Use of hydroxychloroquine and azithromycin combination to treat the COVID-19 infection
2022-05-18JyotiBajpaiAkshyayaPradhanAjayKumarVermaSuryaKant
Jyoti Bajpai, Akshyaya Pradhan, Ajay Kumar Verma, Surya Kant
Jyoti Bajpai, Ajay Kumar Verma, Department of Respiratory Medicine, King George's Medical University, Lukcnow, Lucknow 226003, Uttar Pradesh, India
Akshyaya Pradhan, Department of Cardiology, King George's Medical University, Lukcnow,Lucknow 226003, Uttar Pradesh, India
Surya Kant, Department of Respiratory Medicine, King George Medical University, Lucknow 226003, Uttar Pradesh, India
Abstract Coronavirus disease 2019 (COVID-19) infection is unequivocally the worst crisis in recent decades, which is caused by a severe acute respiratory virus 2. Currently, there is no effective therapy for the COVID-19 infection. Different countries have different guidelines for treating COVID-19 in the absence of an approved therapy for COVID-19. Therefore, there is an imminent need to identify effective treatments, and several clinical trials have been conducted worldwide. Both hydroxychloroquine [HCQS], chloroquine, and azithromycin (AZ) have been widely used for management based on in vitro studies favoring antiviral effects against the COVID-19 virus. However, there is evidence both in favor and against the use of hydroxychloroquine and azithromycin (HCQS+AZ) combination therapy to manage the COVID-19 infection. The combination of hydroxychloroquine and azithromycin was significantly associated with increased adverse events. However, the inference of these findings was from observational studies. Therefore, large randomized trials are imperative to show the future path for the use of HCQS+AZ combination therapy. However, owing to the ban on HCQS use in COVID-19, this may no longer be essential. This review is on the pharmacology, trials, regimens, and side effects of hydroxychloroquine and azithromycin combination therapy.
Key Words: Hydroxychloroquine; Azithromycin; Antiviral effects; QT interval;Randomized controlled trial
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic is possibly one of the most severe we all have witnessed in recent decades. The COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) and was initially reported in Wuhan, China in December 2019[1]. Currently, more than 250 million cases have occurred across the world, and a total of approximately 5 million deaths have been reported thus far[2]. Social distancing, infection control measures, frequent hand washing, and wearing a mask are the cornerstone of the COVID-19 prevention and control. Currently, there are no known effective therapies (e.g., antiviral medications and vaccines) for the disease apart from vaccines which have been shown to be effective against prevention of COVID infection.
A lack of effective therapy against COVID-19 has led the clinicians to rethink the use of repurposed drugs as an effective treatment for COVID-19. The first repurposed drug to be used was the antimalarial drug chloroquine. It is an analog of hydroxychloroquine that is used to treat autoimmune diseases such as systemic lupus erythematous and rheumatoid arthritis. These drugs have shown antiviral activity and immune-modulatory effects underin vivoconditions[3,4]. However, the use of the above mentioned repurposed drug for COVID-19 is based on the results of a small number of observational studies and non-randomized trials, which have been inconclusive. The combination of hydroxychloroquine with a second-generation macrolide (e.g., azithromycin) has also been used, despite limited evidence for its effectiveness[5]. Different studies have shown that treatment with the hydroxychloroquine and azithromycin combination may have an adverse cardiovascular effect of prolonging the QT interval, which may result in predisposition to ventricular arrhythmias[6,7].
Many therapies have been tried for treating COVID-19; however, there have been no long-term studies on the use of these approaches[8]. This review briefly describes the pharmacology, trials, regimens, and adverse effects of the hydroxychloroquine and azithromycin combination therapy.
Methods
We systematically searched the PubMed and Clinical trials.org databases up to December 25, 2021 using several specific keywords (i.e., “COVID-19”, “HCQS and azithromycin”, or “SARS-COV-2”) and retrieved all articles published in the English language that reported efficacy, safety, clinical outcome, and pharmacology for the hydroxychloroquine and azithromycin combination in patients with COVID-19. We compiled all the data and narrated the past, present, and future of HCQS and azithromycin combination in the context of COVID-19.
HCQS
HCQS is a 4-aminoquinolone that is widely used to treat certain autoimmune diseases and dermatological conditions. HCQS is a less toxic by-product of chloroquine, which had been used to treat COVID-19. It is a more soluble hydroxy-analog of chloroquine, which Hans Andersag first synthesized in 1934 and confirmed by military testing during World War II as a safe anti-malarial drug. HCQS has been successfully used during the 20th century to prevent and treat malaria in endemic areas.
According toin vitrostudies, HCQS can inhibit virus entry, transmission, and replication[9]. HCQS increases the pH of cellular endosomes, which inhibits viral entry and replication. Another primary mechanism is the glycosylation of the virus surface receptor ACE-2[10]. In addition to the antiviral activity, various actions of HCQS consist of immune modulation, anti-inflammatory properties, regulation of proinflammatory cytokines [e.g., tumor necrosis factors, interleukin (IL) 1 and 6], and additional antioxidant activities. Currently, there are irrefutable data on cytokine storm in severe cases of COVID-19, which also affects the prognosis of disease[11]. In such cases, the immunomodulatory effect of HCQS can be used for mechanical benefit. HCQS is a less expensive and readily available drug.
Azithromycin
Azithromycin (AZ) (azithromycin dehydrate) is a macrolide; it is an azalide congener of erythromycin and has shown activity against the Zika virus[12-14]. Azithromycin has an expanded spectrum, better tolerability, and superior drug interaction profile. It is more active against gram-negative bacilli H. influenzae. Its pharmacological properties include acid stability, large tissue distribution, rapid oral absorption (from an empty stomach), high attained concentration inside macrophages and fibroblast, and a long terminal half-life > 50 h. It is primarily excreted unchanged in bile, and renal excretion is 10%. There is a molecular similarity between azithromycin and the sugar moiety of ganglioside; a lipid raft ganglioside acts as host attachment cofactor for respiratory viruses. Owing to this similarity, azithromycin interacts with the ganglioside binding domain of the COVID-19 spike protein[15].
An additional advantage may be the prevention of secondary bacterial infection in cytokine-affected alveoli. Macrolide inhibits the CYP-3A4 enzyme with consequent elevation of hydroxychloroquine levels. In vitro studies have shown that the hydroxychloroquine and azithromycin combination has a synergetic effect on SARS-CoV-2-infected cells[15].
HCQS PLUS AZITHROMYCIN CLINICAL DATA
Studies in favor of the combination therapy -the rise of the Roman empire
A study by Gautretet al[16] showed that HCQS was efficient in decreasing the viral nasopharyngeal carriage of COVID-19 in most patients in 3-6 days. On day six post-inclusion, 70% of HCQS-treated patients were virologically cured compared to 12.5% in the control group. The six COVID-19 patients received HCQS+AZ combination therapy for five days (to prevent bacterial superinfection). Five out of six patients' viral load cleared on day three, and all six patients (100%) were virologically cured at day six post-inclusion. Four patients had a lower respiratory tract infection (RTI), and the rest were in the upper RTI group. The adverse effects of the combination therapy were not well documented in the study.
A second pilot study by Gautretet al[17] was performed on 80 patients, who received 200 mg of HCQS three times a day for ten days and 500 mg of azithromycin on day one, and 250 mg for the rest 2-5 d. The majority (65/80, 81.3%) of patients had a favorable outcome. Only 15% of the patients required oxygen therapy, and three patients were transferred to the intensive care unit (ICU), of whom two improved. Only one 74-year-old patient died.
A study by Chenet al[18] initially demonstrated the efficacy of the drug against COVID-19. The use of HCQS resulted in a significant improvement of clinical symptoms, such as fever (2.2 ± 0.4 d) compared to the control group (3.2 ± 1.3 d), cough (2.0 ± 0.2 dvs3.1 ± 1.5 d), and significant radiological improvement.
Millionet al[19] evaluated 1061 COVID-19 patients treated with the HCQS+AZ combination therapy for three days and eight-day follow-ups. The majority of patients had mild COVID-19 disease at admission. The primary outcome was to check for worsening of the condition and access to the intensive care unit. Only ten patients (0.9%) were transferred to the intensive care unit. In addition, this therapy prevented death; only eight patients (0.75%) died.
Based on various observational and non-randomized studies, HCQS+AZ has been recommended in other guidelines and national consensus statements.
Studies that did not favor the combination therapy - the emperor has been dethroned!
A study by Chenet al[20] enrolled 30 COVID-19 patients; 15 patients were treated with 400 mg of HCQS daily for five days, and the remaining 15 patients were in the control group. The study found no significant differences between patients treated with HCQS and the control group in terms of the pharyngeal carriage of viral RNA at day seven. However, the patients also received other antiviral drugs.
Molinaet al[21] performed a retrospective study of 368 patients with confirmed COVID-19, who were categorized into three groups based on the treatment with hydroxychloroquine alone (n= 97, HCQS), hydroxychloroquine with azithromycin (HCQS+AZ,n= 113), and no HCQS (n= 158) in addition to supportive background management for COVID-19. The two primary outcomes were death and the need for mechanical ventilation. The abovementioned study found no benefit for the use of HCQS either with or without azithromycin. This therapy did not reduce the risk of mechanical ventilation in hospitalized patients. Increased overall mortality was observed in patients treated with HCQS alone.
Laneet al[22] found that short-term HCQS treatment is safe, but adding azithromycin may potentially produce heart failure and arrhythmia owing to the synergistic effect on QT interval. The abovementioned study included 956374 and 310,350 users of hydroxychloroquine and sulfasalazine as well as 323122 and 351956 users of hydroxychloroquine-azithromycin and hydroxychloroquine-amoxicillin, respectively. No excess risk of severe adverse effects was identified when 30-d hydroxychloroquine and sulfasalazine use was compared. However, long-term hydroxychloroquine usage was linked to a higher risk of cardiovascular death (HR 1.65) ,when azithromycin was added to hydroxychloroquine, an increased risk of 30-d cardiovascular mortality, chest pain/angina, heart failure, and a two-fold risk of cardiovascular mortality in the first month of treatment were observed(HR 2.19).
Rosenberget al[23] conducted a retrospective multicenter cohort study of patients from a random sample of all admitted patients with laboratory-confirmed COVID-19 in 25 hospitals. Among 1438 hospitalized patients with a COVID-19 diagnosis, the probability of death for patients receiving hydroxychloroquine and azithromycin was (25.7%), hydroxychloroquine alone, (19.9%), azithromycin alone (10.0%), and neither drug (12.7%). In logistic models, compared to patients receiving neither drug, cardiac arrest was significantly more likely in patients receiving hydroxychloroquine and azithromycin but not hydroxychloroquine alone or azithromycin alone.
Chorinet al[24] found that in COVID-19 patients treated with HCQS+AZ, the corrected QTc interval was significantly prolonged. This discrepancy suggests that QT prolongation may be influenced by patient attributes such as co-morbidities and disease severity.
Mercuroet al[25] published data on 90 hospitalized COVID patients in Boston. Corrected QT (QTc) was measured before and after HCQS administration (dosage after day 1: 400 mg/d); 53 received concomitant AZ (dosage not given). The baseline median QTc was longer than average (HCQS-alone group: 472 ms; HCQS+AZ group: 442 ms). Seven patients (19%) receiving HCQS alone developed QTc ≥ 500 ms, a generally agreed-upon measure to discontinue QT-prolonging drugs. For patients on combination treatment, 21% developed QTc ≥ 500 ms.
Bessièreet al[26] reported on 40 French patients in an intensive care unit who were administered HCQS (400 mg/d for ten days) either alone (45%) or combined with AZ (250 mg/d for five days; 55%). Baseline QTc was not prolonged in this cohort (median: 414 ms). Q ≥ 500 ms was observed in 5% of those receiving HCQS alone and 33% of those receiving both medications. No arrhythmias were observed.
A recently published meta-analysis also showed that the HCQS and AZ combination therapy increased mortality (RR = 1.27; 95%CI 1.04-1.54,n= 7 studies)[27].
A solidarity trial included 11330 patients; five arms of 2750 received remdesivir, 954 HCQS, 1411 lopinavir, 2063 interferon, and 4088 no drug. There was no mortality benefit observed in any drug group[28]. Magagnoliet al[29] study retrospectively evaluated 804 patients and found no significant reduction in mortality and need for mechanical ventilation with hydroxychloroquine with or without Azithromycin (Table 1).

Table 1 Different studies on the use of the hydroxychloroquine and azithromycin combination to treat coronavirus disease 2019 infection

HCQS: Hydroxychloroquine; AZ: Azithromycin; COVID-19: Coronavirus disease 2019.
SIDE EFFECTS OF THE COMBINATION THERAPY
HCQS can cause QT prolongation and increases the risk of polymorphic ventricular arrhythmia,Torsades de pointes(TdP), in susceptible individuals. However, this side effect is uncommon; however, other drugs (e.g., azithromycin) can aggravate this risk. Many other drugs (e.g., quinolones and antihistamines) are frequently used, which adds to the risk[30]. It is advised to have baseline ECG to estimate QT interval using Bazett’s formula. Those with baseline QTc > 500 ms should have a clinical evaluation if they have risk factors, and the use of HCQS should be preferably avoided (Figure 1). Some clinical factors and QTc interval that predisposes an individual to HCQS toxicity should be evaluated[31] (Figure 2).
Different studies reported that the rate of QT prolongation varied between 10% and 20%. Thus, the addition of AZ to HCQS increased the risk of QTc prolongation. Chorinet al[24] found that 11% of patient had QTc > 500 with the combination, while 30% had QTc increase of > 60 ms. Expectedly, some precautions are needed when using both HCQS and drugs, which requires regular monitoring of hematological parameters (RBC, WBC, and platelet count), serum electrolyte levels, blood glucose level owing to the hypoglycemic potential of HCQS and its hepatic and renal functions. The safety of these drugs can be maintained by close monitoring. A risk score by Tisdaleet al[32] has been used to predict drug-induced QT prolongation (Table 2).
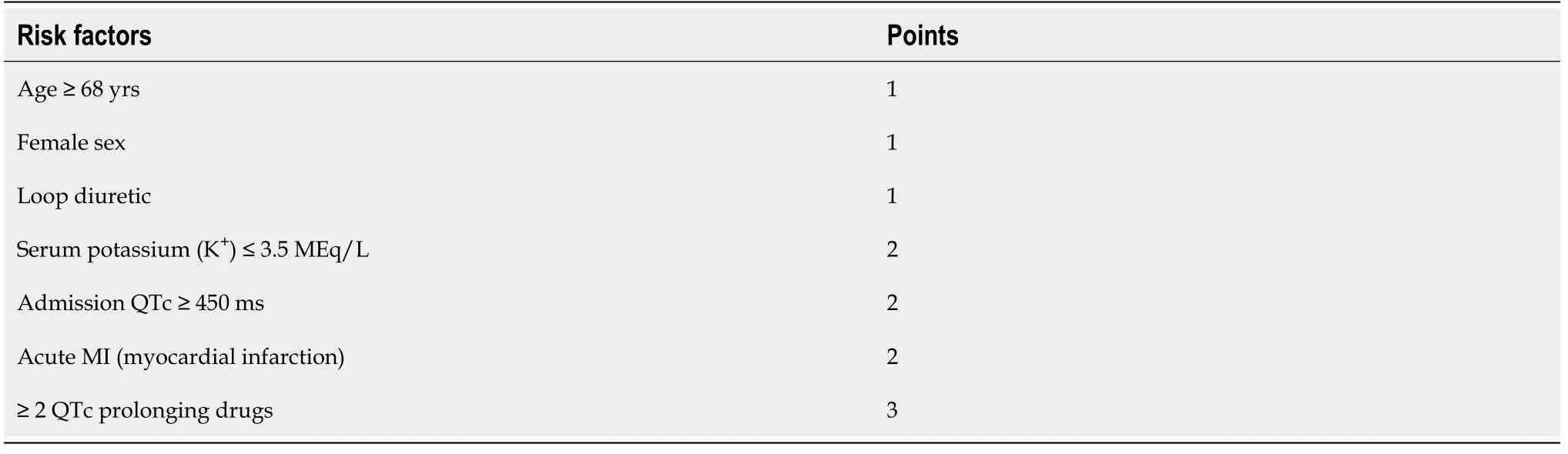
Table 2 Tisdale assessment risk score for drug-associated QTc prolongation. A Tisdale score of < 6 predicts low risk, 7-10 medium risk, and > 11 high risk of drug-associated QT prolongation [Adapted from reference 30]
ROLE OF HYDROXYCHLOROQUINE AND AZITHROMYCIN COMBINATION IN HIGH-RISK PATIENTS
COVID-19 is a systemic disorder with a widespread inflammation and hypercoagulable state. During the COVID-19 pandemic, D-dimer has been identified as one of the most common and rapidly detected laboratory results related to coagulopathy. Higher mean blood D-dimer levels have been associated with increased in-hospital mortality in hospitalized patients due to COVID-19. According to a previous study, the ideal mean D-dimer cut-off value for predicting in-hospital mortality was 779 g/L, with 77% sensitivity and 83% specificity (AUC 0.87; 95%CI 0.81-0.94;P= 0.001)[33]. Fibrinogen, which is also known as one of the acute phase proteins, is produced in large amounts by the liver in response to IL-1- and IL-6-derived stimulation and is implicated in fibrin production as the final step of a triggered coagulation activity. The fibrinogen levels and degradation products of D-dimer [FSE1] were higher in critical COVID-19 patients compared to those in mild or moderate cases. The values were also higher in critical COVID-19 patients compared to healthy controls[34].
The overactivation of the immune system, which causes a complement release syndrome, is the key underlying mechanism responsible for the increased coagulation tendency in COVID-19 patients. Increased cytokines, such as IL-6, are a major regulator of the cellular immune response and a trigger for coagulation disorders. Because a hypercoagulable state has been confirmed at both cellular and organ levels, anticoagulant therapy has shown encouraging results in COVID-19 patients[35].
By comparing the data from 101 adult COVID-19 patients hospitalized for mild to moderate ARDS with the data from 92 similar patients, Lambacket al[36] found that HCQS in conjunction with azithromycin was ineffective in treating mild to moderate COVID-related ARDS. In the study group, the mean D-dimer value was 758 ng/mL at baseline and peaked at 1193 ng/mL. Fibrinogen levels were also higher in the treatment group compared with the controls. However, enrolling high-risk patients (based on D-dimer and fibrinogen) in combination therapy failed to improve any of the clinical outcomes (i.e., transfer to ICU, death, duration of non-invasive ventilation, and duration of hospitalization).
GUIDELINE RECOMMENDATION
HCQS was an essential part of the treatment regimen in almost all recommendations across the globe. However, it should not be used as a stand-alone therapy in the management of COVID-19 because there is a lack of unequivocal data on effectiveness[30]. The Government of India, Ministry of Health and Family Welfare Guidelines on clinical management of COVID-19 (March 31, 2020) recommended the administration of 400 mg of hydroxychloroquine BD at day one followed by 400 mg OD for the next four days in combination with 500 mg of azithromycin. The revised guideline by the Ministry of Health and Family Welfare on Clinical Management of COVID-19 recommended the administration of 400 mg of hydroxychloroquine (without concomitant AZ) BD at day one followed by 400 mg OD for the next four days[37]. However, the recent iteration of MOHFW before the second wave removed HCQS for use in COVID-19. USFDA also issued a black box warning for its use in COVID-19 infection. After the SOLIDARITY trial, HCQS was removed from the list of essential drugs in COVID-19 disorder[28]. Recently, all major guidelines released have obviated the use of HCQS when treating COVID-19.
LIMITATIONS
Most studies and trials had a small sample size, different drug dosing, duration, varied inclusion criteria, and endpoints, which led to exaggerated study results. In addition, most trials did not include severely ill patients with other organ dysfunction, which may alter drug clearance from the body, leading to toxicity. In addition, non-randomized trials and lack of placebo were areas of concern.
CONCLUSION
Because there is no definitive and promising treatment against COVID-19 and cases are yet to reach the peak, any treatment is better than no treatment. Data from preliminary studies showed that the HCQS+AZ combination was beneficial in virological clearance and was initially used as a possible treatment option for COVID-19. In later studies, combination therapy did not significantly improve, although side effects were higher in the combination arm. Moreover, the combination therapy in hospitalized COVID-19 patients, many of whom may have had concurrent renal or hepatic dysfunction, could have aggravated the QT-prolonging potential of these drugs. This could have led to enhanced morbidity and mortality, which was observed in more recent studies using HCQS combination. In more recent studies, the benefit of using HCQS alone is being questioned, and combination therapy is not warranted. Thus, treating the COVID-19 infection with HCQS, either alone or with AZ, is no longer recommended. Therefore, based on the current evidence, HCQS and its combination with azithromycin are not suitable for the management of COVID-19.
FOOTNOTES
Author contributions:Bajpai J and Verma AK conceptualized the article design; Bajpai J, Pradhan A, and Verma AK searched the literature; Bajpai J and Pradhan A drafted the manuscript; A critical revision was done by Kant S, Verma AK, Pradhan A, and Bajpai J.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Jyoti bajpai 0000-0001-6337-856X; Akshyaya Pradhan 0000-0002-2360-7580; Ajay Kumar Verma 0000-0002-2973-1793; Surya Kant 0000-0001-7520-5404.
S-Editor:Liu JH
L-Editor:A
P-Editor:Liu JH
