Carbon potentials of different biochars derived from municipal solid waste in a saline soil
2022-05-11HamnaSALEEMMahtabAHMADJamshaidRASHIDMunirAHMADMohammadALWABELandMemunaAMIN
Hamna SALEEM,Mahtab AHMAD,Jamshaid RASHID,Munir AHMAD,Mohammad I.AL-WABEL and Memuna AMIN
1 Department of Environmental Sciences,Faculty of Biological Sciences,Quaid-i-Azam University,Islamabad 45320(Pakistan)
2 Soil Sciences Department,College of Food&Agricultural Sciences,King Saud University,P.O.Box 2460,Riyadh 11451(Saudi Arabia)
ABSTRACT There are numerous studies conducted on biochar for its carbon(C)sequestration potential;however,there are limited studies available on the behavior of salt-affected soils related to biochar application.Therefore,more studies are needed to elucidate the mechanisms through which biochar affects saline soil properties.In this study,biochars were produced from solid waste at pyrolysis temperatures of 300,500,and 700°C(BC300,BC500,and BC700,respectively)and applied to a saline soil to evaluate their impacts on soil carbon dioxide(CO2)efflux,C sequestration,and soil quality.A soil incubation experiment lasting for 107 d was conducted.The results showed that soil CO2 efflux rate,cumulative CO2 emission,active organic C(AOC),and organic matter(OM)significantly increased with BC300 application to a greater extent than those with BC500 and BC700 as compared to those in the no-biochar control(CK).However,soil C non-lability did not significantly increase in the treatments with biochars,except BC700,as compared to that in CK.Besides improving the soil quality by increasing the soil AOC and OM,BC300 showed positive impacts in terms of increasing CO2 emission from the saline soil,while BC500 and BC700 showed greater potentials of sequestering C in the saline soil by increasing the soil non-labile C fraction.The recalcitrance index(R50)values of BC500 and BC700 were>0.8,indicating their high stability in the saline soil.It could be concluded that biochars pyrolyzed at high temperatures(≥500°C)could be suitable in terms of C sequestration,while biochars pyrolyzed at low temperatures(≤300°C)could be suitable for improving saline soil quality.Key Words: carbon sequestration,carbon storage,climate change,CO2 efflux,CO2 emission,pyrolysis temperature,recalcitrance index,soil quality
INTRODUCTION
The world is presently facing many environmental problems,including increasing atmospheric greenhouse gas(GHG)emissions and climate change,which leads to global warming,drought,and decreasing organic carbon(C)in soil(Zhuet al.,2017).The overall warming impact of GHGs introduced to the ecosystem through human activities has increased by nearly 35%,whereas the warming impact related to carbon dioxide(CO2)alone has accelerated by 56%(Jiang and Green,2017;US-EPA,2017).Farming practices,such as the repetitive use of excessive quantities of organic fertilizers,as well as their mineralization and fast decomposition,contribute tremendously to global warming(Srivastavaet al.,2016).Moreover,excessive quantities of salt negatively influence soil microbial activity,as well as soil chemical and natural properties,consequently decreasing soil productivity.Hence,over an extended period of soil salinization,decreases in C storage capacity of these saline soils have been reported(Rengasamy,2006;Wonget al.,2010).To meet the worldwide standards for food protection,it is important to revitalize salt-affected barren soils for cultivation(Biswas and Biswas,2014).Thus,it is critical to utilize saline soils for agricultural production without deteriorating the ecosystem health.
The sustainable management of waste is another critical issue worldwide.Approximately 54%of the global population live in urban areas(Rathoreet al.,2020),generating 6 million tons of waste per day(Hoornweget al.,2013).Improper waste management,particularly in developing countries,is posing serious public health risks and increasing environmental pollution worldwide(Aliet al.,2017).Open dumping is considered one of the most common waste management technologies in the developing world,which results in soil and groundwater pollution through leachate generation.Moreover,the decomposition of solid waste generates a huge quantity of GHGemissions in an uncontrolled manner(Jacobi and Besen,2011).Additionally,numerous other health and environmental problems are associated with the open dumping of solid waste,including the generation of waterborne pollutants,waste leachate,vector-borne diseases,and bad odors(Nizamiet al.,2015;Rahmanianet al.,2015).Therefore,there is a need to alternatively use solid waste,particularly its organic fraction,to avoid the health and environmental problems mentioned above.
If no sustainable policy is applied,the effect of climate change might minimize the yearly GDP growth in emerging countries to 2%—4%by 2040 and to 10%till 2100(IPCC,2014).There are many efforts worldwide to limit the CO2emission from decaying biomass(Lanzaet al.,2015).Several techniques have been proposed to sequester C in soil with chemical and biological strategies,some of which encompass significant physical aspects,aiming at offsetting GHG emissionsviathe sequestration of C within the surroundings(El-Mahroukyet al.,2015).However,the majority of suggested technologies are ineffective for C sequestration over long periods.Therefore,the sustainability of C sequestration needs to be assured(Nogiaet al.,2016).
Recently,the pyrolytic conversion of solid waste into biochar has been introduced as a sustainable strategy for climate change alleviation,proper waste management,saltaffected soil revitalization,and C sequestration(Kwapinskiet al.,2010;Wanget al.,2017).Biochars have been reported to boost soil fertility and immobilize soil pollutants(Caoet al.,2009;Ahmadet al.,2014).Biochars have recently acquired growing interest owing to their potentials to store massive quantities of C in soil,act as a soil conditioner,enhance crop productivity,decrease soil GHG emissions,enhance soil quality,lower nutrient leaching,and decrease irrigation practices and fertilizer demand(Wuet al.,2019).Biochar,a pyrolytic product,is generally low in labile C for microbial usage because it has higher C/N ratio than its feedstock and is hence hard to degrade through microorganisms owing to the low N supply.Therefore,biochar application to soil can increase the recalcitrant soil organic C(SOC)pool and enhance soil C sequestration(Yanardağet al.,2015).Producing biochar from biomass may withdraw additional CO2from the atmosphere into soil,subsequently combating worldwide climate change.The pyrolysis process and biochar application to soil assist in C sequestration,thus making the environment C emission negative(Nandaet al.,2015).There are numerous studies conducted on biochar for its C sequestration potential;however,there are limited studies available on the behavior of salt-affected soils related to biochar application.Therefore,more studies are needed to elucidate the mechanisms through which biochar affects saline soil properties.This study explored the positive potential of biochar derived from municipal solid waste for C sequestration in a saline soil and evaluated the soil quality improvement with biochar amendment.
MATERIALS AND METHODS
Soil collection,processing,and characterization
Samples of a saline soil were collected from the Koont Research Farm(33°07′00.6′′N,73°00′40.1′′E)of Arid Agriculture University,Rawalpindi,Pakistan.The soil samples were air dried and sieved through a 2-mm aperture to remove gravels and any other debris to obtain homogenized soil particles.Soil pH and electrical conductivity(EC)were measured with a glass electrode in 1:5 soil/water suspensions after shaking at 150 r min-1for 1 h.Soil texture was measured using the hydrometer method(Estefanet al.,2013)through which the percentages of silt,clay,and sand were determined,and the USDA soil textural triangle was used to allocate the textural class.Soil water-holding capacity(WHC)was determined using the method described by Estefanet al.(2013).Soil total organic C(TOC),oxidizable organic C(OOC),and organic matter(OM)contents were determined using the oxidation-reduction method(Estefanet al.,2013).Soil calcium carbonate(CaCO3)content was measured titrimetrically following the method of FAO(2020).
Solid waste collection and processing
The organic fraction of the municipal solid waste(after removing inorganics such as plastics,glass,and metals),comprising mainly food waste(>60%),was collected from different dumping sites at the Quaid-i-Azam University campus(33°44′50′′N,73°08′20′′E),Islamabad,Pakistan.The waste comprised vegetable and fruit peels,bread pieces,litter,leaves,lettuce,straw,sugarcane bagasse,etc.The collected waste was sun dried to reduce the moisture to<10%,crushed,ground,and homogenized.
Biochar production and characterization
The crushed feedstock was pyrolyzed in a muffle furnace,at a heating rate of 5°Cmin-1(to ensure slow pyrolysis),and then three holding temperatures,300,500,and 700°C,were employed.The feedstock was placed in a lidded container and carbonized at the desired temperatures for 2 h under limited oxygen(O2)conditions.After the completion of pyrolysis,the weights of the biochars produced at 300,500,and 700°C(BC300,BC500,and BC700,respectively)were recorded,and then the biochars were stored in sealed polyethylene bags.The yield of each biochar was calculated by dividing the weight of biochar by the weight of the feedstock used for the pyrolysis process.
The biochars derived from the municipal solid waste were characterized using proximate and ultimate analyses.In the proximate analyses,moisture,mobile matter,resident matter,and ash contents were determined following the methods of Ahmadet al.(2012).All samples were analyzed in duplicate.Ultimate analyses of the biochars were performed to determine the surface structure,surface chemistry,and thermal stability.The microstructure and surface physical morphology of the biochars were examined using scanning electron microscopy(SEM)(EFI S50 Inspect,EFI,The Netherlands).Specifically,gold-coated biochar particles were visualized by SEM under a high vacuum and 30-kV voltage.Photographs were taken at different resolutions and magnifications.Fourier transform infrared spectroscopy(FTIR)(Spectral 65 FT-IR,Perkin Elmer,USA)was used to determine the surface chemistry of the biochars.Total C was analyzed with a LECO CS-300 analyzer(LECO,USA)according to the manufacturer’s manual.The pH and EC of the biochars were measured in 1:20 biochar/water suspensions with a digital meter(AD8000,Adwa,Hungary).The TOC,OOC,and OM contents in the biochars were determined using the oxidation-reduction method(Estefanet al.,2013).
Biochar stability and C sequestration potential
Thermal gravimetric analysis(TGA)(DTG-60H,Shimadzu,Japan)was used to determine the recalcitrance potential of the biochars according to the method of Windeattet al.(2014).The C sequestration potential(%)of the biochars was calculated using Eq.1(Zhaoet al.,2013):
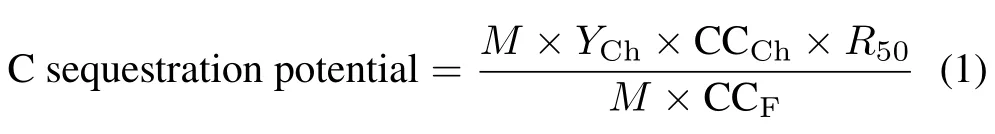
whereMis the weight of the feedstock(g),YChis the yield of the biochar(%),CCChis the C content in the biochar,R50is the recalcitrance index,and CCFis the C content of the feedstock.The value ofR50was calculated using Eq.2(Harveyet al.,2012):

whereT50is the temperature at which 50%of the biochar sample was thermally oxidized(obtained from a moisturecorrected TGA curve)andT50,Gris theT50of graphite(886°C)(Harveyet al.,2012).The correction in the TGA data was made using Eq.3(Harveyet al.,2012;Ahmadet al.,2019):
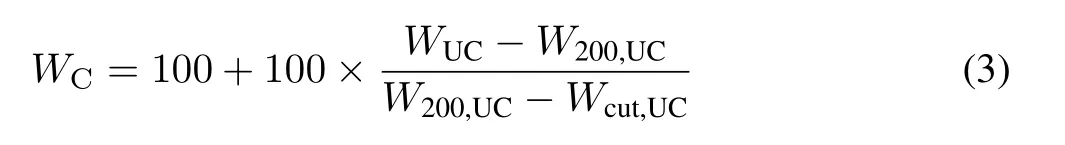
whereWCandWUCare the corrected and uncorrected weight losses in the initial sample,respectively,W200,UCis the weight loss at 200°C,andWcut,UCis the temperature at which no further weight loss was observed.
Soil incubation experiment and determination of CO2 efflux
A soil incubation experiment was conducted at application rates of 2.0%and 5.0%(weight/weight)as suggested by Wanget al.(2019)for the three biochars produced,including a no-biochar control.Specifically,soil samples(250 g each)were mixed with a specific amount of each biochar and put into high-density polyethylene containers.The mixture in each container was moistened with distilled water to attain the field capacity(55%of the soil WHC).The experiment was conducted in triplicate.A small vial containing 10 mL of 2 mol L-1sodium hydroxide(NaOH)solution was placed in each container to trap CO2and evaluate the CO2efflux rate.Three containers(without soil and biochar)each containing a small vial with 10 mL of 2 mol L-1NaOH solution were used as a blank in triplicate.The soil moisture was maintained with distilled water periodically throughout the experiment by weighing each container.The containers were tightly closed,and incubation was performed in the dark at room temperature(27±3°C)for 107 d.
The NaOH solution in the vial was changed at 0(1 h),1,3,5,7,10,15,30,45,60,75,90,97,102,and 107 d.The trapped CO2in the NaOH solution was determined using the gravimetric titration process.Briefly,the NaOH vials were taken out of the container,and after adding BaCl2and a few drops of phenolphthalein indicator,the solution was titrated with 1 mol L-1HCl(Awadet al.,2012).The mineralized C was calculated as the efflux rate of CO2(mg C g-1soil d-1),and the cumulative CO2emission was calculated as g CO2-C kg-1soil(Usmanet al.,2013).
Soil quality analysis
To determine the effect of biochar treatments on soil quality,the soil was sampled after 0(1 h),5,10,30,60,90,and 107 d of incubation.These soil samples were air dried before analysis.Soil pH,EC,and OM were measured as described above.Soil labile and non-labile C fractions were analyzed using the method described by Weilet al.(2003).Soil labile C was designated as active organic C(AOC)that powers soil nutrient cycles and biological characteristics.Soil AOC is considered significant to soil active C pool and also serves as a susceptible indicator of alterations in soil quality.Soil C non-lability was calculated as the ratio of non-labile C to labile C.
Statistical analysis
Mean values with standard deviations were reported,where applicable.One-way analysis of variance(ANOVA)was used to determine the significant differences between different treatments at the 0.05 significance level followed by Tukey’s honestly significant difference(HSD)test.
RESULTS AND DISCUSSION
Characteristics of the saline soil tested
The soil pH was 9.02(Table I),indicating that the soil was alkaline.The soil was a sandy clay loam,and its EC was 6.50 dS m-1,which refers to a highly saline soil.Generally,soils with EC values>4 dS m-1are characterized as saline soils(Aminiet al.,2016).The soil was also deficient in OM(4.4 g kg-1),making it less suitable for plant growth.It is generally reported that SOC in arid and semi-arid regions is normally less than 15 g kg-1.Thus,the soil used in this study with SOC of 2.5 g kg-1was typical for the arid areas of Pakistan.Furthermore,the CaCO3content in the soil was 33.8 g kg-1,indicating that the soil was calcareous.The soil WHC was 3.01%,corresponding to its sandy clay loam texture.

TABLE I General characteristics a)of the saline soil used in this study

TABLE II Yield and proximate and chemical analyses of biochars derived from municipal solid waste at pyrolysis temperatures of 300,500,and 700°C(BC300,BC500,and BC700,respectively)
Characteristics of the biochars produced
The biochar yields decreased with increasing pyrolysis temperatures from 300 to 700°C(Table II).This could be due to the lignin and cellulose decomposition at high temperatures(Sunet al.,2017).The removal of H2O,CO,CH4,CO2,and H2from the feedstock has been recognized to contribute to a drop in biochar yield with increasing temperatures(Tomczyket al.,2020).The mobile matter gradually decreased from 42.0%to 14.5%with increasing pyrolysis temperatures from 300 to 500°C.However,beyond 500°C,no further decrease in the mobile matter was observed,which was due to the maximum removal of volatile compounds from the feedstock.In contrast,the resident matter increased from 34.5%in BC300 to 43.0%and 40.0%in BC500 and BC700,respectively.The significantly larger amount of resident matter in BC500 and BC700 revealed their ability to serve as a C sink in soil.The presence of mobile matter in biochar could provide OM to microorganisms in soil and improve soil quality(Lehmannet al.,2011).The ashes in the biochars increased with increasing temperatures,which was due to the accumulation of OM and alkaline mineral residues during combustion(Domingueset al.,2017).
The pH values of BC300,BC500,and BC700 were 7.16,11.90,and 12.40,respectively(Table II).During pyrolytic conversion,the acidic functional groups are removed,and the biochars become highly alkaline.The increases in pH value with increasing temperatures were particularly because of the accumulation of more alkali salts from organic waste at higher carbonization temperatures(Yuanet al.,2011).The EC values of 5.55,6.08,and 13.36 dS m-1for BC300,BC500,and BC700,respectively,also increased with increasing temperatures,which could again be due to more accumulation of alkali salts in BCs at high temperatures.High ash contents also contributed to high EC values of the biochars produced.The total C contents,470,500,and 520 g kg-1in BC300,BC500,and BC700,respectively,also increased with increasing temperatures,which was due to the removal of H-and O-containing functional groups.
The surface profile of BC300 was non-uniform,and coarse particle aggregation was observed(Fig.1a).However,the surface profiles of BC500 and BC700 exhibited a distinctive aligned structure in which thin walls had appeared(Fig.1b,c),which showed that OMprogressively vanished from these biochars.The bright surfaces indicated that the surfaces of these biochars were loaded with nutritional and mineral elements.The typical porous surface structure of plant-derived biochar(Marmiroliet al.,2018)was not observed in the three biochars produced because of the nature and composition of their feedstock.Solid waste-based feedstocks generally comprise various components,such as paper,grass clippings,and food waste,while plant-based feedstocks generally contain a single component.

Fig.1 Scanning electron microscopy(SEM)images of biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300)(a),500°C(BC500)(b),and 700°C(BC700)(c).
The FTIR spectra of the three biochars produced are shown in Fig.2.The pyrolysis temperature had a significant effect on the presence or absence of various functional groups and their intensities in different biochars.It was observed that at the low temperature,the biochar(BC300)contained many functional groups,while the intensity of functional groups decreased for the biochars at high temperatures(BC500 and BC700),owing to the removal of several functional groups.Carboxylates groups were found at 1 400—1 578 cm-1for BC300 and 1 394 cm-1for both BC500 and BC700.The additional H-and O-containing functional groups,such as phosphines and thiols,were identified in the overlapped spectrum region chiefly between 1 251 and 780 cm-1.A unique aryl—O band at 1 251 cm-1was found for BC500.Aryl—O stretches comprise large ring aromatic compounds(C—O stretch),indicating aromaticity associated with stabilization of biochar.Another aliphatic phosphate(P—O—C),a non-aromatic compound of high intensity,appeared at 1 032 cm-1for BC300 and BC500,whereas it was absent for BC700.A supplementary functional group carbonyl(C—O—O—)was observed at 875 cm-1for BC500.Similarly,a C—S group(aryl thioethers)was identified at 780—670 cm-1for BC500.The stretching vibrations of C—S tend to offer strong transmittance in the FTIR spectrum.As shown in Fig.2,most of the functional groups were diminished in BC700.
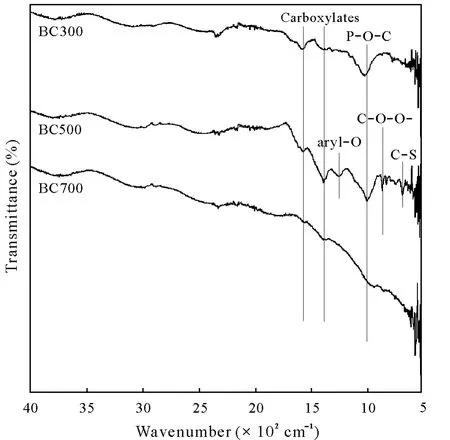
Fig.2 Fourier transform infrared(FTIR)spectra of biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300),500°C(BC500),and 700°C(BC700).
Stability and C sequestration potentials of the biochars produced
Compared to BC500 and BC700,which were thermally stable up to 1 000°C,with weight losses of 39.66%and 48.18%,respectively,BC300 showed a greater loss in weight(82.34%)at 1 000°C(Fig.3).A particularly sharp decrease in weight loss was observed beyond 250°C,which could be due to the presence of high amount of mobile matter in BC300(Table II).
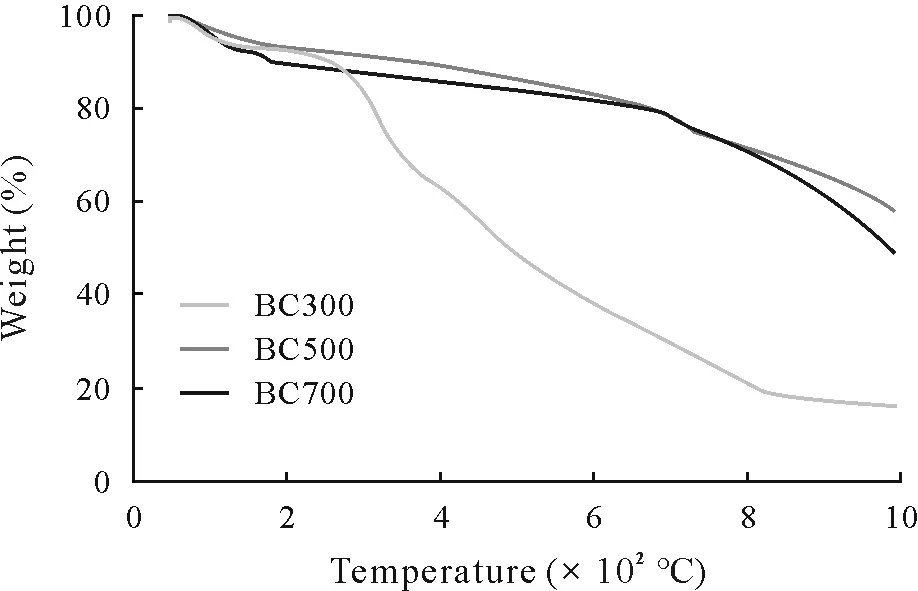
Fig.3 Thermogravimetric analysis(TGA)of biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300),500°C(BC500),and 700°C(BC700).
Biochars act as a C sink in soil because of their relatively high resistance or recalcitrance to biotic and abiotic degradation.A recalcitrance index,R50,was used to quantify the thermal recalcitrance of different biochars and screen them for their Csequestration potentials.The C sequestration potential is an imperative property for determining the soil quality parameters;it provides a measure of the original biomass C that would be retained in biochar for long periods.The calculatedR50and C sequestration potential values are listed in Table III.
TheR50values for BC300,BC500,and BC700 were 0.521,0.821,and 0.925,respectively(Table III).The higher theR50value,the greater the recalcitrance potential of the biochar(Harveyet al.,2012).The recalcitrance of biochar indicates its stability toward thermal,physical,and chemical degradation(Ahmadet al.,2019).Harveyet al.(2012)categorized biochars into three classes according to theirR50values:R50≥0.7,highly recalcitrant biochar;0.5≤R50<0.7,slightly degradable biochar;andR50<0.5,degradable biochar.Accordingly,BC300 was slightly degradable,while BC500 and BC700 were highly recalcitrant.Biochar recalcitrance is associated with aromatic C,which increases with increasing pyrolysis temperature.Carbon in biochar is more recalcitrant than that in raw feedstock,owing to the greater number of aromatic structures of biochar.Recalcitrant C pool in biochar can have a mean residence time ranging from 362 to 2 273 years(Lenget al.,2019).However,the recalcitrant index of biochar should not be equated with its C sequestration potential in soil because of variations in the design of experimental methods(Lenget al.,2019).The C sequestration potentials of BC300,BC500,and BC700 in this study were 36.14%,33.29%,and 34.53%,respectively.The high C sequestration potential of BC300 could be due to its higher yield compared to those of the other biochars,since biochar yields are directly proportional to their C sequestration potentials(Eq.1).No relationship was observed between theR50and C sequestration potential values for any of the biochars,which indicates that the two parameters should be used carefully.TheR50values were calculated with relevance to the graphite stability(highly stable form of C),while the C sequestration potential values depended not only on theR50values,but also on the biochar yields in addition to the C content in the feedstock.Hence,other methods,such as isotopic technology,field experiment,and long-term incubation experiment,should be considered for assessing biochar stability in soil(Lenget al.,2019).

TABLE III Recalcitrance index(R50,calculated using Eq.2)values and Csequestration potentials of biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300),500°C(BC500),and 700°C(BC700)
Effects of biochars on soil CO2efflux rate and cumulative CO2emission
Changes in the soil respiration with various biochar treatments throughout the incubation period were traced as the CO2efflux rate and cumulative CO2emission.As shown in Fig.4a,significantly higher amounts of CO2emitted in all biochar treatments than in CK after 1 d of incubation.This may be due to the decomposition of readily available OM present in the biochars.The highest CO2efflux rates after 1 d of incubation were recorded as 0.013±0.002 and 0.020±0.002 mg g-1soil d-1for BC500 at 2%and 5%,respectively.After 107 d of incubation,the CO2efflux rates for all the biochar treatments were not significantly different from that of CK,except for the treatment with BC300 at 5%(Fig.4b).The significantly higher CO2efflux rates in the treatment with BC300 at 5%than in CK,even after 107 d,could be due to the high OMcontent in BC300(Table II),which promoted the microbial activity and OMdegradation,resulting in greater CO2emission(Chenget al.,2017).
In general,a decreasing trend was observed in the CO2efflux rate until day 77,after which a slight increase in the CO2efflux rate was observed until day 92 and then the rate became constant(Fig.4c).The CO2efflux rates were generally higher in all of the treatments with biochars than in CK;however,at the end of the incubation period,the CO2efflux rates in all of the treatments with biochars became equal to that of CK.The initially more CO2emissions from all of the treatments with biochars than in CK were due to the microbial degradation of the OMpresent in the biochars.The addition of organic amendments into soil generally enhances the organic C and nutrients available in soil,which can generate suitable conditions for soil microorganisms,and consequently increase microbial activity and respiration in the soil(Usmanet al.,2013).Overall,it can be concluded that the addition of the biochars to the saline soil increased CO2emission into the atmosphere.
The treatment with BC300 showed the highest CO2efflux rate until day 92,particularly at the high(5%)application rate(Fig.4c).The lowest CO2efflux rates were observed in the treatment with BC700,almost equal to that of CK.These results are consistent with other studies,which reported that biochars formed at lower temperatures mineralized quicker than those formed at higher temperatures with enhanced aromaticity(Zhaoet al.,2017;Sarfarazet al.,2020).
After 1 d of incubation,soil cumulative CO2emission was the highest in the treatment with BC500 at 5%(0.032±0.001 g CO2-C kg-1soil)and the lowest in the treatment with BC700 at 5%(0.013±0.002 g CO2-C kg-1soil)(Fig.5a).It was observed that the BC300 and BC500 treatments significantly increased the cumulative CO2emission,while the BC700 treatments did not show any significant changes,as compared to CK.This suggested that BC700,owing to its greater recalcitrance index value(Table III),prevented the cumulative CO2emission,even after 1 d.After 107 d(Fig.5b),the treatment with BC300 at 5%showed the highest cumulative CO2emission(2.76±0.06 g CO2-C kg-1soil),compared to the lowest value in CK(0.016±0.001 g CO2-C kg-1soil).This could be attributed to the greater microbial activity as a result of enhanced organic and mobile matter contents in BC300(Table II).Generally,all the biochar treatments significantly increased the cumulative CO2emission compared to CK,particularly after 40 d of incubation(Fig 5c).These results further confirmed that the biochar applications to the saline soil could elevate the emission of CO2from soil into the atmosphere.However,the BC500 and BC700 treatments showed no significant di-fferences in cumulative CO2-C emission and produced much lower CO2emission than the BC300 treatments,indicating their greater stability in soil compared to that of BC300.The biochars pyrolyzed at the high temperatures(≥500°C)were more resistant to the release of CO2from soil into the atmosphere than the biochar pyrolyzed at the low temperature.At high temperatures,most of the OMfractions are lost from biochar,consequently lowering the cumulative CO2emission from amended soils(El-Mahroukyet al.,2015;Yanget al.,2020;Rizhiyaet al.,2020).

Fig.5 Cumulative CO2 emission in a saline soil under different treatments with biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300),500°C(BC500),and 700°C(BC700)at application rates of 2%and 5%(BC300-2%,BC300-5%,BC500-2%,BC500-5%,BC700-2%,and BC700-5%,respectively)after 1 d(a),after 107 d(b),and throughout the 107-d incubation period(c).Values are means with standard deviations shown by vertical bars(n=3),and different letters above the bars indicate significant differences between treatments at P<0.05.CK=no-biochar control.
It could be concluded that the biochar applications to the saline soil increased CO2emission into the atmosphere,thus contributing to increased GHG emission.However,the incubation experiment of this study only lasted for 107 d,and it is also obvious that the enhanced soil microbial activity upon the application of organic soil amendments could result in increased CO2emission due to the biodegradation of organic components of the biochars.
Effects of biochars on non-lability of C in soil
Generally,soil C lability is determined to evaluate the impact of soil amendments on soil fertility.However,nonlability of Cin soil indicates the long-term storage of C.At all of the time intervals throughout the incubation period,soil C non-lability increased in all biochar treatments compared to CK(Fig.6).Specifically,the BC500 and BC700 treatments showed greater soil C non-lability than the BC300 treatments,which was attributed to the greater stability of BC500 and BC700 as indicated by their C sequestration potentials(Table III).At the end of the incubation period(107 d),all biochar treatments showed greater soil C non-lability than CK.Particularly,the treatments with BC500 and BC700 at 5%resulted in remarkable increases in soil C non-lability than the other biochar treatments.These results indicated that the biochar treatments could store C for a long time in the saline soil.In particular,the biochars produced at the high temperatures(BC500 and BC700)were more stable,thus exhibiting greater C sequestration potential than that produced at the low temperature(BC300).
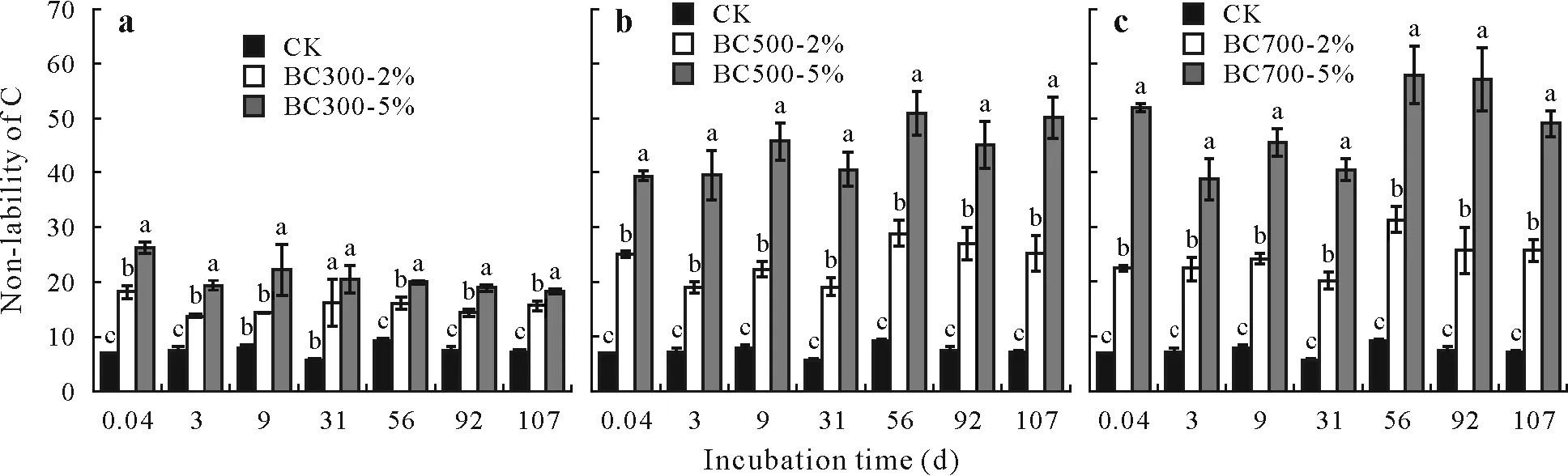
Fig.6 Non-lability of C,the ratio of non-labile C to labile C,in a saline soil under different treatments of biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300)(a),500°C(BC500)(b),and 700°C(BC700)(c)at application rates of 2%and 5%(BC300-2%,BC300-5%,BC500-2%,BC500-5%,BC700-2%,and BC700-5%,respectively)throughout the 107-d incubation period.Values are means with standard deviations shown by vertical bars(n=3),and different letters above the bars indicate significant differences between treatments at P<0.05.CK=no-biochar control.
The labile and non-labile C fractions were fractionated to more clearly evaluate the C storage potentials of the biochars produced.The non-labile C contents increased with the application of biochars compared to CK(Fig.7).The non-labile C contents were higher in the BC500 and BC700 treatments than in the BC300 treatments,confirming that the biochars produced at 500 and 700°C exhibited greater C storage potentials in soil than the biochar produced at 300°C.The fixed matter contents in the biochars are closely linked to their stability and decomposition rates.The non-labile C fraction is associated with the long-term sequestration of C into soil.The low contents of the degradable fraction or mobile matter and high C and resident matter contents of the biochars pyrolyzed at the high temperatures(Table II)resulted in their high C sequestration potentials.It was imperative to estimate the effect of pyrolysis temperature on the extended stability of biochars as well as the microbial degradation.The passive C pool/recalcitrant fraction of organic C augmented with escalating carbonization temperature enhanced the C storage into soil.Subsequently,it could be concluded that the biochars produced at the higher temperatures(≥500°C)could more reliably sequester C in soil than the biochar produced at the lower temperature.
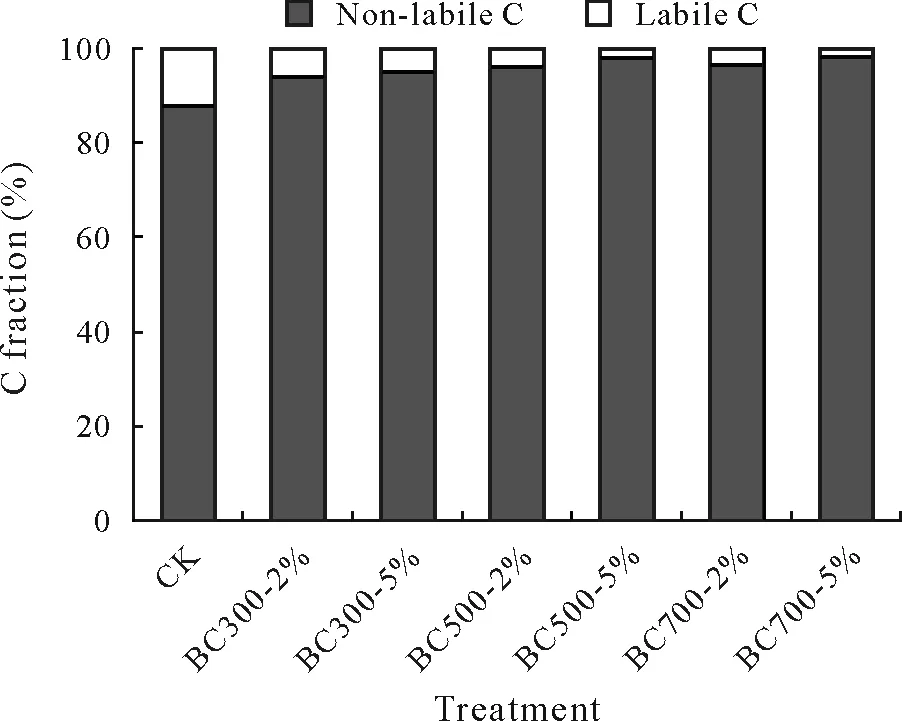
Fig.7 Percentages of labile and non-labile C fractions in a saline soil under different treatments with biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300),500°C(BC500),and 700°C(BC700)at application rates of 2%and 5%(BC300-2%,BC300-5%,BC500-2%,BC500-5%,BC700-2%,and BC700-5%,respectively)at the end of the 107-d incubation period.CK=no-biochar control.
Effects of biochars on soil quality
Since biochar is a nutrient-rich soil amendment that enhances soil fertility,the biochars produced in this study were also evaluated for their impacts on the quality of the saline soil tested.There was only a slight increase in soil pH in the treatments with BC500(9.35±0.03)and BC700(9.26±0.03)at 5%as compared to CK(8.97±0.02)at the end of the incubation period(data not shown),which could be attributed to the highly alkaline nature of these biochars(Table II).The BC300 treatments,however,did not cause any significant change in the soil pH relevant to CK.Similarly,no significant effect of the biochars on the soil EC was observed throughout the incubation period,which indicated that the biochars did not further increase the soil salinity.
Soil AOC is considered one of the commonly recognized indicators of soil quality(Bünemannet al.,2018).The BC300 treatments significantly increased the soil AOC compared to the BC500 and BC700 treatments and CK throughout the incubation period(Fig.8a).The high OM in BC300 emanating from the feedstock contributed to the greater soil AOC content.This implied that BC300 could be a potential candidate for soil quality improvement.Nevertheless,the BC500 and BC700 treatments did not show significant decreases in soil AOC content as compared to CK,indicating that despite their high stability in the soil,these amendments did not deteriorate the soil quality.
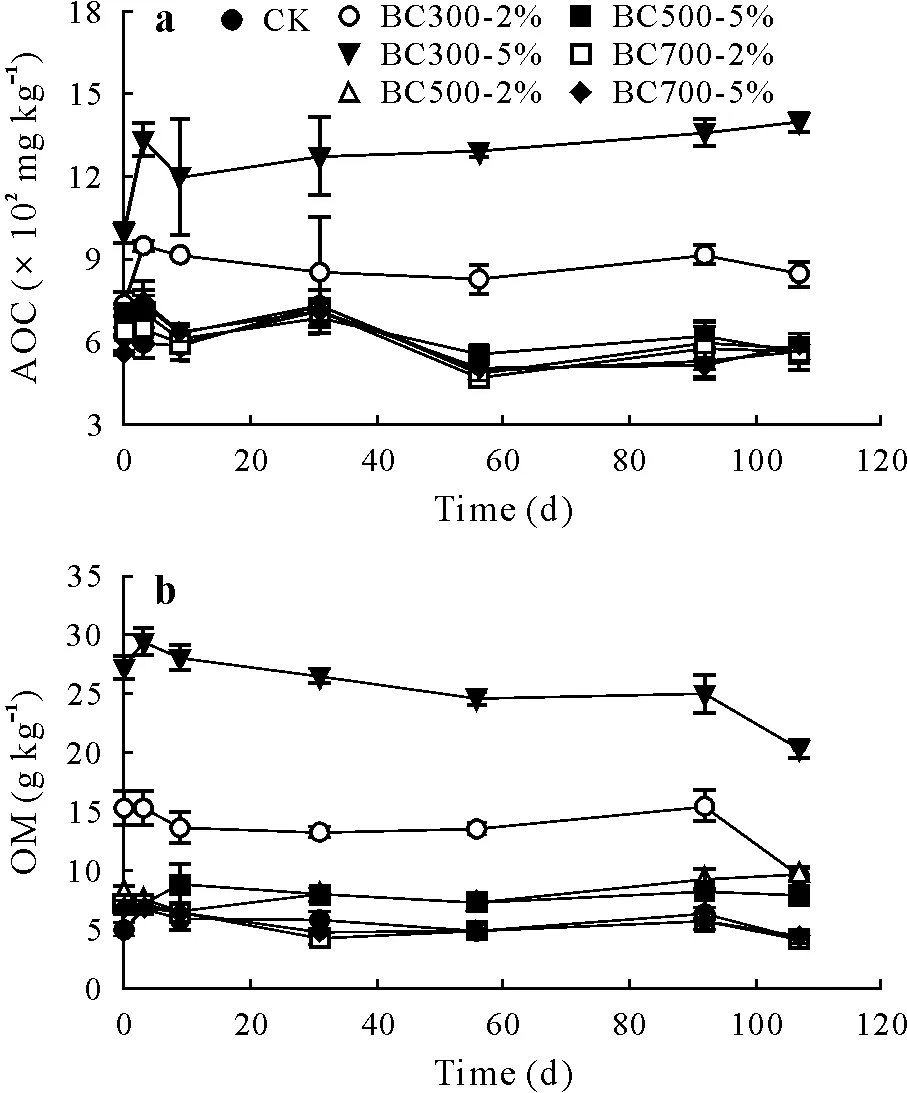
Fig.8 Active organic C(AOC)(a)and organic matter(OM)(b)in a saline soil under different treatments with biochars derived from municipal solid waste at pyrolysis temperatures of 300°C(BC300),500°C(BC500),and 700°C(BC700)at application rates of 2%and 5%(BC300-2%,BC300-5%,BC500-2%,BC500-5%,and BC700-2%,BC700-5%,respectively)throughout the 107-d incubation period.Values are means with standard deviations shown by vertical bars(n=3).CK=no-biochar control.
Soil OMis another important parameter in determining soil quality.At the end of incubation(107 d),soil OM contents in the treatments with BC300 at 2%and 5%(9.5±0.8 and 20.3±0.9 g kg-1,respectively)were significantly higher than that in CK(4.3±0.6 g kg-1)(Fig.8b).No significant differences were found between the treatments with BC500 and BC700.This was due to the high amount of OMin the BC300(Table II)and a greater soil CO2efflux rate and cumulative CO2emission in the treatments with BC300(Figs.4 and 5).The addition of OM-rich biochar stimulates soil biological activity,which can further result in increased emission of CO2from soil.Soil fertility could be improved by improving soil OM content.The alkaline and nutrient-deficient soil used in this study had a low OM content of 4.4 g kg-1(Table I);however,after applications of the biochars,particularly BC300,soil OM increased remarkably,indicating that the biochar pyrolyzed at the low temperature could be more useful in improving soil quality than the biochars pyrolyzed at the high temperatures(≥500°C).
The soil OMcontents were the highest for the treatments with BC300 and lowest for the treatments with BC700 during the 107 d incubation period.The soil OMresults were positively linked with the CO2efflux rate and cumulative CO2emission results.All of the biochar treatments released more CO2into the atmosphere than CK(Fig.5).This was due to the OMpresent in the biochars,which was decomposed by soil microbes over time.Additionally,the highest CO2emission was due to the selected soil that was nutrient poor(OM=4.4 g kg-1).Thus,application of the biochar pyrolyzed at the low temperature to the alkaline saline soil may negatively affect the climate in terms of CO2emission.However,application of the biochars carbonized at the high temperatures(≥500°C)may also affect the climate positively in terms of sequestering C in the soil for a long period of time.
CONCLUSIONS
The pyrolysis temperature greatly affected the properties of the biochars produced.The biochars produced at high temperatures(500 and 700°C)were more recalcitrant than that produced at a low temperature(300°C).The treatments with BC300 increased CO2efflux rate in the tested saline soil to a greater extent than the treatments with BC500 and BC700.Soil cumulative CO2emission was higher in the biochar treatments than in CK.The addition of biochars to the saline soil may increase the CO2emission into the atmosphere because of the biological decomposition of the OMpresent in these biochars.Addition of the biochars pyrolyzed at the high temperatures(≥500°C)to the saline soil caused a higher soil non-labile C content than that of the biochar pyrolyzed at the low temperature,indicating their greater C storage potentials.Moreover,soil AOC and OM increased with addition of biochars,which indicated the improved quality of the nutrient-deficient saline soil.The biochar produced at the low temperature could aid in improving the soil fertility;however,the biochars produced at the high temperatures(≥500°C)exhibited greater potentials to sequester C in the saline soil studied.Therefore,it is recommended to select an appropriate biochar for a specific soil application depending on the particular outcome required.
CONTRIBUTION OFAUTHORS
Hamna SALEEM and Mahtab AHMAD contribute equally to this work.
ACKNOWLEDGEMENT
The present work was partially supported by the University Research Fund Program of the Quaid-i-Azam University,Pakistan.
杂志排行
Pedosphere的其它文章
- Potential of plant growth-promoting rhizobacteria-plant interactions in mitigating salt stress for sustainable agriculture:A review
- Influence of kaolinite and montmorillonite on benzo[a]pyrene biodegradation by Paracoccus aminovorans HPD-2 and the underlying interface interaction mechanisms
- Mutualistic fungus Piriformospora indica modulates cadmium phytoremediation properties of host plant via concerted action of enzymatic and non-enzymatic biochemicals
- Reducing residues of tetracycline and its resistance genes in soil-maize system and improving plant growth:Selecting the best remediation substance
- Soil texture prediction through stratification of a regional soil spectral library
- Evaluation of immobilizing agents as soil quality conditioners in addition to their metal(loid)immobilizing effect
