Electrochemical Reduction Determination of N-Nitrosodiphenylamine in Food Based on Graphene Electrode Material
2022-05-09XINGWenqian邢文倩HUJianmei胡建梅ZHANGXuan
XING Wenqian(邢文倩), HU Jianmei(胡建梅), ZHANG Xuan(张 煊)
Key Laboratory of Science and Technology of Eco-Textiles, Ministry of Education, College of Chemistry, Chemical Engineering & Biotechnology, Donghua University, Shanghai 201620, China
Abstract: As a group of the most notorious carcinogens, N-nitrosamines (NAs) are highly toxic and usually involved in healthy issue of human daily life. An electrochemical sensor for N-nitrosodiphenylamine (NDPhA) detection was constructed based on graphene electrode material. The graphene was facilely obtained by direct reduction of graphene oxide (GO) with hydrazine hydrate, and further coated on the surface of the glassy carbon electrode (GCE) to fabricate the electrochemical sensor for NDPhA analysis in food samples. The present sensor showed excellent sensitivity and selectivity for the electrochemical determination of NDPhA under a reduction manner with the detection limit of 0.6 μmol/L. It was also successfully used in beer and ham food samples with satisfactory recovery results.
Key words: electrochemical sensor; N-nitrosamine (NA); graphene; food sample
Introduction
N-nitrosamines (NAs) represent a group of compounds in which the nitrogen atom of nitroso group (—NO) is directly connected with the amine group. NAs have been considered as one of the most notorious carcinogens due to their high toxicity, mutagenicity and teratogenicity even existing in trace amount. Now it has been recognized that these compounds could be formed during treatment of meats by smoking, curing, and nitrite salt, as well as chlorination and ozonation processes of drinking water[1-2]. Therefore, sensitive detection of NAs in food samples becomes highly desirable because NAs are closely relative to the public health and food safety issues of human daily life. Various analysis methods, such as gas/liquid chromatography coupled with mass-spectrometry, fluorimetric, chemiresistive and electrochemical sensors, have been widely used for NA detection[3-13]. Among these approaches, the electrochemical analysis method has attracted much recent attention due to its advantages of sensitivity, rapidity, and low cost[1, 12-14]. As a non-volatile NA,N-nitrosodiphenylamine (NDPhA) has been usually used as a typical NA compound for the performance evaluation of the electrode material in the electrochemical sensor. The first report on electrochemical oxidation of NDPhA in aqueous solution appeared in 1994 based on ruthenium polymer electrode material[6]. To solve the problem of poor water solubility, Yangetal.[7]developed an electrochemical sensor for determination of NDPhA based on porous Au electrode in ionic liquid. The poly (diallyldimethylammonium chloride)-stabilized graphene/platinum electrode material has also been employed to detect NDPhA in tap water samples[12]. However, most of these previous electrochemical sensors for NDPhA required noble metals and worked in an oxidation manner at a high potential (>1.1 V) in aqueous solution. The analysis of NDPhA on noble metal electrodes generally becomes difficult in aqueous solution due to the formation of oxide and the evolution of oxygen at such high potentials[7]. Recently, a graphite-polyurethane composite electrode was used for electrochemical reduction determination of NDPhA at -0.7 V in a phosphate buffer (pH=2.1), and the sensor was capable of detecting NDPhA in synthetic urine samples[13].
In this work, a novel electrochemical sensor for NDPhA detection is developed in aqueous solution based on graphene electrode material. The present electrochemical sensor is facilely fabricated and successfully used for NDPhA detection in beer and ham samples.
1 Experiments
1.1 Reagents and methods
Graphene oxide (GO) and NDPhA were purchased from XFNANO (Nanjing, China) and Aladdin (Shanghai, China), respectively. Common chemicals as well as solvents were supplied by Sinopharm Chemical Reagent Corp. (Shanghai, China). Reduced graphene oxide (RGO) was obtained by direct reduction of GO with hydrazine hydrate as described previously[15].
Electrochemical measurements were thoroughly carried out on the CHI-660D electrochemical workstation (Chenhua, Shanghai, China) by using a conventional three-electrode system, where the glassy carbon electrode (GCE with a diameter of 3 mm), the saturated calomel electrode (SCE) and Pt wire were used as the working electrode, the reference electrode and the counter electrode, respectively. The electrical conductivities of electrodes were tested by electrochemical impedance spectroscopy (EIS). The X-ray powder diffraction (XRD) was performed on D/max 2550 (Rigaku, Japan). Centrifugation and ultrasonication were finished on Optima MAX-TL (Beckman, USA) and KQ3200 (Kunshan, China), respectively.
1.2 Detection procedure for NDPhA
Before the electrochemical test, the GCE was firstly polished with 0.30 μm and 0.05 μm aluminium oxide powder, respectively, until the redox peak potential separation of the potassium hexacyanoferrate redox probe was lower than 80 mV in cyclic voltammetry (CV). Then 5 μL RGO suspension (2 mg/mL in ethanol) was transferred onto GCE surface to fabricate the working electrode (RGO/GCE) for NDPhA detection. The quantitative analysis of NDPhA was performed in phosphate buffer solution (PBS, pH=7.4) by the differential pulse voltammetry (DPV) method, with continuously increase in concentration of analyte that was further linearly correlated with the peak current intensityIat -0.95 V. The potentialEwindow was selected to be -1.2-0.6 V (versusSCE), with pulse amplitude of 50 mV, pulse width of 0.2 s, and pulse period of 0.5 s, respectively.
For a practical application experiment, beer and ham samples were purchased from a local supermarket and treated simply. The beer sample (10 mL) was treated by ultrasonication for 5.0 min and then a certain amount of the sample was added into 0.1 mol/L PBS for analysis. The ham sample (10 g) was firstly crushed and ultrasonicated in 20 mL methanol for 20.0 min. Then after centrifugation, the supernatant was collected for electrochemical analysis by following the above detection procedure for NDPhA. The sample solution was also spiked with a certain amount of NDPhA to evaluate the recovery rate.
2 Results and Discussion
2.1 Electrochemical performance of electrode
The transformation of GO into RGO was firstly confirmed by the XRD pattern analysis, where the sharp diffraction peak attributed to (002) in GO disappeared with appearing of a broad pattern (002) around 10° in RGO (Fig. 1). RGO was then coated on GCE to fabricate the RGO/GCE, and its electrochemical performance was studied by CV and EIS with the potassium hexacyanoferrate redox probe in 0.1 mol/L KCl solution. As shown in Fig. 2(a), a pair of well-defined redox peaks was observed on both GCE and RGO/GCE electrodes with a potential separation of 70 mV. The peak current on the RGO/GCE is higher than that on the bare GCE, suggesting larger electrochemical surface area of the RGO/GCE[16-18]. EIS measurements were performed to evaluate the electrical conductivity and the Nyquist plots were shown in Fig. 2(b) in whichZ’ is the real part of impedance andZ″ is the imaginary part of impedance. It can be noticed that the diameter of the semicircle at high frequencies is smaller on the RGO/GCE than that on the bare GCE, indicating the higher conductivity of the RGO/GCE electrode[19]. The advantages of larger electrochemical surface area and higher electrical conductivity could make the RGO/GCE electrode promising for NDPhA detection.
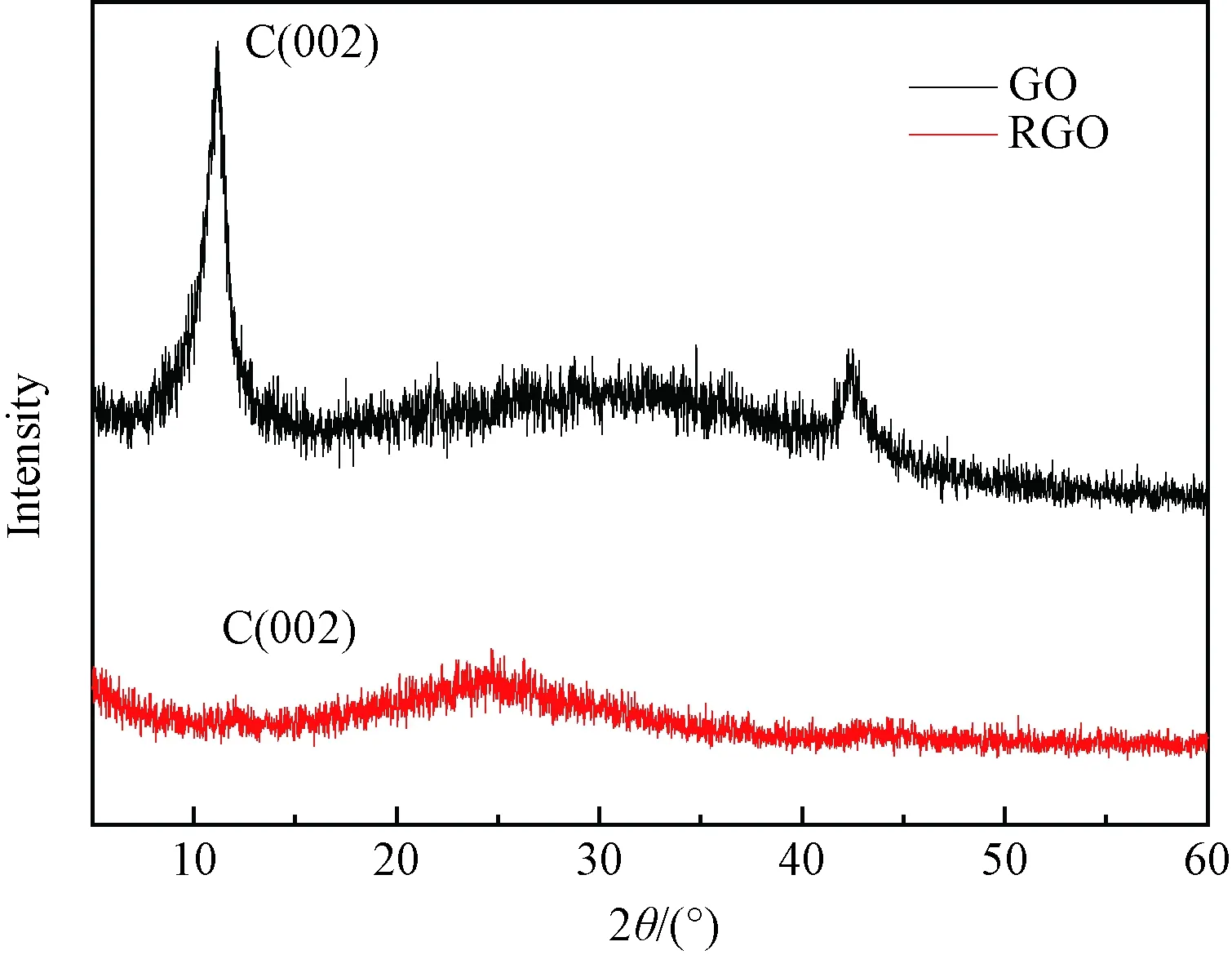
Fig. 1 XRD pattern of GO and RGO
The electrochemical response towards NDPhA (50.0 μmol/L) in PBS (pH=7.4) was therefore conducted by DPV. The results are shown in Fig. 2(c). The reduction peak current intensity at -0.95 V is substantially higher on the RGO/GCE than that on the bare GCE, demonstrating the more sensitive response performance in the former electrode. The reduction mechanism could be described according to the proposed two-step reduction processes of NDPhA, where the nitroso group was firstly reduced to hydroxylamine, and further reduced to amine group[12-13].
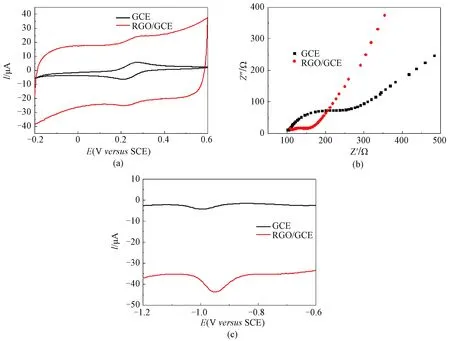
Fig. 2 (a) CV curves and (b) EIS plots of 0.5 mmol/L K3[Fe(CN)6] on GCE and RGO/GCE; (c) DPV curves of NDPhA (50.0 μmol/L) in 0.1 mol/L PBS (pH=7.4) on GCE and RGO/GCE
The kinetics of the electrochemical reduction of NDPhA on the RGO/GCE was further investigated by CV at different scan rates (5-100 mV/s). As shown in Fig. 3(a), with the increasing scan ratev, the reduction peak potential is continuously negative-shifted and the peak current increases. Furthermore, the peak currents are linearly proportional to the square roots of scan rates (Fig. 3(b)), implying that the electrochemical reduction reaction of NDPhA on the RGO/GCE is a diffusion controlled process[18].

Fig. 3 (a) CV curves of NDPhA (50.0 μmol/L) in 0.1 mol/L PBS (pH=7.4) measured on RGO/GCE at various scan rates (5-100 mV/s); (b) corresponding plots of peak currents versus square root of scan rates
2.2 Optimization of determination conditions
To gain the best determination performance, experimental conditions such as pH and accumulation time were optimized by DPV measurements. As shown in Figs. 4(a) and 4(c), the reduction peak potentialEof NDPhA depends greatly on the pH value, revealing that the proton is involved in the redox process[18], whereas the peak currentIincreases and reaches the maximum at pH of 7.4. Therefore, the optimal pH was selected to be 7.4 for the determination of NDPhA. The effect of accumulation times on the reduction of NDPhA is also examined and the results are presented in Figs. 4(b) and 4(d). It can be seen that the peak current at -0.95 V increases with increasing accumulation time within 60.0 min, but the change is more drastic at the first 10.0 min. Therefore, the optimal accumulation time was chosen to be 10.0 min in this work.
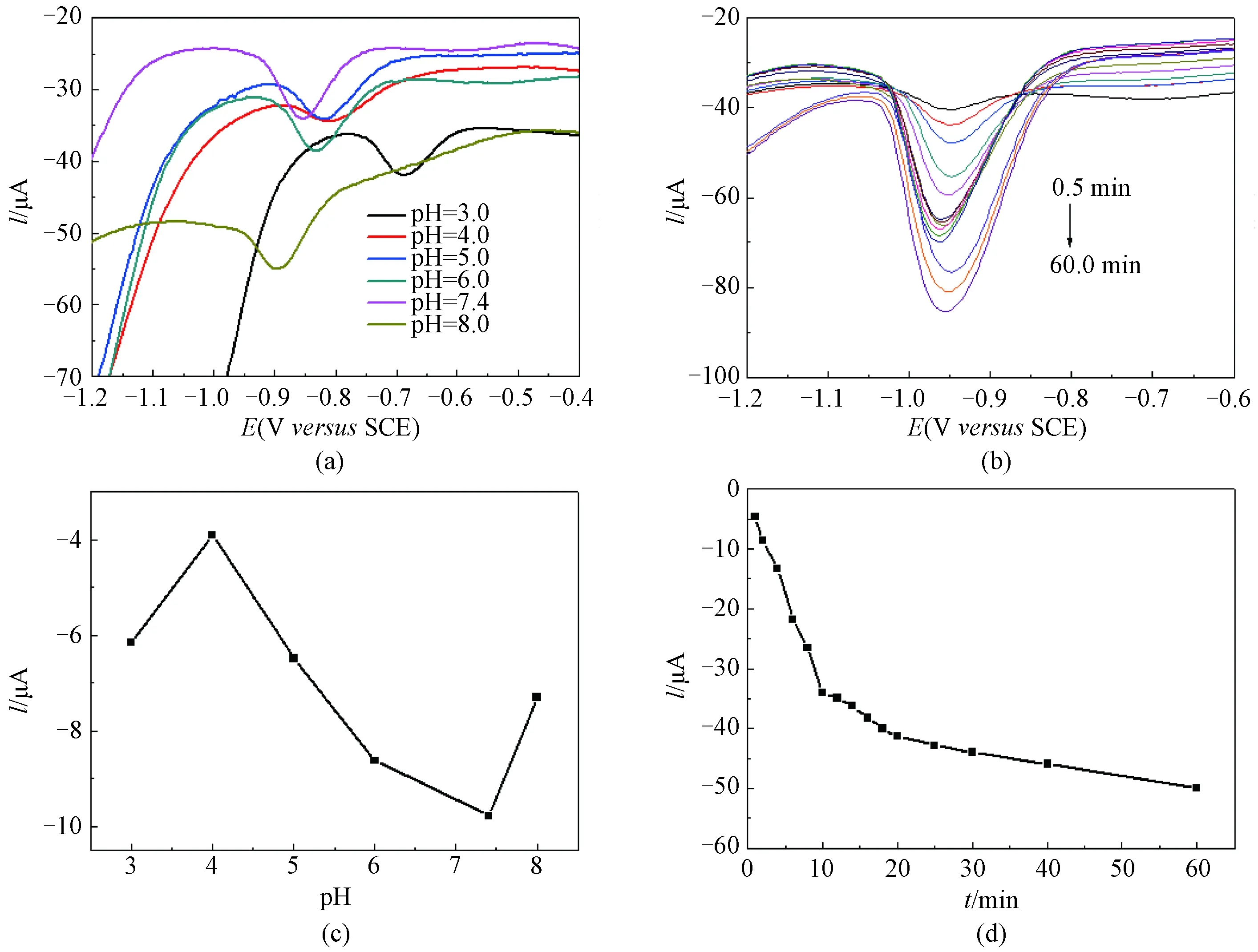
Fig. 4 DPV curves of NDPhA (100.0 μmol/L) on RGO/GCE in various (a) pH and (b) accumulation time; the corresponding peak current versus (c) pH and (d) time, respectively
2.3 Performance of RGO/GCE for NDPhA determination
Under the optimal determination conditions, the electrochemical titration for NDPhA was carried out in 0.1 mol/L PBS (pH=7.4) by DPV. The results are shown in Fig. 5. With increasing the NDPhA concentration (0-150.0 μmol/L), the peak current at -0.95 V increased (Fig. 5(a)), and a linear relationship between the currentIand the concentrationCwas found within 0.8-20.0 μmol/L (Fig. 5(b)). The detection limit was estimated to be 0.6 μmol/L (signal-to-noise ratio is 3), and was comparable or even better than that of those reported electrochemical sensors for NDPhA previously[12-13].
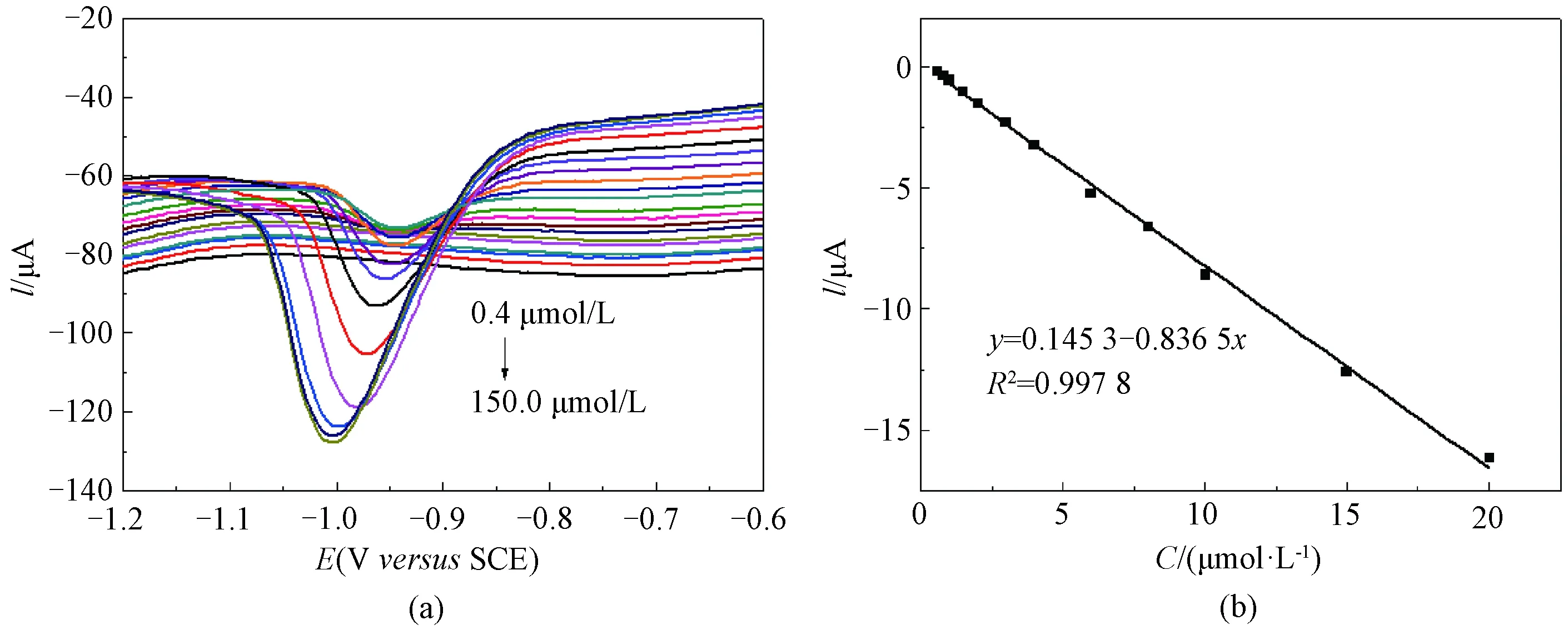
Fig. 5 (a) DPV titration curves of NDPhA (0.4-150.0 μmol/L) on RGO/GCE in 0.1 mol/L PBS (pH=7.4); (b) corresponding calibration plots between peak current intensity and NDPhA concentration
The reproducibility was examined by independently making five RGO/GCE electrodes and used for 50.0 μmol/L NDPhA detection in the PBS (pH=7.4). As shown in Fig. 6(a), the DPV curves almost overlapped, and the peak currents had little change with a relative standard deviation (RSD) of 2.3%, indicating the good repeatability. The anti-interference study was performed by introducing potential interfering species for 50.0 μmol/L NDPhA detection (Fig. 6(b)). It was revealed that the existence of 250.0 μmol/L KCl, K2SO4, CuSO4, NaCl, CaCl2, MgSO4, FeSO4, and NaNO2, and 500.0 μmol/L glucose, ascorbic acid, citric acid, triethylamine, and p-phenylenediamine, have no significant influence on NDPhA detection with peak current signal deviations below 10%, respectively.
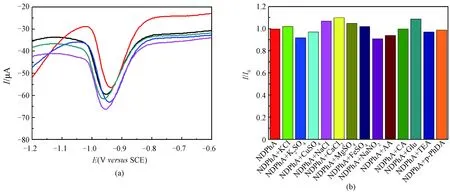
Fig. 6 (a) DPV curves of NDPhA (50.0 μmol/L) measured on RGO/GCEs in 0.1 mol/L PBS (pH=7.4); (b) peak current intensity change (I/I0) of 50.0 μmol/L NDPhA in absence and presence of various species
2.4 Application in food samples
The practical potential application of RGO/GCE in food samples was demonstrated by determination of NDPhA in beer and ham samples. The sample solution was also spiked with a certain amount of NDPhA to evaluate the recovery rate. The results were summarized in Table 1. Obviously, the recovery rates of NDPhA in the samples are between 90.8% and 108.0%. These results demonstrated that RGO/GCE could serve as a novel electrochemical sensor for NDPhA detection in food samples with a satisfying recovery rate.
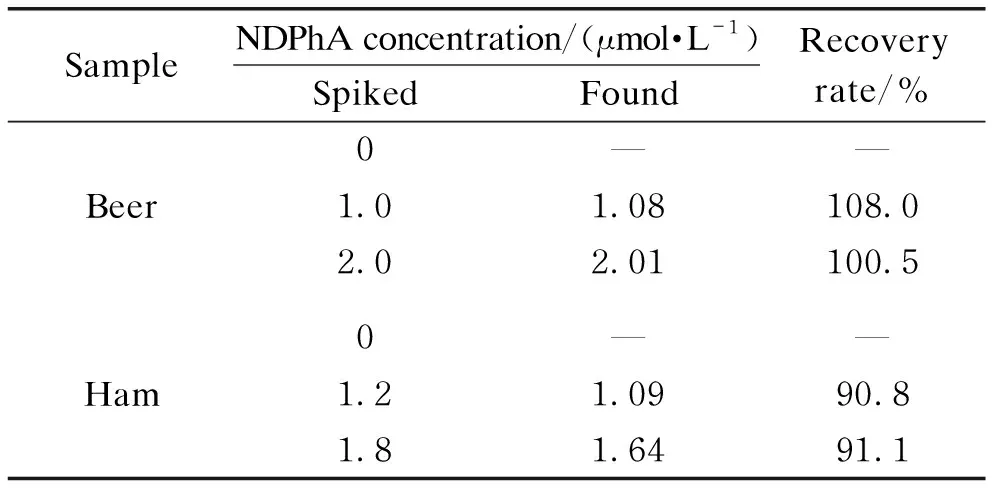
Table 1 Detection results in real samples
3 Conclusions
In summary, RGO was used to construct an electrochemical sensor for NDPhA detection under a reduction manner. The present sensor showed excellent sensitivity with the detection limit of 0.6 μmol/L, and satisfying anti-interference ability, as well as reproducibility. It was also successfully used for NDPhA detection in food samples such as beer and ham with satisfactory recovery rates (90.8% -108.0%).
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Electrospinning of Bead-on-String Sodium Alginate Nanofibrous Membrane
- Polysaccharides Based Random and Unidirectional Aerogels for Thermal and Mechanical Stability
- Eco-Friendly pH Indicator Based on Natural Anthocyanins from Lycium ruthenicum
- Formulating Novel Halogen-Free Synergistic Flame Retardant Epoxy Resins for Vacuum Assisted Resin Infusion Composites
- Hydrothermal Synthesis of Ordered ZnO Nanorod Arrays by Nanosphere Lithography Method
- Temperature-Dependent Growth of Ordered ZnO Nanorod Arrays
