 Wang J, Song Y, Wang Q, Kralik PM, Epstein PN. Causes and characteristics of diabetic cardiomyopathy.
2006; 3: 108-117 [PMID: 17487334 DOI: 10.1900/RDS.2006.3.108]
119 Acar E, Ural D, Bildirici U, Sahin T, Yılmaz I. Diabetic cardiomyopathy.
2011; 11: 732-737[PMID: 22137942 DOI: 10.5152/akd.2011.196]
120 Monnier VM, Sell DR, Genuth S. Glycation products as markers and predictors of the progression of diabetic complications.
2005; 1043: 567-581 [PMID: 16037280 DOI: 10.1196/annals.1333.065]
121 Xu J, Chen LJ, Yu J, Wang HJ, Zhang F, Liu Q, Wu J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy.
2018; 48: 705-717 [PMID: 30025404 DOI:10.1159/000491897]
122 Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications.
2014; 18: 1-14 [PMID: 24634591 DOI: 10.4196/kjpp.2014.18.1.1]
123 Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins.
1992; 267: 14998-15004 [PMID:1378843]
124 Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications.
2007; 9: 1146-1155 [PMID: 18023248 DOI:10.1016/j.ejheart.2007.09.009]
125 Lepetsos P, Papavassiliou KA, Papavassiliou AG. Redox and NF-κB signaling in osteoarthritis.
2019; 132: 90-100 [PMID: 30236789 DOI: 10.1016/j.freeradbiomed.2018.09.025]
126 D'Souza A, Howarth FC, Yanni J, Dobryznski H, Boyett MR, Adeghate E, Bidasee KR, Singh J. Left ventricle structural remodelling in the prediabetic Goto-Kakizaki rat.
2011; 96: 875-888 [PMID: 21622965 DOI:10.1113/expphysiol.2011.058271]
127 Watanabe K, Thandavarayan RA, Harima M, Sari FR, Gurusamy N, Veeraveedu PT, Mito S, Arozal W, Sukumaran V,Laksmanan AP, Soetikno V, Kodama M, Aizawa Y. Role of differential signaling pathways and oxidative stress in diabetic cardiomyopathy.
2010; 6: 280-290 [PMID: 22043204 DOI: 10.2174/157340310793566145]
128 Mandavia CH, Pulakat L, DeMarco V, Sowers JR. Over-nutrition and metabolic cardiomyopathy.
2012; 61:1205-1210 [PMID: 22465089 DOI: 10.1016/j.metabol.2012.02.013]
129 Hayden MR, Tyagi SC. Myocardial redox stress and remodeling in metabolic syndrome, type 2 diabetes mellitus, and congestive heart failure.
2003; 9: SR35-SR52 [PMID: 12883468]
130 Schreier B, Rabe S, Schneider B, Ruhs S, Grossmann C, Hauptmann S, Blessing M, Neumann J, Gekle M.Aldosterone/NaCl-induced renal and cardiac fibrosis is modulated by TGF-β responsiveness of T cells.
2011; 34: 623-629 [PMID: 21346767 DOI: 10.1038/hr.2011.16]
131 Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling.
2004; 79: 187-205 [PMID: 15005177]
132 Athanasiou A, Clarke AB, Turner AE, Kumaran NM, Vakilpour S, Smith PA, Bagiokou D, Bradshaw TD, Westwell AD,Fang L, Lobo DN, Constantinescu CS, Calabrese V, Loesch A, Alexander SP, Clothier RH, Kendall DA, Bates TE.Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death.
2007; 364: 131-137 [PMID: 17931597 DOI:10.1016/j.bbrc.2007.09.107]
133 Sarafian TA, Habib N, Oldham M, Seeram N, Lee RP, Lin L, Tashkin DP, Roth MD. Inhaled marijuana smoke disrupts mitochondrial energetics in pulmonary epithelial cells in vivo.
2006; 290: L1202-L1209 [PMID: 16414979 DOI: 10.1152/ajplung.00371.2005]
134 Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce?
2008; 7: 438-455[PMID: 18446159 DOI: 10.1038/nrd2553]
135 Chanda D, Neumann D, Glatz JFC. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target.
2019; 140: 51-56 [PMID: 30553404 DOI: 10.1016/j.plefa.2018.11.016]
136 Hiley CR. Endocannabinoids and the heart.
2009; 53: 267-276 [PMID: 19276990 DOI:10.1097/FJC.0b013e318192671d]
137 Rabino M, Mallia S, Castiglioni E, Rovina D, Pompilio G, Gowran A. The Endocannabinoid System and Cannabidiol:Past, Present, and Prospective for Cardiovascular Diseases.
2021; 14 [PMID: 34577636 DOI:10.3390/ph14090936]
138 Sun Y. Myocardial repair/remodelling following infarction: roles of local factors.
2009; 81: 482-490[PMID: 19050008 DOI: 10.1093/cvr/cvn333]
139 Defer N, Wan J, Souktani R, Escoubet B, Perier M, Caramelle P, Manin S, Deveaux V, Bourin MC, Zimmer A,Lotersztajn S, Pecker F, Pavoine C. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy.
2009; 23: 2120-2130 [PMID: 19246487 DOI:10.1096/fj.09-129478]
140 Wagner JA, Hu K, Karcher J, Bauersachs J, Schäfer A, Laser M, Han H, Ertl G. CB(1) cannabinoid receptor antagonism promotes remodeling and cannabinoid treatment prevents endothelial dysfunction and hypotension in rats with myocardial infarction.
2003; 138: 1251-1258 [PMID: 12711625 DOI: 10.1038/sj.bjp.0705156]
141 Liao Y, Bin J, Luo T, Zhao H, Ledent C, Asakura M, Xu D, Takashima S, Kitakaze M. CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice.
2013; 167: 1936-1944[PMID: 22656047 DOI: 10.1016/j.ijcard.2012.05.033]
142 Weis F, Beiras-Fernandez A, Sodian R, Kaczmarek I, Reichart B, Beiras A, Schelling G, Kreth S. Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure.
2010; 48: 1187-1193 [PMID: 19931541 DOI: 10.1016/j.yjmcc.2009.10.025]
143 Duerr GD, Heinemann JC, Dunkel S, Zimmer A, Lutz B, Lerner R, Roell W, Mellert F, Probst C, Esmailzadeh B, Welz A, Dewald O. Myocardial hypertrophy is associated with inflammation and activation of endocannabinoid system in patients with aortic valve stenosis.
2013; 92: 976-983 [PMID: 23567807 DOI: 10.1016/j.lfs.2013.03.014]
144 Cortes CJ, La Spada AR. TFEB dysregulation as a driver of autophagy dysfunction in neurodegenerative disease:Molecular mechanisms, cellular processes, and emerging therapeutic opportunities.
2019; 122: 83-93[PMID: 29852219 DOI: 10.1016/j.nbd.2018.05.012]
145 Shao BZ, Han BZ, Zeng YX, Su DF, Liu C. The roles of macrophage autophagy in atherosclerosis.
2016; 37: 150-156 [PMID: 26750103 DOI: 10.1038/aps.2015.87]
146 Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex.
2013; 14: 759-774 [PMID: 24201109 DOI: 10.1038/nrm3696]
147 Klionsky DJ. Look people, "Atg" is an abbreviation for "autophagy-related." That's it.
2012; 8: 1281-1282[PMID: 22889836 DOI: 10.4161/auto.21812]
148 Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae.
1993; 333: 169-174 [PMID: 8224160 DOI: 10.1016/0014-5793(93)80398-e]
149 Suzuki H, Osawa T, Fujioka Y, Noda NN. Structural biology of the core autophagy machinery.
2017; 43: 10-17 [PMID: 27723509 DOI: 10.1016/j.sbi.2016.09.010]
150 Chan EY. Regulation and function of uncoordinated-51 Like kinase proteins.
2012; 17: 775-785[PMID: 22074133 DOI: 10.1089/ars.2011.4396]
151 Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 Lipid kinase.
2013; 15: 741-750 [PMID:23685627 DOI: 10.1038/ncb2757]
152 Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L,D'Amelio M, Nardacci R, Romagnoli A, Piacentini M, Cecconi F, Fimia GM. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy.
2010; 191: 155-168 [PMID: 20921139 DOI:10.1083/jcb.201002100]
153 Dooley HC, Razi M, Polson HE, Girardin SE, Wilson MI, Tooze SA. WIPI2 Links LC3 conjugation with PI3P,autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1.
2014; 55: 238-252 [PMID:24954904 DOI: 10.1016/j.molcel.2014.05.021]
154 Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy.
2007; 282: 37298-37302 [PMID: 17986448 DOI:10.1074/jbc.C700195200]
155 Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 Localize to autophagosomal membrane depending on form-II formation.
2004; 117: 2805-2812 [PMID:15169837 DOI: 10.1242/jcs.01131]
156 Fujita N, Hayashi-Nishino M, Fukumoto H, Omori H, Yamamoto A, Noda T, Yoshimori T. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure.
2008; 19: 4651-4659[PMID: 18768752 DOI: 10.1091/mbc.E08-03-0312]
157 Park S, Choi SG, Yoo SM, Son JH, Jung YK. Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy.
2014; 10: 1906-1920 [PMID: 25483962 DOI: 10.4161/auto.32177]
158 Kim YM, Jung CH, Seo M, Kim EK, Park JM, Bae SS, Kim DH. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation.
2015; 57: 207-218 [PMID: 25533187 DOI:10.1016/j.molcel.2014.11.013]
159 Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: A comprehensive review.
2018; 104: 485-495 [PMID: 29800913 DOI: 10.1016/j.biopha.2018.05.007]
160 Wang GY, Bi YG, Liu XD, Zhao Y, Han JF, Wei M, Zhang QY. Autophagy was involved in the protective effect of metformin on hyperglycemia-induced cardiomyocyte apoptosis and Connexin43 downregulation in H9c2 cells.
2017; 14: 698-704 [PMID: 28824303 DOI: 10.7150/ijms.19800]
161 Yang F, Zhang L, Gao Z, Sun X, Yu M, Dong S, Wu J, Zhao Y, Xu C, Zhang W, Lu F. Exogenous H2S Protects Against Diabetic Cardiomyopathy by Activating Autophagy
the AMPK/mTOR Pathway.
2017; 43:1168-1187 [PMID: 28977784 DOI: 10.1159/000481758]
162 Denaës T, Lodder J, Chobert MN, Ruiz I, Pawlotsky JM, Lotersztajn S, Teixeira-Clerc F. The Cannabinoid Receptor 2 Protects Against Alcoholic Liver Disease Via a Macrophage Autophagy-Dependent Pathway.
2016; 6: 28806[PMID: 27346657 DOI: 10.1038/srep28806]
163 Ke P, Shao BZ, Xu ZQ, Wei W, Han BZ, Chen XW, Su DF, Liu C. Activation of Cannabinoid Receptor 2 Ameliorates DSS-Induced Colitis through Inhibiting NLRP3 Inflammasome in Macrophages.
2016; 11: e0155076 [PMID:27611972 DOI: 10.1371/journal.pone.0155076]
164 Shao BZ, Wei W, Ke P, Xu ZQ, Zhou JX, Liu C. Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis
activation of autophagy and inhibiting NLRP3 inflammasome.
2014; 20: 1021-1028 [PMID: 25417929 DOI: 10.1111/cns.12349]
165 Pei Z, Deng Q, Babcock SA, He EY, Ren J, Zhang Y. Inhibition of advanced glycation endproduct (AGE) rescues against streptozotocin-induced diabetic cardiomyopathy: Role of autophagy and ER stress.
2018; 284: 10-20 [PMID:29174818 DOI: 10.1016/j.toxlet.2017.11.018]
166 Xiao Y, Wu QQ, Duan MX, Liu C, Yuan Y, Yang Z, Liao HH, Fan D, Tang QZ. TAX1BP1 overexpression attenuates cardiac dysfunction and remodeling in STZ-induced diabetic cardiomyopathy in mice by regulating autophagy.
2018; 1864: 1728-1743 [PMID: 29476905 DOI: 10.1016/j.bbadis.2018.02.012]
167 Wu A, Hu P, Lin J, Xia W, Zhang R. Activating Cannabinoid Receptor 2 Protects Against Diabetic Cardiomyopathy Through Autophagy Induction.
2018; 9: 1292 [PMID: 30459625 DOI: 10.3389/fphar.2018.01292]
168 Xu K, Liu XF, Ke ZQ, Yao Q, Guo S, Liu C. Resveratrol Modulates Apoptosis and Autophagy Induced by High Glucose and Palmitate in Cardiac Cells.
2018; 46: 2031-2040 [PMID: 29723857 DOI: 10.1159/000489442]
169 Zhou B, Leng Y, Lei SQ, Xia ZY. AMPK activation restores ischemic postconditioning cardioprotection in STZinduced type 1 diabetic rats: Role of autophagy.
2017; 16: 3648-3656 [PMID: 28765969 DOI:10.3892/mmr.2017.7033]
170 Böckmann S, Hinz B. Cannabidiol Promotes Endothelial Cell Survival by Heme Oxygenase-1-Mediated Autophagy.
2020; 9 [PMID: 32708634 DOI: 10.3390/cells9071703]
171 Lin TK, Chen SD, Chuang YC, Lin HY, Huang CR, Chuang JH, Wang PW, Huang ST, Tiao MM, Chen JB, Liou CW.Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy.
2014; 15: 1625-1646 [PMID: 24451142 DOI: 10.3390/ijms15011625]
172 Surolia R, Karki S, Kim H, Yu Z, Kulkarni T, Mirov SB, Carter AB, Rowe SM, Matalon S, Thannickal VJ, Agarwal A,Antony VB. Heme oxygenase-1-mediated autophagy protects against pulmonary endothelial cell death and development of emphysema in cadmium-treated mice.
2015; 309: L280-L292 [PMID: 26071551 DOI: 10.1152/ajplung.00097.2015]
173 Waltz P, Carchman EH, Young AC, Rao J, Rosengart MR, Kaczorowski D, Zuckerbraun BS. Lipopolysaccaride induces autophagic signaling in macrophages
a TLR4, heme oxygenase-1 dependent pathway.
2011; 7: 315-320[PMID: 21307647 DOI: 10.4161/auto.7.3.14044]
174 Schwartz M, Böckmann S, Hinz B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features by cannabidiol in vascular smooth muscle cells.
2018; 9: 34595-34616 [PMID: 30349652 DOI:10.18632/oncotarget.26191]
杂志排行
World Journal of Diabetes的其它文章
- Admission hemoglobin level and prognosis of type 2 diabetes mellitus and possible confounding factors: Correspondence
- Concomitant dysregulation of androgen secretion and dysfunction of adipose tissue induced insulin resistance
- Changes and significance of retinal blood oxygen saturation and oxidative stress indexes in patients with diabetic retinopathy
