Complex diseases demand novel treatment strategies:understanding drug combination
2022-05-12RenataSiqueiradeMelloVanessaFernandesArnaudSampaioLucasFerreiraMacielVanessadeTalitaGlaserHenningUlrichClaudianaLameu
Renata Siqueira de Mello,Vanessa Fernandes Arnaud-Sampaio,Lucas Ferreira Maciel,Vanessa de Sá,Talita Glaser,Henning Ulrich,Claudiana Lameu*
1Departamento de Bioquímica,Instituto de Química,Universidade de São Paulo,SP,Brazil.
Abstract For many multigene or multifactorial diseases,the one-drug therapy for inhibiting a defined molecular target is often less effective than combined treatments.Typically,drug combination therapies are multitargeted,so the mechanisms or even interactions are often complementary.These drug-drug interactions may promote alteration of pharmacokinetic or pharmacodynamic activities of one drug by another drug.Other interactions may change the expected effect of medications through polymorphisms that alter the expression or activity of the drug-mediated enzyme and the cell signaling cascade,such as drug-gene interactions and drug-drug-gene interactions.The number of possible existing interactions requires appropriate methods of study.In this review,we summarized combination therapies for cancer,as well as for viral,cardiovascular,and neurological diseases.Here,we also highlight known methodologies,such as in vitro methods based on Loewe’s and Bliss’s pioneer models and in silico methods based on online available data.With more elaborate methods and reliable results,multitarget therapies through drug combinations may increasingly benefit patients suffering from complex diseases.
Keywords:drug combinations;multitarget therapy;combination therapy;drug-drug interaction;in vitro;in silico; methods and models
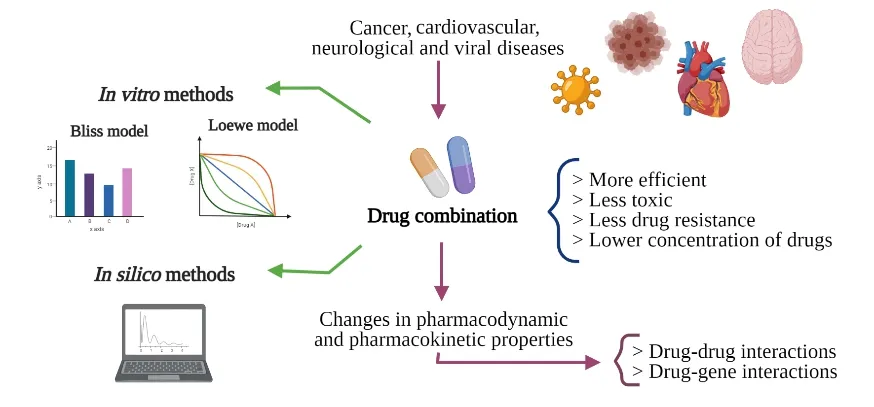
Introduction
Throughout history drug development research has been focused on identifying new agents directed against individual molecular targets,in an attempt to avoid any unspecific effects caused by mistargeting to other biological structures [1,2].However,biological organisms have many systems operating interconnectedly to protect the organism from malfunctioning [3].Therefore one-drug to inhibit a defined molecular target is often less effective than combined treatments [3,4].Moreover,the complexity of some pathologies has challenged this paradigm,and the study of molecules hitting more than one target is now rising [5].
Multiple targeting strategies are used in clinical studies through a combination of various drugs that act distinctly on different targets of a sickness [6].Multitarget therapies are repeatedly considered more efficacious,less toxic,and less vulnerable to resistance development,as a lower concentration of drugs is administered to the patient to reach similar or greater effect [2,4].This strategy is now standard in cancer,hypertension and viral infections,and is also of great promise for diseases such as Alzheimer’s and tuberculosis [7–16].
Combination treatments are often multitargeted,but the two denominations are not synonyms.While a multitargeted agent may be a unique molecule with a suitable combination of activities,combination therapies combine two or more drugs that can act through the same molecular targets,and have complementary mechanisms or even unknown interactions [4].
Combining drugs raises many challenges,as each drug has different properties,such as bioavailability and metabolism,which leads to an exponential interest in the development of medicines based on a single molecule that acts on more than one specific target of disease [6].The search for combined drugs targeting complementary mechanisms requires a large-scale search of a vast world of possible target combinations,but can also reveal potential synergies or unexpected interactions between disease-relevant pathways,leading to a greater understanding of disease biology [4].
According to Zimmerman et al.,there are three categories of multitarget drugs that can be described by their relationship with the target [4].In the first type,the therapeutic effect is given by separate targets that trigger individual signaling pathways in the cell;in the second type,the effect on one target favors the action on a specific second target;and in the third type,the coordinated action on a single target or a cell complex produces the therapeutic effect.
Therefore,when the interaction between two drugs reaches an effect larger than the sum of the independent effects,it is synergistic;if equal,it is addictive,and if it is smaller than the sum,it is antagonistic.However,defining the sum of two independent effects is actually more challenging than it seems:it is not feasible to simply consider the algebraic sum,for instance,when cytotoxic effects are expressed as fractions or percentages.The definition of additivity is then crucial for establishing mathematical and empirical models to study drug-drug interactions.Despite the existing mathematical approaches to predict interactions between drugs,the increase in drug combination treatments for known diseases has spurred the development of new computational methods to foresee efficient combinations [17].
Currently used drug combination therapies
Cancer
Most cancer treatment strategies include methods such as surgery and radiotherapy-for locoregional tumors-and systemic therapies for advanced and aggressive tumors that include chemotherapy,targeted therapies,endocrine therapy,and/or adjuvant therapy.Most clinically relevant chemotherapy drugs used to fight lung,breast,ovarian,and pancreatic cancers primarily target DNA molecules,such as platinum-based drugs (i.e.,cisplatin,oxaliplatin,and carboplatin).These drugs cause a plethora of DNA lesions that,if not promptly repaired,induce tumor cell death.In other words,the main cytotoxicity mechanism of antitumor agents occurs through DNA damage.
Although some malignant tumors respond well to only one treatment strategy,other tumors require combined treatment with synergistic or additive effects for antitumor efficacy,since cytotoxic effects to healthy body tissues can overcome the effectiveness limited by primary and acquired drug resistance [18].
Furthermore,resistance to chemotherapy is estimated to be the cause of therapeutic failure in 90% of patients with metastatic cancer[19].These limitations are based on the traditional use of chemotherapy to control the disease in a systematic way;therefore,radical and/or strategic changes in the treatment protocol for these tumors are necessary,to achieve more effective clinical results.
Certainly,if we could overcome drug resistance,the impact on patient survival would be immense.An interesting alternative often used in the clinic is the combination of different chemotherapies to enhance the therapeutic effect of these drugs.The modalities of drug combinations are based on their complementary mechanisms of action,to create an appropriate therapeutic program considering the characteristics of the patient and tumor.What is sought with this therapeutic strategy is that the cytotoxic effect of the drugs together is synergistic.Thus,it is possible to administer lower doses and obtain the same or better therapeutic effects and,at the same time,reduce side effects often associated with chemotherapy.
Neoadjuvant treatment for the most aggressive breast cancer subtype,triple-negative breast cancer,is an example of a current regimen that consists of drug combinations of anthracyclines plus cyclophosphamide followed by platinum compounds and taxanes which significantly increase pathologic complete response and reduce the risk of recurrence [20].
Anticancer therapeutic regimens must also consider the impact of systemic therapies on the tumor microenvironment,which is often established by the tumor-host relationship.The components of the microenvironment have great importance in tumor growth and progression.Endothelial cells,stromal fibroblasts,specific natural killer cells,tumor-associated macrophages,and specific lymphocytes stand out in the tumor microenvironment.Currently,the understanding of the tumor-host interaction has been used as a determinant of clinical evolution and types of responses to treatment for human neoplasms [19–22].
Some drugs classified as antiangiogenic,such as sunitinib,sorafenib,and bevacizumab,are used in combination to manipulate the tumor-host interaction favoring the reduction of the tumor,through the biological targeting of the endothelial cell and interruption of the vascularization associated with the tumor [23].
Furthermore,new methods of therapy based on activation of the immune system have added many benefits to patients with several types of cancer in relation to the duration of response and survival time,especially when combined with chemotherapeutic drugs.
Nevertheless,chemotherapy is not restricted to being cytotoxic to cancer cells;it also affects other cells,such as endothelial cells and immune cells in general [24].The same happens with antiangiogenic and tumor-specific monoclonal therapies [25,26].Any changes in the cancer biology process will affect other elements present in the tumor microenvironment and host elements.
Thus,a careful assessment of the impact of combinations between drugs already established,including chemotherapy,and new drugs designed to modulate the immune system,such as vaccines,checkpoint inhibitors,and adoptive cellular therapy,has been necessary to generate a more effective multimodal therapeutic integration in the treatment of cancer.
Moreover,genetic variability in combination or not with other factors,such as medical conditions or even environmental influences,can alter the response of an individual to treatment [27,28].Thus,each patient in a large population can respond differently to the same medicine.
Pharmacogenomics studies the variability in response to drugs due to genetic variations that are often related to single-nucleotide polymorphisms (SNPs) causing mutations in genes,for example,multidrug resistance (MDR) 1,multidrug resistance protein (MRP) 1,and MRP2,which are associated with resistance to drugs or other genes involved in drug metabolism and transport [29,30].Such mutations can change the gene expression or structure of proteins involved in pharmacokinetic and pharmacodynamic parameters,which explains why a treatment never has the same effect in several patients and why different patients can present distinct adverse drug events [28].
Adverse drug events may significantly change the expected effect of prescribed medications and are often related to so-called drug-gene interactions (DGIs) and/or drug-drug-gene interactions (DDGIs) [30,31].The genome affects metabolism through polymorphisms that alter a drug’s metabolic enzyme by decreasing or increasing its function.If a SNP causes the expression of a low function enzyme,for example,this enzyme may reduce the metabolism of the administered drug through a DGI (Figure 1A) [30].Then,this low-function drug’s metabolic enzyme can interact with another drug by DDGIs by inhibitory or inductive interactions,or also an interaction of phenoconversion [30].The interaction of the enzyme with an enzyme-inhibitor drug or an enzyme-inducing drug may promote reductions or increases in drug metabolism,respectively,leading to pharmacokinetic and pharmacodynamic changes in the medication(Figure 1B) [30].
Considering that DDIs and DDGIs are linked with adverse drug responses in the treatment of several diseases,the identification of clinically relevant interactions becomes increasingly important.Currently,pharmacogenomics enables the investigation of genes and genetic variants and the identification of particular genetic loci of an individual and/or related to a disease that determines the drug response of patients [28].Thus,information such as this will allow individual development and planning of more effective and accurate therapeutic approaches for each patient shortly.
Cancer vaccine and chemotherapy combinationCurrently,the potential use of therapeutic combinations that incorporate chemo and radiotherapy has been discussed with a view to synergy with antitumor vaccines.In general,chemotherapy is considered immunosuppressive because it acts on dividing cells,including bone marrow cells and peripheral lymphoid tissue.The use of cancer vaccines as an immunotherapeutic strategy is based on their ability to capture and present tumor proteins,triggering a strong and effective immunogenic response,through the activation and expansion of effector cells such as Th1 lymphocytes,cytotoxic T lymphocytes(CTLs),and natural killer cells.The recognition of peptide complexes of tumor cells via major histocompatibility complex class-I (MHC-I),through the T-cell receptor,promotes CTL activation and releases cytotoxins (perforin and granzyme),destroying malignant cells.However,this treatment alone is insufficient to generate tumor regression,especially in advanced diseases [32,33].
Several studies have shown benefits in the combination of vaccines and some chemotherapy drugs in low doses against some tumors,including breast lymphomas [34].The first Food and Drug Administration (FDA) approved antitumor dendritic cells (DCs) is a first-generation vaccine,called Sipuleucel-T (Provenge®),which was approved for all types of cancer.In a randomized phase III study,patients with prostate cancer hormone-resistant or metastatic castrating were treated with DCs autologous cells obtained from peripheral blood cells cultured in vitro with granulocyte-monocyte colony-stimulating factor (GMCSF) and a prostate-specific antigen,acid phosphatase (PA2024).The cell immunotherapy induced an immune response in these patients,reducing the risk of death by 22%and increasing overall survival (OS) in 4.1 months compared with the placebo group.Despite the substantial difference in OS,the single use of Sipuleucel-T does not alter disease progression in the short term.A phase III trial showed more benefit in survival when patients received docetaxel after vaccine treatment [32,33].
T-regulatory cell modulation and chemotherapy combination
Several clinical studies have demonstrated an important synergistic effect between chemotherapy and checkpoint inhibitors when used in combination.By causing the death of tumor cells,chemotherapy promotes the release of antigens that are presented by antigen-presenting cells,making the tumors more immunogenic for the performance of immunotherapeutic agents.This combination has been reflected in the increased response rate and progression-free survival of several types of tumors [25,32,33,35–38].With technological development and greater knowledge of the immune system,inhibitory pathways which attenuate this immune response was discovered.Drugs acting on this interface of the immune system are called immunological checkpoint inhibitors [34].
The cytotoxic T lymphocyte antigen 4 (CTLA-4) molecule expressed in T lymphocytes after being activated,has great homology with the CD28 molecule.It also has a greater affinity for CD80/CD86 than the CD28 molecule,activating an immunoregulatory response that inactivates the T4 lymphocyte response [39–41].Ipilimumab was the first monoclonal antibody against CTLA-4.Its use proved to be effective in the regression of melanoma,renal carcinoma,prostate cancer,urothelial carcinoma,and ovarian cancer [42,43].In addition,the combination of ipilimumab with other immune checkpoint inhibitors had positive anti-cancer effects for metastatic melanoma,metastatic colorectal cancer,and advanced renal cell carcinoma [44].
The programmed death 1 (PD-1) pathway is a checkpoint that limits mediated immune response by T cells.Its ligands,PD-L1 and PD-L2 on cancer cells bind to the PD-1 receptor on immune cells and induce PD-1 signaling for T cell“exhaustion”,reversible inhibition of T cell activation and proliferation [43,45].Multiple antibodies (PD1 or PDL1-inhibitors) are in clinical phase studies [46,47].Pembrolizumab and Nivolumab are PD1-inhibitors approved for the treatment of advanced melanoma,renal cell carcinoma,and non-small cell lung cancer (NSCLC),as clinical studies have shown improvements in OS[48,49].However,many patients do not benefit from PD1 or PDL1-inhibitors.Thus,the combination of immunotherapy and chemotherapy are options for first-line treatment in patients with metastatic NSCLC without epidermal growth factor receptor (EGFR)mutation or anaplastic lymphoma kinase (ALK) translocation which has improved OS,progression-free survival (PFS),and overall response rate [44,50].
Although immunotherapy has benefited cancer patients,it is worth mentioning the high cost of this therapy,which can limit access to patients [51].
Targeted therapy plus immunotherapyTargeted therapies have been used in several types of tumors with very expressive clinical responses rates.Targeted therapy uses small molecules or monoclonal antibodies to attack features unique to cancer cells as growth,division,and spread.Nevertheless,the response rate is often transient,causing the escape by tumor cells through the generation of resistance mutations,months after starting treatment.By contrast,immunotherapies have shown more durable responses in several types of tumors,mainly in combination with other available therapies.Several clinical studies corroborate this new approach-mainly through a deeper understanding of the mechanisms of tumor immunity and their interaction with genomic mutations,generating an important impact on therapy directed to the tumor microenvironment.To date,translational studies have shown clear benefits in combining target therapies with checkpoint inhibitors (anti-PD1 and PDL-1) [52].
The results of preclinical and translational studies revealed interesting effects of combining mitogen-activated protein kinase(MAPK) inhibition,as BRAF ± MEK inhibitors with checkpoint inhibitors (anti-CTLA-4 and/or anti-PD-1/PD-L1).Such combinations had a significant effect on the immunomodulation of melanoma patients increasing the proportion of CD8+,CD4+,and PD-1+T lymphocytes in the tumor microenvironment,consequently increasing the anti-tumor immune response [48].
Currently,clinical trials combining anti-vascular endothelial growth factor (VEGF) and anti-PD-1 therapies have shown good clinical benefits compared to monotherapy,in advanced NSCLC and melanoma patients [37,53].The therapeutic atezolizumab(anti-PD-L1) and bevacizumab (anti-VEGF) combination can increase the immune system’s potential by the ability of atezolizumab to activate T cell responses against the tumor [53].Another study evaluated the combination of ipilimumab and bevacizumab versus monotherapy with ipilimumab alone in advanced melanoma.The combination therapy increased the expression of adhesion molecules and intratumoral CD8+T-lymphocytes,improving the clinical responses [54].
In renal cancer cells,the combination of nivolumab (check-point inhibitor;anti-PD1 antibody) and ipilimumab (check-point inhibitor;anti-CTLA-4 antibody) was tested versus sunitinib in metastatic patients not previously treated (Checkmate 214 study).The combined treatment was able to reduce the risk of death by 37%,increasing the response rate from 27% to 42% and from 8.4 to 11.6 months PFS in the intermediate-risk and poor population.PD-L1 expression seems to be related to the best response to immunotherapy.Meanwhile,in a subgroup analysis,patients at favorable risk responded better to sunitinib,with a higher response rate and higher PFS [54].Another phase III study,IMmotion 151,evaluated the combination of atezolizumab (anti-PDL1 antibody) and bevacizumab (anti-VEGF antibody) versus sunitinib at the first line,showing an increase in PFS manly in immunohistochemical positive PDL-1 (≥ 1%) samples [55].
Viral diseases
In human viral diseases,such as acquired immunodeficiency syndrome (AIDS) and hepatitis,the progress of treatment has also relied on the use of combination therapies.The strategies for treatment of human immunodeficiency virus (HIV) infection,the agent behind the AIDS disease,changed with the knowledge that viral replication of the causative virus.HIV replication occurs during the years before the development of the clinical disease,with a progressive decline of the immune function by latent infection of CD4 T cells [56,57].Antiretroviral therapy (ART) also called HIV regimen,consists of treatment with the combination of two or three medicines.By 1998,drugs licensed in the US for use in combination therapy to HIV included nucleoside and non-nucleoside reverse transcriptase inhibitors,integrase inhibitors,and protease inhibitors [56,58].ART,outstandingly,improves the prognosis of individuals infected with HIV,being efficient by suppressing viral load,preventing viral resistance demonstrating low toxicity in a short time,and good tolerance by patients [58,59].After some years of studies,ART consisting of combined drugs,such as tenofovir alafenamide,ritonavir,zidovudine,dolutegravir,bictegravir among others,has resulted in a survival range for patients living with HIV similar to HIV-uninfected people,and reduction of the viral transmission [57,58].Other antiviral therapies of combined drugs have only been explored in recent years for conditions such as chronic hepatitis C and B [60].
A leading cause of severe liver disease,hepatitis C virus (HCV)infection can result in cirrhosis and hepatocellular carcinoma in patients [61].After the discovery of interferon alfa as the principal agent in HCV therapy,the development of direct-acting antiviral agents (DAAs) has provided an important advance in the treatment[62].Although interferon-based HCV therapies offer a 40% cure for the most difficult to treat genotype-1 infection,treatment with interferon-free DAA regimens,such as combinations of DAAs and medicines,has been successful in most patients [61].Nevertheless,the implementation of DAAs is still a challenge due to the different six clinical genotypes of HCV.The first oral regimen of sofosbuvir and ribavirin that was approved by the US for genotypes 1–4 became the standard treatment for genotypes 2 and 3,but its efficacy was not sufficient in genotype 1 and did not have sufficient evidence to genotypes 5–6 [61].In the case of hepatitis B virus (HBV) infection,various combination therapies have been evaluated over time but just a few of them have been shown to induce higher rates of response as compared to monotherapy [60].Currently available therapeutic options include medications such as interferon or pegylated interferon,nucleotide,and nucleoside analogues but although when combined they achieved good results it is still needed more trials [63,64].Many agents available for the treatment of HBV are also part of combination regimens for HIV infection.Thus,the combination therapy option is especially attractive for HBV,since interferon monotherapy has therapeutic effectiveness limited to 25% to 40%[63].
Cardiovascular diseases
Hypertension is considered one of the strongest risk factors for different cardiovascular diseases because of the direct relationship between blood pressure and cardiovascular events [65].Decades of research have offered antihypertensive treatment a lot of medications that can reduce elevated blood pressure by being used alone or in combination with one another [66].Since the treatment demands a quick normalization of blood pressure rate,the achievement of the ideal blood pressure is difficult in most patients [67].Recently,evidence indicates that the use of combined drugs at the beginning of treatment can normalize blood pressure more quickly,reducing significantly major adverse cardiac and cerebrovascular events [65].The fact that hypertension is multifactorial,therapies based on drug combination may be favorable since the combination of the agents may lead to better blood pressure control by the action of a supposed complementary mechanism [67].Nowadays,the combination of a blocker of the renin-angiotensin system,generally one of the angiotensin-converting enzyme (ACE) inhibitors of first-generation as captopril,enalaprilat,and lisinopril,with a calcium channel blocker of the class of dihydropyridine or non-dihydropyridine;or an ACE inhibitor with a diuretic for reducing sodium and water levels in the body are prescribed.These drug combinations have been successful to promote the best cardiovascular protection and good therapeutic results for providing greater antihypertensive effectiveness and fewer side effects than the use of high doses of monotherapy [65,66].
Neurological diseases
Diverse neurological disorders are multifactorial,thus single-target drugs are mostly inadequate to achieve satisfactory therapeutic effect[68].In addition,the therapeutic strategy available for some well-described neurodegenerative diseases is still based on the treatment of symptoms.In this topic,we will highlight some common drug interactions that are beneficial or harmful to affected patients by neurological diseases,such as depression and Alzheimer’s disease.
Major depressive disorder,also commonly called depression,affects increasingly amount of the population year after year.The cause is still unclear;however,many drugs are available to fight the disorder.One of the most recent challenges on psychopharmacology is the drug-drug interactions (DDIs),since psychiatrists are treating patients with increasing complexity medication [69,70].DDIs are defined by the alteration of the pharmacokinetic or pharmacodynamic activities of one drug by another drug [71,72].In pharmacokinetic interactions,a drug can change the absorption,distribution,metabolism,and elimination (ADME) of other drugs reciprocally,besides affecting its concentrations at the sites of action (Figure 2).Knowledge of the mechanisms behind DDIs and their consequences is crucial for the development of combination therapies,since the most widely used treatments may have secondary pharmacologic characteristics that could induce or inhibit the activity of drug-metabolizing enzymes or transport proteins [73].According to Greenblatt,metabolic inhibition results from an inhibitory DDI that may reversibly or irreversibly affect the drug-metabolizing enzymes themselves,frequently the cytochrome P450 enzyme (CYP450) (Figure 1) [73].For instance,the administration of fluoxetine to a patient that already has a titrated dose for Nortriptyline will produce clinical tricyclic toxicity,because fluoxetine is an inhibitor of the enzyme that metabolizes Nortriptyline,which the consequence is higher levels of Nortriptyline[74].In addition,the reverse situation will also induce toxicity,for example,the administration of Phenytoin (degradation enzyme-substrate) to fluoxetine or fluvoxamine-treated patient [75,76].
Furthermore,some drugs can induce the degradation of another one,by activating its metabolic enzyme,as previously discussed [77–79].Such a situation happens in the co-administration of Phenytoin and Risperidone [75].The first is an inducer of CYP450 3A4 enzyme,the same that metabolize risperidone.Therefore,the patient will need higher doses of risperidone to achieve the same effect.As well as Alprazolam addition to the carbamazepine-treated patient [80].
Another classic situation is the change of habit of a smoker.Smoking tobacco is a potent and ubiquitous inducer of CYP4501A2,which metabolizes some drugs such as Clozapine [81].Once the patient decides to quit smoking,the blood levels of clozapine will rise causing morbidity.Accordingly,it is highly recommended for clinicians to prescribe medicaments within a given class with a low likelihood of producing DDIs,likewise citalopram/escitalopram among the selective serotonin reuptake inhibitors (SSRIs),mirtazapine or venlafaxine among the non-SSRI antidepressants,pravastatin among the statins,and azithromycin among the macrolides [82–84,77].
Although current medications cannot cure Alzheimer’s disease or stop it from progressing,they may help lessen symptoms,such as memory loss and confusion,for a limited time.Therefore,in order to treat Alzheimer’s symptoms in early to moderate stages,there is a class of drugs called cholinesterase inhibitors.
Cholinesterase inhibitors are usually prescribed to treat symptoms related to memory,thinking,language,judgment,and other cognitive processes.Some examples of this category are donepezil,galantamine and,rivastigmine (see Miranda et al.for a complete review on Alzheimer’s drug discovery) [85].However,cholinesterase inhibitors are not effective enough for advanced stages of Alzheimer’s disease,given that,the current FDA-approved treatment is the combination of donepezil with memantine [86].
Memantine regulates the activity of the neurotransmitter glutamate,by antagonizing one of its receptors,the NMDA receptor [68,13,86].Though powerful,this treatment still displays notable disadvantages,including increased toxicity such as seizures,irregular heartbeat,dizziness,and stomach ulcer;and treatment costs [68,86].Opposing,a clinical trial led by Robert Howard in 2012 did not show any significant benefits of the combination of donepezil and memantine over donepezil alone [87].Taken together,these data emphasize that the combination treatment for neurological diseases should be well studied.
Studying drug combinations
In vitro studies
Combining drugs,resolving their individual and combined effects,and translating the results into clinically plausible recommendations is indeed a challenge.In pharmacokinetic DDI studies,it is possible to measure the expression of enzymes,transporter systems,and human cellular fractions,and their specific interactions with drugs of interest[88].On the other hand,pharmacodynamic interpretations of synergism and antagonism demand well-described theoretical approaches,usually based on a reference model,which establishes an index of additivity or null interaction [89,90].
Null models define an ideal“non-interacting”system,from which definitions of additivity and synergism are derived.In 1992,scientists from all over the world reunited to reach a common understanding of the terminology employed in drug combinations research,leading to the Saariselkä agreement [91].The consensus reached was that there was no one-best model and that the two approaches predominantly used in the research field are appropriate for different sets of applications.Next,we will briefly discuss these approaches:the Loewe additivity and the Bliss independence models [92,93].
Loewe’s modelThe null model proposed by Loewe is based on the sham principle,which postulates that one agent cannot interact with itself [92].Thus,if two drugs do not interact,effects obtained by their combination should be equivalent to those observed if one drug is combined with itself.This is the principle of concentration addition,the reason why Loewe’s additivity model is also known as the concentration addition method [92].For determining a reference non-interactive scenario among two compounds,one should determine complete dose-effect or dose-response curves for both,which will correspond to the first line and column of a matrix of combinations [94].
Some criteria must be met so that these assumptions are accurate:the principle of dose equivalence,similar pharmacodynamic properties,and a constant potency ratio.In summary,dose-response curves must be parallel,as shown in Figure 3.This ensures that a concentration α of a compound A (Aα) able to disclose an effectyis equivalent to a concentration β of a compound B (Bβ) able to disclose the same effecty.Let’s assume that both concentrations individually result in the half-maximum effect (i.e.y=0.5;α and β are the IC50 values of compounds A and B,respectively).If a concentrationa=is used in a co-exposure experiment and the principle of dose equivalence holds up,to produce the same half-maximum effect,the concentration of the compound B must beb=B-xB,so that the following mathematical correlation applies:
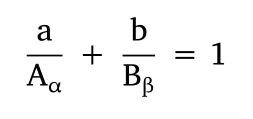
This equation is known as the median-effect equation.If this correlation applies,the combination is considered additive.Thenceforth,it is possible to define relationships between pairs of several dosages tested.In 1983,Chou and Talalay proposed an algorithm based on this equation called“combination index”(CI),which categorizes the combinations as synergic (CI <1),antagonistic(CI >1),or additive (C=1) [95,96].Moreover,when several values ofyare considered,the isobole equation arises:

A graphical representation called isobologram plots growing concentrations of the compounds analyzed to draw a straight-line distinguishing additive non-interaction from synergistic and antagonistic interactions (Figure 3A) [97–99].
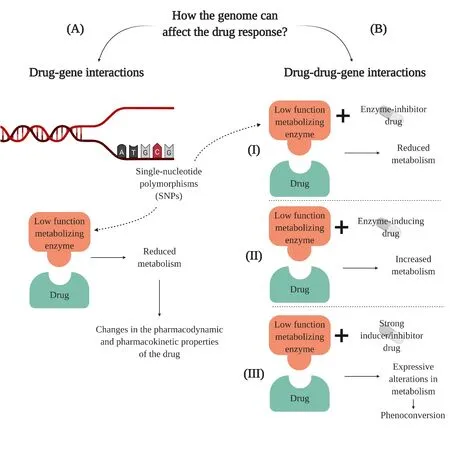
Figure 1 Representative scheme of drug-gene and drug-drug-gene interactions. (A) In drug-gene interactions,a single-nucleotide polymorphism (SNPs) in DNA can cause dysfunction of a drug-metabolizing enzyme,such as CYP450.For instance,a reduction in its activity can result in a reduction in the metabolism and also in the response of the drug once its pharmacokinetic and pharmacodynamic properties would be changed [28].(B) In drug-drug-gene interactions,the effect of one drug can be affected by the interactions between the mutated drug-metabolizing enzyme and another drug,causing (I) inhibitory,(II) inductive,or (III) phenoconversion interactions[30].Interactions of inhibitory and inductive character are caused by drugs that promote a reduction or increase of the metabolism of the drug,respectively,leading to changes in the pharmacokinetics and pharmacodynamics properties [30].The phenoconversion interaction is also inhibitory or inductive,but it is usually related to strong drugs that can promote expressive changes in metabolism resulting in a change of the phenotype [30].Basically,drug-gene interactions occur when the genome affects the ability of a patient to metabolize a drug,while drug-drug-gene interactions occur when the genome and another drug together affect the metabolism of a medication [123].Image created with BioRender.
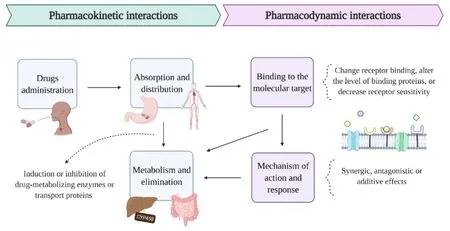
Figure 2 Pharmacokinetic and pharmacodynamic drug interactions. In pharmacokinetic interactions,orally administered drugs may change the absorption in the gastrointestinal system and the consequent distribution into the bloodstream [71].Even after being absorbed,the action of the drug may change before its elimination from the body,since the metabolism of drugs in the liver can pass through alterations if one of the drugs inhibits or induces transport proteins and/or drug-metabolizing enzymes,such as CYP450 [73].In pharmacodynamic interactions,the effect of one drug can be affected by the administration of another drug through synergistic,additive,or antagonistic effects that can directly affect the binding of the drugs to their molecular receptors and also the levels or sensitivity of the receptor [72].These interactions can cause an alteration in the cell signaling cascade responsible for the mechanism of action and response of the drug in the body.Image created with BioRender.
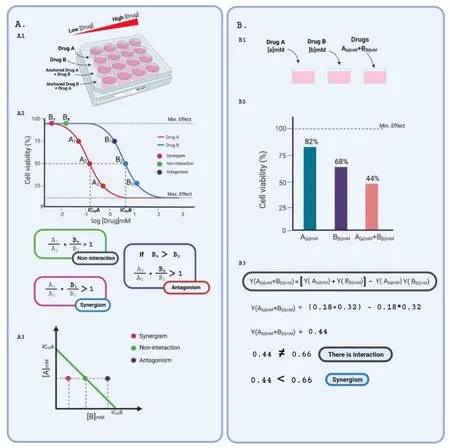
Figure 3 Summary of the two main mathematical approaches used to determine drug interactions,from which several other models were derived.(A) Loewe’s additivity method.The A1-Typical experiment design employed in studies that apply Loewe’s model.The drugs under evaluation (A and B in the picture) are individually administered to the cell culture in crescent concentrations.Also,dose-effect curves are drawn for an anchored (non-varying concentration) drug plus a varying drug.A2-Hypothetical dose-response curves for drugs A and B that aim to decrease cell viability,for example,chemotherapy,meeting the principles of drug equivalence,a pharmacodynamic similarity,constant potency ratio.Drug equivalence relies on the existence of doses that produce equivalent effects.In the figure,doses A1 and B1 are equivalent,as well as doses A2 and B2,and doses A3 and B3,as they produce the same percentage of reduction in cell viability,as the measured effect.Pharmacodynamic similarity is demonstrated by the same minimal and maximal effects reached by both drugs,and similar slope of the curves.Finally,the constant potency ratio is shown by the constant ratio observed between drug concentrations that produce the same effects.In simple words,A1/B1=A2/A2=A3/B3.Finally,combination index calculations originate dots throughout the graph representing the concentration of compound B required to achieve half-maximal effect in different combination scenarios:additive,synergic or antagonistic interactions.A3-An isobologram drawn from IC50 concentrations of compounds A and B represent,for an anchored concentration of drug A,varying concentrations of B hypothetically required to produce the half-maximal effect in combination with A.Interactions are interpreted as additive (when the null model applies),synergic (when the concentration of B required for half-maximal effect is lower than expected),and antagonistic (when the concentration of B required for half-maximal effect is higher than expected).(B) Bliss’s independence method.B1-Typical single-dose experiment design used to evaluate drug interactions applying Bliss’s independence model.Drugs A and B are used at fixed concentrations,either individually or combined.B2-Histogram showing hypothetical cell viability measurements following treatments with drugs A and B drugs A and B that aim to decrease cell viability,for example,chemotherapy at fixed concentrations.B3-Mathematical interpretation of the hypothetical results represented in B2.As the action of both drugs is not independent (observed combined effects are different (lower) from the expected product of both individual effects),an interaction is detected and categorized as synergism.Image created with BioRender.
Derived from Loewe’s model several others emerged,such as Tallarida’s model and Hand’s model [97,100–102].For constant potency ratio drugs,the Loewe,Hand,and Tallarida models are considered very similar [94].Although,Hand suggests building dose-effect curves for the combination of full and partial agents in instantaneous time.This characteristic is essential in biochemical models where molecular reactions happen all the time even though do not give knowledge about them [94].
Bliss’s modelThe Bliss model follows an effect-based null strategy,relying on the principle that drugs act independently through different sites of action and together assemble a combined response [93,94,99].Based on a probabilistic assumption and considering the effect as a fraction of the maximal possible response,the additive effect of a drug A on a dose α (Aα) and a drug B on a dose β (Bβ) would correspond to the observed effect of Aαplus the effect of Bβon the remaining fraction.For didactic purposes,consider that the measured effect is cell survival.If Aαimpairs the viability of a fractionxof the observed cells while Bβreaches a fractiony,the combined effect in a non-interaction context would correspond to the sum of:
a.The cell viability reduction produced by Aαalone=x,and
b.The cell viability reduction produced by Bβin the remaining
population of cells=(1 -x) *y
Thus,a general formula could be written as:
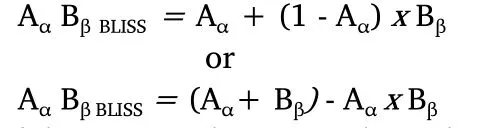
If doses Aαand Bβact independently,the surviving fraction of the cells after simultaneous administration will be the same as cells that survived only drug A or drug B treatments.In other words,after the effect of drug A,the remaining fractionxof cells is then affected by drug B that produces the fractiony,acting independently.However,if there is synergy,the fraction of surviving cells after the simultaneous administration of the drugs will be smaller than cells that have been treated only with drug A or drug B [103].If there is antagonism,the fraction of surviving cells will be bigger than cells treated only with drug A or drug B [103].Thus,there are three basic types of drug combinations in the Bliss model [93]:independent,similar,and synergistic/antagonistic.If the combined drugs present different modes of action and act independently,can be defined as independent;if the drugs present similar effects and interact independently,can be classified as similar;finally,if the effect is more or less potent than individual effect,the response is considered synergistic or antagonistic [3,93].
Such Bliss’s model suggests that two drugs reach their effects independently,the Highest Single Agent (HSA) model also suggests that the expected combination effect should be equal to the higher effect of the individual drugs [104].Therefore,any additional effect over the higher single drug is considered synergy [105].
In silico methods
To identify new combinations,systematic high-throughput in vitro testing of pairwise drug combinations can be applied.But this can be time and resource-consuming as for every 100 drugs there are 4,950 possible drug pairs combinations that could be tested;if we are searching for combinations of three drugs the number rises to 161,700 possibilities.Thus,to decrease the number of combinations to be tested in vitro,computational approaches can be used to tackle this issue and prioritize more promising combinations,although the development of models to foresee drug combinations with high accuracy is not an easy task.
In order to improve the understanding of drug combination synergy and facilitate the development of novel and better computational tools,in 2015 a challenge called the DREAM Challenge was launched and brought relevant advances on the field [106].For this challenge the AstraZeneca dataset was created,containing 11,576 experiments from 910 combinations across 85 cancer cell lines,that were provided to the 160 participating teams to train new models.The best performing team applied a random forest algorithm,a Machine Learning (ML) approach,to perform the predictions and achieved a high performance compared to the theoretical limit,using as input monotherapy data,gene interaction networks,drug target information,and the molecular profile of the 85 cell lines to calculate the synergy scores [107,108].ML are algorithms that can derive models for classification,prediction,and pattern recognition from existing data;so,data such as the AstraZeneca set can be used to identify patterns on known synergic drugs to predict new and unknown synergic drugs based on these patterns [109].
ML algorithms have been widely applied to predict drug synergy for cancer treatment,as exemplified by the winning team of the DREAM challenge,especially due to the considerable amount of available data from this disease,which is essential and limiting to train new models[110–113,107,108].Another example is DeepSynergy,which was the first method to apply Deep Learning to predict anti-cancer drug synergy,requiring the genomic profiles of cancer cell lines and compounds represented by their chemical descriptors to perform the predictions [114].Further,to overcome the cancer data scarcity from some understudied tissues,such as bone,prostate,and pancreas,a deep neural networks model was developed to utilize information from the data-rich tissues (breast,kidney,skin,and lungs) and improve prediction accuracy [115].
Nevertheless,ML algorithms can also be applied to predict combinations for the treatment of other diseases,as shown by the computation model INDIGO-MTB that uses drug-gene associations inferred from transcriptomic data from Mycobacterium tuberculosis and experimentally measured drug-drug interactions to infer interactions between new combinations of drugs for the treatment of tuberculosis [116].This model could also be applied to other pathogens with transcriptomic data available.
Network-based methods,which generally use information from genomic,chemical,and pharmacological properties to build a network representing the associations among drugs,proteins,and pathways,can also be very powerful tools [117].One example is the method developed by Cheng et al.to identify clinically efficacious drug combinations that provided novel insights to understand drug synergy[118].This method is based on the relationship between drug-target pathways and the disease pathways via network proximity in the human protein interactome built based on protein-protein interactions(PPI) experimentally confirmed.They found,using known drug combinations for hypertension and cancer,that to have synergism with lower adverse effects each drug must target different groups of interacting genes that are affected by the disease,and that drugs that target a similar group of genes have more adverse effects [118].These results are corroborated by Qian et al.,which also indicated that known adverse drug pairs tend to have more genetic interaction on their targets [119].
The method developed by Cheng et al.was later applied for drug repurposing for treatment of the novel coronavirus SARS-CoV-2,using virus-host interactome information from 4 human coronaviruses(SARS-CoV,MERS-CoV,HCoV-229E,and HCoV-NL63) and other viruses,and transcriptomics data in human cell lines [118,120].From 2000 FDA-approved or experimental drugs,three potential drug combinations were identified that now could be prioritized for experimental testing.Another in silico approach was capable of prioritizing 73 combinations of 32 drugs with potential activity against SARS-CoV-2,from which 16 synergistic and 8 antagonistic combinations were found in vitro,showing the usefulness of in silico approaches in the combat of the novel coronavirus outbreak [121].Among the interactions identified we may highlight the remdesivir and hydroxychloroquine combination that demonstrated strong antagonism,and the nitazoxanide and remdesivir combination with a strong synergistic interaction [121].The nitazoxanide-remdesivir combination to treat COVID-19 patients is very promising from a clinical perspective because both drugs are FDA approved,and the concentrations with synergistic effect identified in vitro can be achievable in plasma and lung [121].
Conclusion
The multiple targeting strategies through drug combination therapy raises many challenges since each drug has different properties [6].The search for combined drugs targeting complementary mechanisms requires a large-scale search of several possible target combinations,but can also reveal potential synergies or interactions between disease-relevant pathways,leading to a greater understanding of the disease biology [4].Despite the constant effort to quantify the effect of the combination of compounds in biological systems,there is still no agreement on which approach should generally be used since Loewe,Tallarida,Hand,Bliss,and HAS methods have limitations and problems [98,122].Although,each model of study has a different mathematical interpretation of results that should be considered in the study of possible combinations.In silico methods are extremely useful to lower the number of combinations to be tested in vitro,since you can prioritize more promising combinations and predict drug combinations with more accuracy.ML algorithms have been widely applied to predict drug synergy in the treatment of several diseases.
Knowledge of the mechanisms and consequences of pharmacokinetic and pharmacodynamic drug-drug interactions is crucial to the development of therapies since treatments can have secondary pharmacologic characteristics.Thus,it is important to consider that these effects generally depend on the concentration of the drugs through the dose-response effect,by the binding to specific receptors and the activity of drug-metabolizing enzymes or transporters.These effects can also change through DGI and DDGIs since genetic variability can alter the response of an individual to treatment,highlighting the importance of identifying possible drug interactions in different individuals to accurate therapeutic approaches [28].
Finally,although combined therapy has made advances in studies of a vast number of diseases as previously mentioned,it is clever to remember that treatments,despite improving the quality of life of patients,are hardly curative and further studies are always needed.The combined therapies have the potential to play an essential role in still unexplored therapies of several diseases,but it requires knowledge about the properties of the drugs,the multi-targets to be treated,and the possible genetic polymorphisms to better predict pharmacokinetics and pharmacodynamics and potential interactions[68].
杂志排行
Drug Combination Therapy的其它文章
- Mechanism of Zixinyin oral liquid in the treatment of insomnia based on network pharmacology and molecular docking
- Meta-analysis of curative effect of Sacubitril valsartan combined with Qiliqiangxin capsule in the treatment of patients with chronic cardiac failure
- Study on the anti-inflammatory and analgesic effects of white peony root and mucuna pruriens and their combinations in vivo
- Mechanism of Magnoliae Flos and Xanthii Fructus herb pair in treatment of allergic rhinitis based on network pharmacology
