Synthesis, Structure and Properties of an Iron Complex with Formally Zero Oxidation State①
2022-04-16ZHULinJunHUANGToZHANGTeng
ZHU Lin-Jun HUANG To ZHANG Teng②
a (State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China)
b (University of the Chinese Academy of Sciences, Beijing 100049, China)
ABSTRACT An unusual iron complex with formally zero oxidation state, iron bis(6,6′-dimethyl-2,2′-bipyridine),was synthesized and its crystal structure was determined. Combination of experimental and theoretical studies reveals that the electronic structure of the complex is best described as Fe(II)(Mebipy-)2 with anionic Mebipy- ligands and high-spin Fe(II) center.
Keywords: Fe(Mebipy)2, crystal structure, electronic structure, redox-active ligand;
1 INTRODUCTION
Iron complexes with low oxidation states have been intensively studied in recent years as these complexes are potential catalysts for a wide range of organic transformations such as cross coupling[1], hydrogenation and reduction[2,3], hydroboration[4], olefin oligomerization and polymerization[5,6], C-H activation and C-H functionalization[7], and so on. Moreover,low-oxidation-state iron center also plays a crucial role in natural and artificial nitrogen activation[8-12]. However, most reported Fe complexes with low oxidation state are stabilized by strong field ligands such as phosphines, carbonyl or alkyls. It usually requires harsh conditions to have Fe coordinate with these strong field ligands, which increases the cost of preparation and limits the application of those catalysts. Moreover, as strong field ligands prefer low-spin state of the central metal,high-spin states and spin transition can hardly be achieved by using those ligands. Therefore, it is of interest on fundamental research to prepare low oxidation state Fe complexes with weak field ligands. 2,2′-Bipyridine (bipy) was one of the most widely used ligands in coordination chemistry[13-15]. With medium coordination field strength from bidentate N,N donor set,the first-row transition metal complexes of bipy and bipy derivatives can adopt in either high-spin or low-spin state. While most studies of metal-bipy complexes focus on high oxidation state species, complexes with the formula of M(0)(bipy)xhave been investigated by spectroscopic and electrochemical methods[16-21]. Early reports claimed the synthesis of Fe(0)(bipy)3[22]and Co(0)(bipy)3[23], yet the lack of crystal structure makes their conclusions plausible. It also remains contradictive whether these compounds should be regarded as positive-valent metal coordinated with bipy-anions, or zero-valent metal coordinated with uncharged bipy ligands. Herein, we report the synthesis, structure and characterizations of Fe(Mebipy)2(Mebipy = 6,6′-dimethyl-2,2′-bipyridine). For the first time,we obtained the single crystal of a formal Fe(0) complex with bipy derivative ligands. Combination of experimental and theoretical studies reveal that the electronic structure of Fe(Mebipy)2can be best described as Fe(II)(Mebipy-)2with a high-spin state on the metal center. A formal Co(0) complex with a similar formula of Co(Mebipy)2has been reported by us earlier[24].
2 EXPERIMENTAL
2. 1 Materials
The reaction was carried out in an argon-filled glove box under dry, inert atmosphere. All solvents (dichloromethane,tetrahydrofuran, diethyl ether) were purified according to standard procedure to remove dissolved oxygen and residue water prior to use. Other chemicals were used as received from suppliers without further purification. Fe(Mebipy)Cl2was synthesized according to the literature procedure[25].
2. 2 Synthesis of Fe(Mebipy)2·1/3THF
To a suspension of Fe(Mebipy)Cl2(20.0 mg, 0.063 mmol)in THF, NaBEt3H (0.15 mL, 1.0 M in THF, 2.3 equiv.) was added and the reaction mixture was stirred for another 15 min.A black precipitate formed immediately. The deep green solution was then filtered and concentrated. The resulting solid was recrystallized in THF/diethyl ether at -30 ºC to afford 12 mg(yield 84% w.r.t Fe(Mebipy)Cl2) of the product as dark green crystal.
2. 3 Crystal structure determination
The crystal data were collected at 100 K on a Bruker D8 Venture diffractometer equipped with a graphite-monochromated MoKαradiation (λ= 0.71073 Å). The frames were integrated with the Bruker SAINT© build in APEX II software package using a narrow-frame integration algorithm, which also corrects for the Lorentz and polarization effects. Absorption corrections were applied using SADABS. Structures were solved by direct methods and refined to convergence by leastsquares method onF2using the SHELXTL-2013 software suite[26]. All non-hydrogen atoms are refined anisotropically except for the solvent molecule. Hydrogen atoms were fixed at the calculated positions and refined by using a riding mode.A total of 133520 reflections were collected in theθrange ofθ2.49~33.04°, of which 12554 were independent withRint=0.0491. The refinement process gave the finalR= 0.0459 andwR= 0.1083 (w= 1/[σ2(Fo2) + (0.0497P)2+ 14.2224P], whereP= (Fo2+ 2Fc2)/3) for strong reflections (I> 2σ(I)) andS=1.038.
2. 4 Spectroscopic and electrochemical measurements
UV-vis spectrum was recorded by a Shimadzu UV-2600 spectrometer under the protection of argon. Cyclic voltammetry (CV) was performed with a three-electrode system using CHI1140C electrochemical workstation in an argon-filled glove box. A glassy carbon electrode was used as the working electrode, a platinum foil as the count electrode, and a Ag+/Ag electrode (0.1 M AgNO3/MeCN) as the reference electrode.The measurements were performed in dry THF with 0.1 M tetra-n-butylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte. All voltages were corrected with respect to the Fc+/Fc redox couple.
2. 5 DFT calculation
All calculations were performed using Gaussian 16 software suite[27]. Structure optimization and frequency analysis were conducted with MN15L functional[28]and def2-SVP basis set at the unrestricted level. Single point energy calculations were performed at the MN15L/def2-TZVP levels of theory. The calculation results were further investigated and analyzed by the Multiwfn package[29].
3 RESULTS AND DISCUSSION
3. 1 Synthesis and characterization of Fe(Mebipy)2

Fig. 1. Synthesis of Fe(Mebipy)2
Fe(Mebipy)2was synthesized through the reaction of Fe(Mebipy)Cl2and NaBEt3H. Presumably, the reaction first takes place with the substitution of chloride by the hydride,followed by a reductive elimination of H2from the dihydride(Fig. 1). The Fe(0)(Mebipy) intermediate then undergoes an intermolecular ligand exchange to form Fe(Mebipy)2complex and Fe(0) nanoparticles.
Single crystal suitable for diffraction studies can be obtained through recrystallization from THF/diethyl ether mixture. The exact formula of the crystalline sample was found to be Fe(Mebipy)2·1/3THF. It crystallizes in monoclinic system,C2/cspace group witha= 25.3799(8),b= 12.2753(4),c=22.0876(7) Å,β= 107.9200(10)° andV= 6547.5(4) Å3. The asymmetric unit consists of one and a half Fe(Mebipy)2molecules and a half lattice tetrahydrofuran. Therefore, there are two crystallographically inequivalent Fe(Mebipy)2molecules in the structure, with one sitting on theC2axis and the other locating on the general position. In both molecules, Fe adopts a distorted tetrahedral coordination sphere with two bidentate bipyridine ligands (Fig. 2). Selected bond lengths and bond angles are shown in Table 1. The Fe-N bond lengths fall in the range of 1.99~2.02 Å. The N-Fe-N bond angles are in the range of 80.72~81.14° for N′s from the same bipyridine moiety and 115.88~142.26° from different bipyridine moieties, respectively.
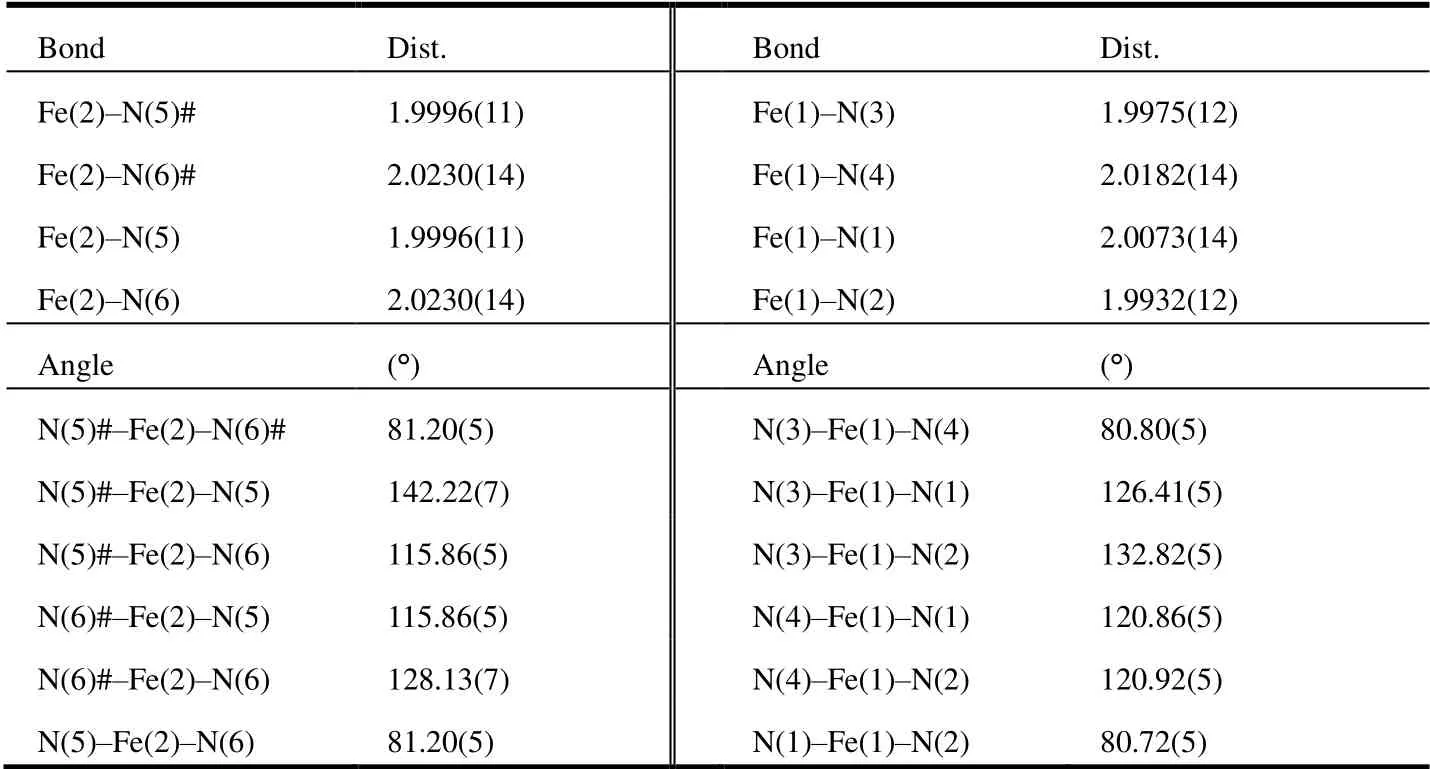
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Fe(Mebipy)2
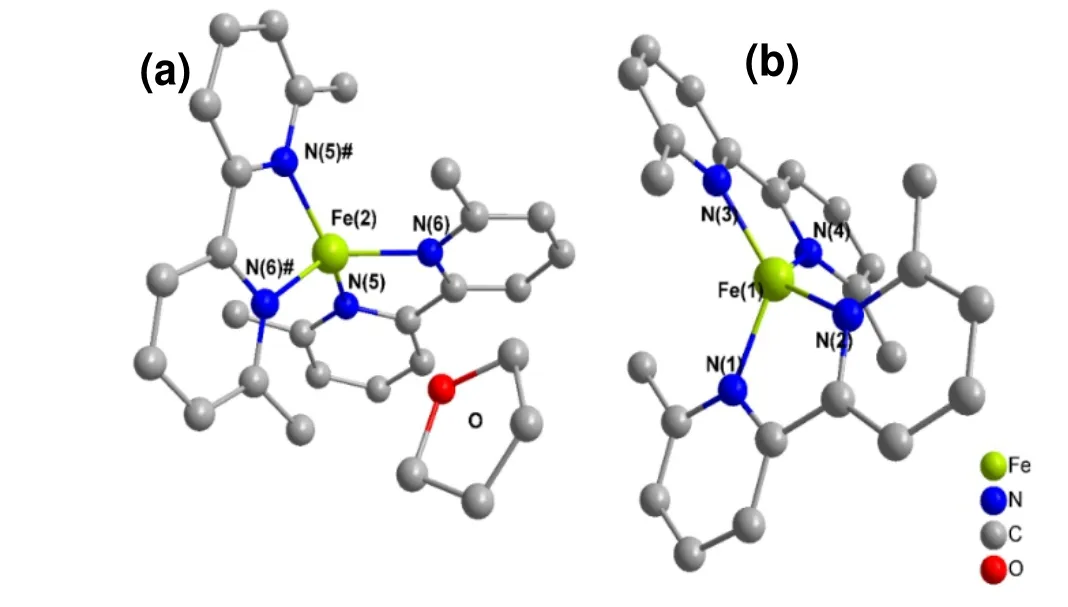
Fig. 2. Coordination environments of Fe(Mebipy)2: molecules a and b
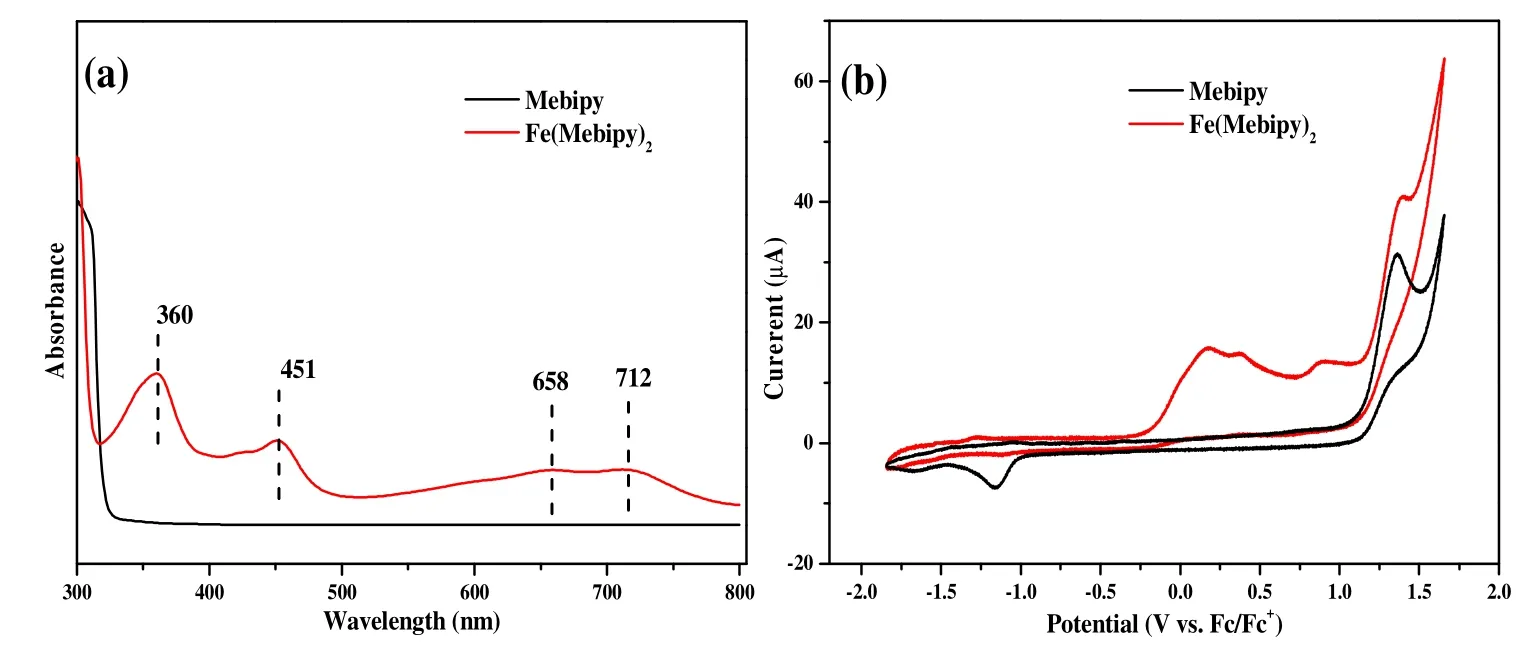
Fig. 3. UV-vis spectra (a) and cyclic volt41z
UV-vis adsorption spectrum of Fe(Mebipy)2was recorded in THF solution under inert atmosphere (Fig. 3a). Fe(Mebipy)2showed adsorption peaks at 360, 451, 658, and 712 nm, while the neutral ligand Mebipy does not show significant adsorption in the range of 330~800 nm. The broad peaks at 658 and 712 nm indicate a strong metal-to-ligand charge transfer adsorption in this low valence complex. The 360 nm adsorption is characteristic forπ-π* transition in anionic bipyridine derivatives,suggesting that the Mebipy ligands adopt -1 other than neutral oxidation state[16,30]. This assignment is consistent with the previously reported Fe(bipy)3[22], Co(bipy)2[17]and Co(Mebipy)2[24].
Fig. 3b shows the CV behavior of Fe(Mebipy)2and Mebipy in THF solution. Fe(Mebipy)2shows a characteristic oxidation peak around 0.2 V vs. Fc+/Fc, which could be assigned to the transition from Fe(Mebipy)2to Fe(Mebipy)22+. Unlike early reports on Fe(bipy)3systems, the oxidation is irreversible,which suggests the instability of Fe(Mebipy)22+probably due to the steric effect of methyl substituents.
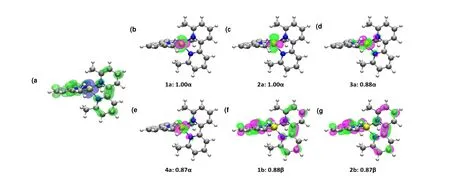
Fig. 4. (a) Spin density map of triplet Fe(Mebipy)2. Alpha and beta spin densities are represented in blue and green, respectively.(b-g) SNOs with significant alpha or beta spin occupancy
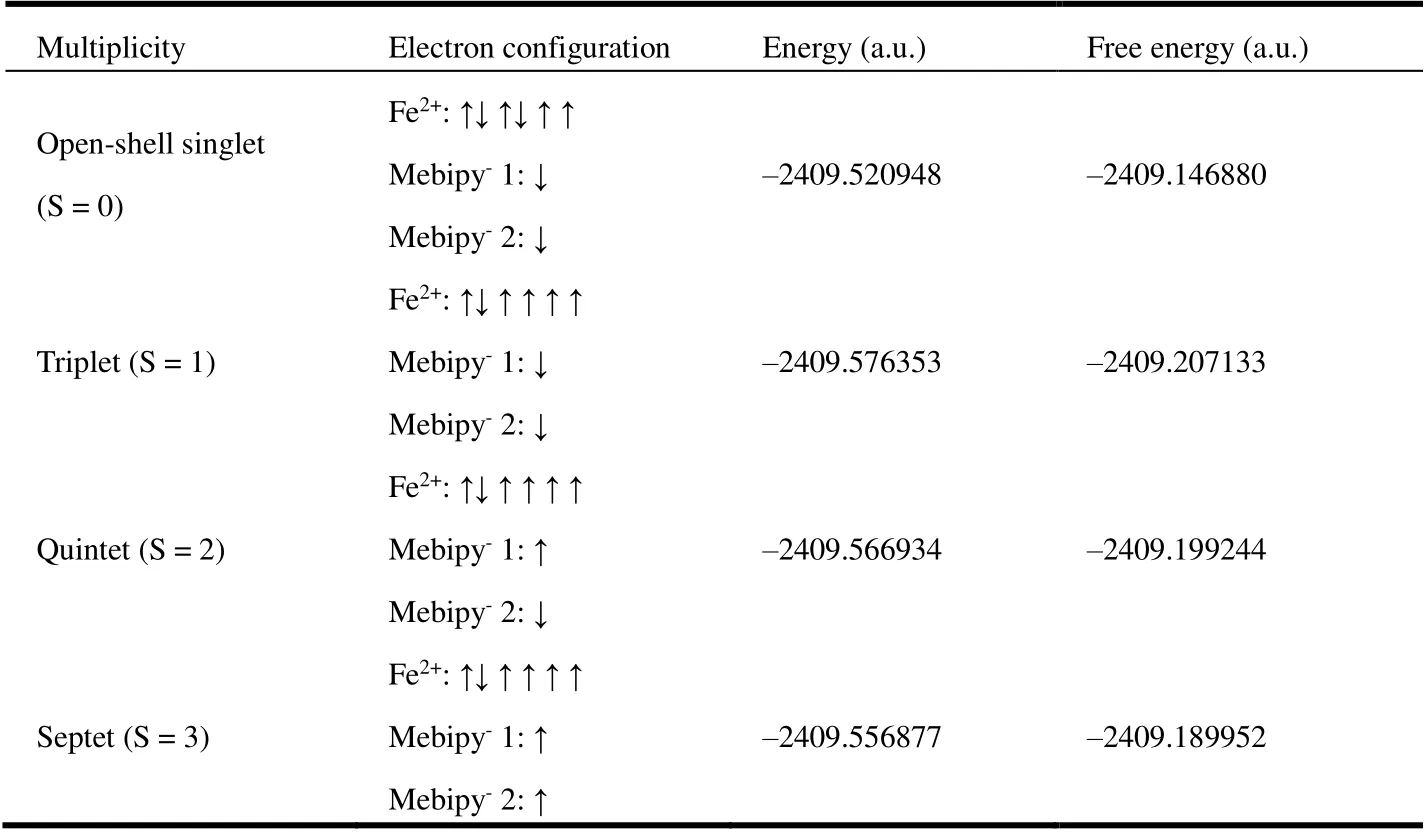
Table 2. Calculated Energy of Different Spin Multiplicity States of Fe(Mebipy)2
3. 2 Electronic structure analysis
While UV-vis spectrum and CV results suggest a bonding mode of Fe(II)(Mebipy-)2of the complex, we next performed density functional theory (DFT) calculations on the Fe(Mebipy)2molecule to gain a better understanding on its electron configuration. We first optimized the structures of Fe(Mebipy)2assuming different spin multiplicity and calculated the free energies accordingly. While the optimized geometric structures of all tested configurations are similar, the spin triplet state has the lowest energy and free energy and thus is assigned as the ground state (Table 2). We next performed spin density analysis on the complex for a deeper understanding of the electronic ground state. As shown in Fig. 4a, the complex shows both alpha and beta spin densities. Mulliken[31-33]and Hirshfeld[34,35]spin population analyses indicate that most alpha spin density locates on the Fe center with a spin population of 3.806 (Mulliken) or 3.497(Hirshfeld), while beta spin density mainly distributes over the Mebipy ligands. Spin natural orbitals (SNOs) were thus constructed to analyze the contribution of MOs to the spin density.SNO analysis reveals four orbitals with significant alpha spin occupation and two with beta spin occupation (Fig. 4). These orbitals can be approximately viewed as single occupied molecular orbitals (SOMOs). The four alpha-spin SNOs all locate on the Fe center withdsymmetry, suggesting a dominant contribution from Fe 3dorbitals. On the other hand, the beta-spin SNOs are mainlyπ* orbitals of Mebipy ligands. Therefore, the structure of Fe(Mebipy)2should be best described as a high spin (S = 2)d6Fe(II) center coordinated by two radical anionic Mebipy-ligands with antiferromagnetic coupling between the Fe(II) center and Mebipy-ligand.
The assignment of oxidation states was further confirmed by population analysis. Mulliken and Hirshfeld atomic charge calculations give positive atomic charges of 0.069 (Mulliken)and 0.248 (Hirshfeld) for Fe center, respectively, suggesting a positive oxidation state of Fe other than the formal Fe(0) (Table 3). Localized orbital bonding analysis (LOBA) also shows that Fe center possesses an oxidation state of +2 and each Mebipy ligand possesses an oxidation state of -1.

Table 3. Atomic Charge and Spin Population of Triplet Fe(Mebipy)2 Based on Mulliken and Hirshfeld Analysis
4 CONCLUSION
In this paper, we report the synthesis, structure and properties of a formal Fe(0) complex Fe(Mebipy)2. Spectroscopic and electrochemical characterizations as well as theoretical calculations support the presence of a high-spind6Fe(II) center coordinated with redox-active Mebipy ligand. The use of redox-active ligand can effectively stabilize the transition metal complexes with formally low oxidation state, and thus will open up possibilities on the synthesis and catalytic applications of those complexes.
杂志排行
结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①
