Effectiveness of non-pyrimethamine-based regimens for toxoplasma encephalitis: A systematic and meta-synthesis study
2022-04-27DavidSusantoArthurMawuntuFinnyWarouwWindyWariki
David Susanto, Arthur H. P. Mawuntu, Finny Warouw, Windy M. V. Wariki
1Neurology Department, Faculty of Medicine, Sam Ratulangi University/R.D. Kandou Hospital, Manado, Indonesia
2Department of Community Medicine, Faculty of Medicine, Sam Ratulangi University, Manado, Indonesia
ABSTRACT
Objective: To examine the differences in effectiveness and side effects between pyrimethamine-based and non-pyrimethaminebased regimens for toxoplasma encephalitis since the availability of pyrimethamine in Indonesia is currently limited due to its withdrawal from the market.
Methods: A systematic review and meta-synthesis study that was carried out by following a protocol guided by the Preffered Reporting Items for Systematic Review and Meta-analysis (PRISMA).Effectiveness measures included clinical improvement, mortality,and radiological improvement. We evaluated selected articles narratively because of the limitations of homogeneity. The risk of bias in RCTs was assessed using the Cochrane Risk of Bias tool for RCT (ROB 2.0) and cohort studies were assessed using the Risk of Bias In Non-Randomized Studies of Interventions (ROBINS-1) tool.Research quality was assessed using the GradePro software.
Results: We included two retrospective cohort studies and one RCT.Narrative outcome assessment in these three studies did not show significant difference in effectiveness between pyrimethamine-based and non-pyrimethamine-based regimens for toxoplasma encephalitis treatment. However, drug side effects were consistently higher in the pyrimethamine-based regimen.
Conclusions: This study has a high risk of bias. The quality of the research also has a low recommendation value. However, the results may be considered for application if a standard regimen is not available.
KEYWORDS: Toxoplasma encephalitis; Alternative treatment;Without pyrimethamine; Systematic review; Meta-synthesis
Significance
Currently, the availability of pyrimethamine is becoming limited both in Indonesia and other countries due to the high rate of pyrimethamine drug resistance. Disease control centers in many countries also no longer recommend pyrimethamine-based drugs. This study investigated whether non-pyrimethaminebased drugs were as effective as pyrimethamnine-based drugs in treatment of toxoplasma encephalitis, so that they can be an option when pyrimethamine is no longer available. Narrative outcome assessment in two retrospective cohort studies and one RCT did not show significant difference in effectiveness between pyrimethamine-based and non-pyrimethamine-based regimens for toxoplasma encephalitis treatment. However, the study has a high risk of bias.
1. Introduction
Toxoplasma encephalitis (TE) is a central nervous system (CNS)infection caused by Toxoplasma (T.) gondii. These microorganisms are obligate intracellular parasites that generally use cats as their definitive host. In immunocompetent patients, this infection usually does not cause any symptoms. Toxoplasmic infections are often caused by reactivation from preexisting diseases. Generally, it attacks patients with a decreased immune system. The number of TE cases is increasing due to a higher number of human immunodeficiency virus (HIV/AIDS) patients[1].
A study from Xiao et al.[2] reported that the number of positive toxoplasma IgG prevalence in China was 12.5% from 2 634 subjects examined. In India, Anuradha and Preethi[3] who studied HIV-positive subjects, reported seropositive rates up to 34.78% of 92 subjects. The study also determined the association between Cluster of Differentiation 4 (CD 4) levels with the prevalence of toxoplasmosis in subjects. About 75% of subjects with CD4 values of 51-100 cells/mm3had an antitoxoplasmic immunoglobulin G(IgG)[2-4].
In Indonesia, the prevalence of toxoplasma infections varies considerably. In Jakarta, research from Terazawa et al. recruiting 1 693 subjects found a seropositive percentage of 70%. Konishi et al.research in Surabaya with 1 761 subjects reported 58% seropositive.Prasetyo et al. in Surakarta with 143 subjects obtained 30.8% seropositive, and research by Tuda et al. in North Sulawesi reported a seropositive percentage of 58.5% from 856 subjects examined from various cities[5-8].
The main regimen for TE therapy is a combination of pyrimethamine and sulfadiazine (P-S), or pyrimethamine and clindamycin (P-C) combination. At present, the availability of pyrimethamine in various health service centers is becoming harder to find and limited due to the issuance from the National Agency of Drug and Food Control in 2020, which stopped the production of pyrimethamine. Drug limitation also occur in other countries because Center of Disease Control and Prevention (CDC) no longer recommends pyrimethamine based treatment, especially for malaria,so drug production has begun to be limited.
There are still several alternatives for TE treatment without pyrimethamine that can be used, such as trimethoprimsulfamethoxazole (TMX) and atovaquone which are less frequently used in Indonesia. We conducted a systematic review and metasynthesis regarding the effectiveness of alternative drug regimens without pyrimethamine in TE patients compared to the main regimen(P-S or P-C) to determine whether treatment without pyrimethamine was as effective as pyrimethamine based regimen.
2. Materials and methods
This research was initially a systematic review and meta-analysis that was regularly and logically conducted according to the correct research protocol, guided by Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) using the Review Manager (RevMan) software version 5.4. However, meta-analysis was not performed due to limited articles with homogeneous populations, interventions, comparisons, and outcomes.
Researchers included randomized controlled trials (RCTs) and cohorts that addressed the comparison of alternative drugs without pyrimethamine (TMX or atovaquone) with the main drug (P-S or P-C) on TE. The included studies recruited subjects:1) aged >18 years; 2) diagnosed with TE through clinical manifestations and radiological features and/or PCR results. The researcher determined three primary outcomes to be examined, namely clinical response,mortality rate, and radiological images improvement. Secondary outcome is drugs’ side effects. Article exploration has been limited in the last fifteen years (from January 2021) to get the latest support articles and distinct from other previous systematic reviews. Articles with complete manuscripts, written in English or Indonesian, and not published in an abstract, proceeding, or not yet published symposium books were included for review.
Article data was searched in Portal Garuda, PubMed/MEDLINE,Wiley Online Library, Science Direct, and Google Scholar database. Keywords used in this study were ‘Ensefalitis toxoplasma’or ‘ET’ or ‘toxoplasma encephalitis’ or 'TE' or ‘toxoplasmosis ensefalitis’ or ’toxoplasmic encephalitis’ or ‘brain toxoplasmosis’or ‘cerebral toxoplasmosis’ or ‘toksoplasmosis serebral’ or‘toksoplasmosis otak’ and ‘trimethoprim and sulfamethoxazole’or ‘trimethoprim plus sulfamethoxazole’ or ‘trimetoprim dan sulfametoksazol’ or ‘cotrimoxazole’ or ‘kotrimoksazol’ or ‘atovaquone’and ‘pyrimethamine and sulfadiazine’ or ‘pyrimethamine and clindamycin’ or ‘pyrimethamine plus sulfadiazine’ or ‘pyrimethamine plus clindamycin’ or ‘pirimetamin dan sulfadiazin’ or ‘pirimetamin dan klindamisin’ and ‘therapy’ or ‘treatment’ or ‘management’ or‘terapi’ or ‘penatalaksanaan’. After keywords widening strategy was carried out, a search narrowing was performed by combining previously obtained keywords using the "and" link.
Articles obtained were identified, then screening was done based on the title and abstract. Articles with appropriate titles and abstracts were sought in full text. Eligibility assessment was conducted in articles in which full manuscripts were available. Thus, relevant articles were selected. This process was carried out by four researchers (DS, AM, FW, and WW).
The risk of bias was assessed in each study described in narrative form. In RCT studies, the Cochrane Risk of Bias Tool for RCT(ROB 2.0) was used to analyze bias risk, while in cohort researches,bias risk was assessed using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-1). Research quality assessment was evaluated using GradePro software. GradePro evaluated the outcome chosen in this article, namely mortality rates, clinical response, radiological features, and drug side effects. All research outcomes were assessed for their quality based on components: the risk of bias, inconsistency, indirectness, and imprecision[9-11].
3. Results
3.1. Research article selection
The initial search resulted in 90 articles from PubMed/MEDLINE,Wiley Online Library, Science Direct, Google Scholar, and Portal Garuda. Articles exploration with hand searching techniques and bibliography checking of each article resulted in two additional articles. A total of 10 duplicated articles were excluded. Thus,there were 82 articles from titles and abstracts screening. Nineteen articles fulfilled research criteria, then studied in their full text.After a complete manuscript assessment, as many as 16 complete manuscripts were excluded because of unfitted with the research criteria, did not meet the outcome criteria, and/or did not match design and research type. Three articles were obtained in the final review (Figure 1).

Figure 1. Research PRISMA flowchart.
3.2. Description of selected research articles
Research articles included were primary research, with two retrospective cohorts and one RCT article. Two retrospective cohort studies were each conducted in India and South Africa, while RCT research was conducted in Thailand (Table 1)[12-14].

Table 1. Selected article description.
Retrospective cohorts had 41 and 43 patients, respectively. The mean age in Goswami et al. study was (32 ± 8.6) years. In Arens et al. study, subjects were between 27-41 years. In Goswami et al.study, the diagnostic criteria consisted of clinical criteria (fever,headache, and focal neurological deficits), radiological features(CT scan and head MRI highly suggestive of TE), and positive toxoplasmia IgG antibodies. Arens et al.'s research chose CT scan and head MRI images characteristic of TE accompanied by clinical and radiological improvements after therapy administration as the diagnostic criteria. Subjects in both studies were divided into two groups. The first group was given P-S combination therapy.The second group in Arens et al. study was given TMX, while in Goswami et al. study was given a combination of TMX and clindamycin. Goswami et al.'s study gave pyrimethamine doses of 50 mg/day (for bodyweight < 60 kg) and 75 mg/day (for bodyweight≥ 60 kg), 4 g/day sulfadiazine, and trimethoprim (20 mg/kg/day)-sulfamethoxazole (100 mg/kg/day). Arens et al.'s study did not provide information about the dose of therapy given. Outcome parameters in both studies were a clinical improvement, mortality,and drug side effects. The outcome in Goswami et al.'s study was measured after being given therapy for two weeks, while Arens et al.'s study did not provide the subject’s length of hospital stay. The outcome in Goswami et al.'s research was divided into complete responses (there was > 50% clinical improvement after two weeks of therapy and a radiological picture showed brain lesion reduction>50% after two weeks of therapy), partial response (there was <50% clinical improvement after two weeks of therapy and a radiological picture showed brain lesion size reduction <50% after two weeks of therapy), and failed therapy (no clinical improvement, radiological picture, death, and major drugs side effects)[12,13].
The RCT article consisted of 30 subjects ranging from 19-62 years. Diagnostic criteria used were clinical manifestations(fever and focal neurological deficits) and imaging that were in accordance with TE coupled with the presence of T. gondii titer in cerebrospinal fluid. Subjects were divided into three groups, namely the group given a combination of pyrimethamine 50 mg/ day and sulfadiazine 4-6 g/ day, a combination of pyrimethamine 100 mg/ day and sulfadiazine 4-6 g/day, and trimethoprim (10 mg/ kg/day)-sulfamethoxazole (50 mg/kg/day). The primary outcome was mortality rate and the secondary outcome was drug side effects.Outcomes were measured after six weeks of therapy.
3.3. Differences in clinical response
The cohort study by Arens et al. showed that clinical response was better with TMX than with P-S but the difference was not statistically significant (full recovery 11/25 vs. 7/18, P=0.738; disability 9/18 vs.10/25, P=0.736). Goswami et al.’s cohort showed a better complete response with TMX-C regimen compared to P-S (complete response 20/25 vs. 5/16, P=0.003). RCT research by Kongsaengdao et al. does not provide clinical response data after therapy. We cannot measure the estimated magnitude of the effect (effect size) in these articles because each article had a patient, intervention, comparison, and outcome (PICO) component that was not homogeneous, so that the assessment was carried out meta-synthetically[12-14].
The selection of alternative TMX drugs compared to P-S can still be considered because it was supported by two cohort studies that showed an equally good clinical response outcome between TMX and P-S, and because is widely available. Moreover, one cohort study showed a better clinical outcome if TMX was combined with clindamycin[12-14].
3.4. Difference in mortality
The cohort study by Arens et al. showed a higher mortality rate on TMX use compared to P-S, but not statistically significant (mortality 4/25 vs. 2/18, P=1.0). In contrast, the cohort by Goswami et al.showed a lower TMX-C mortality rate than P-S even though it was not statistically significant (mortality 3/25 vs. 6/16 , P=0.063). The RCT study by Kongsaengdao et al. showed a higher mortality rate on TMX use than P-S (mortality 3/10 vs. 1/10, P=0.05)[12-14].
The estimated magnitude of the effect cannot measure all three articles due to the limited number of articles and PICO components that are not homogeneous. The assessment was carried out metasynthetically, because assessment using meta-analysis must meet the requirements of having at least >1 article with homogenous PICO component. Therapy with TMX showed a higher mortality rate in one cohort study and in one RCT study, but TMX combined with clindamycin demonstrated a lower mortality rate. All three articles have limitations because they did not clearly describe the cause of death, whether caused by TE or other causes, and the number of subjects was small. Compared to P-S, the selection of alternative TMX drugs can still be considered because current studies showed statistically meaningless mortality rates in both drug groups[12-14].
3.5. Differences in radiological features improvement
The assessment of radiological features improvement was only evaluated in Goswami et al. study. Still, this study combined radiological images with clinical improvement into one unit as a complete response, partial response, and failed therapy. One weakness of this article was that it did not describe in detail the number of subjects who experienced clinical improvement separated from imaging improvement, so researchers could not examine the number of subjects who only had radiological improvement.If only looking at the whole group, this article shows a better complete response with treatment based on TMX-C compared to P-S (P=0.003). This article cannot be measured by the estimated magnitude of the effect due to the limited number of articles so that the assessment was performed meta-synthetically[12].
Compared to P-S, the selection of alternative TMX drugs could still be considered because it was supported by a cohort study that showed a better radiological image of TMX-C use than P-S.
3.6. Differences in side effects
The study from Arens et al. established side effects of the drugs resulting in kidney dysfunction (16.7%, 3/18) and impaired liver function (11.1%, 2/18) on P-S therapy, while there were no side effects using TMX. Studies by Goswami et al. showed severe side effects with the use of P-S, including severe thrombocytopenia(25.0%, 4/16), Steven-Johnson syndrome (6.3%, 1/16), and febrile neutropenia (6.3%, 1/16). In contrast, these side effects did not occurr on TMX-C therapy. Mild side effects were reported with both drug regimens including skin rash, reversible asymptomatic neutropenia, and diarrhea. Studies by Kongsaengdao et al. show side effects of drugs such as severe skin allergies and bone marrow suppression with the use of P-S, while no side effects occurred with the use of TMX[12-14].
Three selected articles consistently showed that P-S therapy was associated with more drug toxicity compared to TMX. Hence,the selection of alternative TMX drugs compared to P-S can be considered because it was supported by two cohort studies and one RCT that presented higher rate of drug toxicity on P-S use.
3.7. Can treatment regimen without pyrimethamine become an alternative toxoplasma encephalitis therapy?
The conclusions of this study could not be assessed using the magnitude of the effect due to the limited number of articles and the PICO component between heterogeneous research articles.Conclusions were assessed meta-synthetically. Based on clinical response outcomes, mortality rates, radiological images, and drug side effects from all selected articles, it can be concluded that alternative treatment options without pyrimethamine can be considered when the standard treatment is not available. This conclusion had limitations because it cannot be assessed statistically,requiring further study with a good research design and a more significant number of subjects to produce strong recommendations.
3.8. Biased risk assessment
The risk of bias in cohort studies was evaluated using the ROBIN-1 device. In a cohort article by Goswami et al, on the domain of confounding variables, researchers have attempted to determine confounding variables such as CD4 levels before therapy application.Still, confounding variables have not been well controlled. The limitations of confounding variables in this article include:1) researchers did not explain the limit of CD4 level within the inclusion criteria; 2) patients who were included in the study were both inpatient and outpatient; 3) researchers did not describe patient’s level of consciousness before intervention was given. Based on these limitations, Goswami et al.'s research on the domain of confounding variables has a moderate level of risk bias. The participant selection domain is low risk because the selection of participants in the study group was not related to the intervention and results. The domain of intervention classification had a low risk because of its accurate presentation. The domain of intervention deviation had a low risk because there was balance in the interventions given in both groups.Researchers consistently determined the interventions given, such as the type of intervention, drug dosage, frequency, intensity, and duration of intervention given. The missing data domain in this article has a low risk. In the article, only one in 41 participants could not be evaluated due to a serious drug side effect, Steven Johnson's syndrome. Therefore, the availability of data in this article is quite good at 97.5%. The bias domain of outcome measurement has a moderate risk of bias because this article was retrospective.The investigator could recognize and be aware of the interventions received by the study participants. The bias on reporting variables for the results had low risk of bias because both groups had a clear and consistent report (Figure 2)[12].
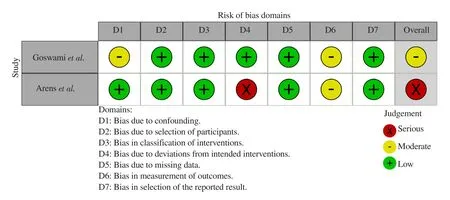
Figure 2. Summary of risk assesment of bias in cohort studies.
In Arens et al.’s cohort, the confounding variable domain had a low risk of bias because researchers attempted to determine confounding variables such as CD4 levels and Glasgow Coma Scale (GCS)score of the participants before therapy. The researcher controlled the confounding variable properly by only including participants admitted to the hospital and diagnosed with TE for the first time. The selection of participants had a low risk of bias because the selection of participants into the research group was not related to intervention and results. The domain of intervention bias classification had a low risk because the allocation of interventions was clearly explained in this article. The domain of bias for intervention deviations had a high risk because this article did not clearly define the completeness of the interventions given, such as drug dosage, frequency, and duration of intervention. The missing data domain had a low risk because there were no subjects who dropped out in the study, so data availability reached 100%. This article's bias domain of outcome measurement had a moderate risk of bias because this article was retrospective.The researcher could recognize and be aware of the interventions received by the study participants. The bias on reporting variables for the results had a low risk of bias because both groups had a clear and consistent report (Figure 2)[13].
The risk of bias in RCT studies was evaluated using ROB2 devices.RCT article by Kongsaengdao et al, in the randomization process domain, had a moderate risk because this article did not provide information about the order and process of sampling. The domain of implementing interventions in this article had a moderate risk because researchers were aware of the interventions given to each group, but on the inclusion criteria is not clear if the testing done on cerebrospinal fluid (CSF) was toxoplasma PCR or IgG serology on CSF. The missing outcome data domain had a low risk because outcome data in all research groups were available. The measurement bias of results in this article had a high risk because investigators knew the interventions assigned to the research participants, and the knowledge of interventions delivered tends to influence the research results. The bias of result’s reporting in this article had a high risk because the results reported were based on the assessment of several measurement results such as mortality rates, patient recovery without severe side effects, and side effects that occurred in study participants. The patient's recovery measurement was not clearly reported, therefore having a high risk of bias (Figure 3)[14].

Figure 3. Summary of risk assesment of bias in RCT studies.
3.9. Proof of research quality
Mortality outcome has a low certainty rate in RCT articles, and certainty is very low in cohort articles. Clinical response outcomes have a low certainty rate in RCT articles. The outcome of drugs’side effects has a low certainty level in RCT articles, and certainty is very low in cohort articles. Cohort studies have received very low certainty in the assessment of imprecision because the cohort article by Arens et al. did not describe the intervention data (Table 2)[12-14].

Table 2. Level of certainty assessment on each outcome research.
3.10. Strength of research recommendations
The strength of research recommendations was evaluated using the GRADE approach. A summary of the article quality evidence has been presented earlier in Table 2 and a narrative summary such as assessing the risk of bias for each article using ROBIN-1 and ROB2 devices, inconsistencies, indirectness, imprecision,and publication bias. The summary explained the assessments of each article transparently. Based on these assessments, it can be concluded that this study has a weak recommendation strength.A weak recommendation implies that not all individuals given the intervention will get the expected results. Careful consideration is needed in the provision of interventions and requires mutual agreement in the provision of interventions regarding potential benefits and risks to patients[11].
4. Discussion
The recommendations that could be given in our study have low grades due to some limitations, such as the small number of published articles and the high risk of overalls bias based on GRADEPro. Low-recommended research means that it can be considered to be used by clinicians with consideration of the limited main drug availability and involve patients and families in making intervention decisions regarding its potential benefits and risks to patients.
We tried to compare meta-analysis studies that have been carried out before, namely researches by Dedicoat et al, Yan et al, and Hernandez et al. The PICO component in all three previous metaanalysis studies had several similarities and differences compared to our study, including the patient component (P) in all studies have similarities, namely TE patients, intervention component (I)has a difference where in this study it is only focused on those without pyrimethamine, while the three previous studies include all interventions, control component (C) in all studies are similar,P-S and P-C, and outcome components (O) has similarities in the therapeutic response, mortality rate, and side effects of the drug[15- 17].
Research by Dedicoat et al. and Yan et al. included only RCT articles. In contrast, research by Hernandez et al. included cohort and RCT studies, thus similar with our current research. In terms of number of articles included, research by Yan et al. had an advantage over other studies because it had the highest number of RCT articles (n=11) with a total of 1 472 patients, but this study included therapeutic interventions using only pyrimethamine, which did not fit the purpose of this study. All studies had similar objectives in terms of outcomes, namely therapeutic response, mortality rates, and drug side effects[15-17].
The role of antitoxoplasma drugs is to inhibit folate metabolism,which is indispensable for the survival of parasites. The combination of anti-folate drugs used must work on different enzymes synergistically and prevent drug resistance. The enzymes that are often inhibited are DHPS and DHFR. In theory, both P-S and TMX are well combined because they work on the two different enzymes,such as pyrimethamine and trimethoprim, which work on the DHFR enzyme, whereas sulfadiazine and sulfamethoxazole work on the DHPS enzyme. Supported by several previous meta-analysis studies,our research showed that the combination of P-S had greater drug side effects than the TMX combination and equally good clinical improvement and mortality outcome. The use of other drugs than pyrimethamine, such as TMX, can be considered an alternative if the standard therapy is not available. One article showed that the TMX-C combination had a better outcome than P-S, but this article had many limitations. Further research is needed on a larger scale and with better methodology[18].
Our research contribution was including articles published within recent years (the last 15 years) compared to previous publications and providing biased assessments with the GRADEPro method in each article. Our research is also different from previous metaanalysis studies because it only includes articles that use therapeutic interventions without pyrimethamine. This study has several limitations: first, the number of articles analyzed was small, hence it is not possible to perform a meta-analysis; second, some of the articles included in this study have low-quality from the GRADEPro evaluation tool; third, radiological imaging improvement outcomes is still very limited because only one study included the outcome;and last, article selection was limited to Indonesian and English language.
We advise researchers to conduct research with larger samples and RCT design as the golden standard to minimize research bias.In addition, in both the intervention and control group therapy must be explained in detail for dosage, duration, and the route of administration. The outcome must also be clearly described to reduce research bias. Better evidence and research strength are needed for supporting alternative treatments of TE patients so that they can overcome the problem of limited availability of P-S drugs,which are currently still the standard treatment for TE.
In conclusion, alternative therapy without pyrimethamine in TE had clinical response outcomes, radiological images improvement,and mortality rates as good as the combination of therapy using pyrimethamine. The side effects of alternative drugs without pyrimethamine were lower than pyrimethamine. The level of recommendation of alternative drugs without pyrimethamine in TE is low. This choice can be considered when the main regimen is not available.
More primary researches are needed on alternative regimens on TE compared to the current primary regimens (P-S and P-C) because of the small number of research. The Indonesian government needs to reconsider providing pyrimethamine for TE therapy since there is no strong evidence of alternative therapy without pyrimethamine.
Conflict of interest statement
We declare that we have no conflict of interest.
Authors’ contributions
This research was conducted by 4 researchers, namely with the initials DS, AM, FW, and WW. Concept and design of the research was carried out by all of us (DS, AM, FW, and WW). Literature search, data collection, and data acquisition was conducted by DS.Data analysis and interpretation was carried out by DS, AM, FW,and WW. Manuscript preparation, editing, and review was carried out by DS and FW. Final approval of the version to be published was evaluated by AM and WW.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Larvicidal activity of the pericarp extract of Garcinia mangostana against dengue vector Aedes aegypti in Sri Lanka
- Comparison of Cox proportional hazards model, Cox proportional hazards with time-varying coefficients model, and lognormal accelerated failure time model:Application in time to event analysis of melioidosis patients
- Prevalence and risks of soil transmitted helminths among Ethiopian school children: A cross-sectional study
- Antimicrobial susceptibility and serotypes of Neisseria meningitidis and Streptococcus pneumoniae in Sri Lanka: Experience from the National Reference Laboratory
- Sex differences in SARS-CoV-2 infections, anti-viral immunity and vaccine responses
