MOF-Conductive Polymer Composite Film as Electrocatalyst for Oxygen Reduction in Acidic Media①
2022-04-16ZHUGERuiXueSHIPengChaoZHANGTeng
ZHUGE Rui-Xue SHI Peng-Chao ZHANG Teng②
a (College of Chemistry and Materials Science, Fujian Normal University, Fuzhou 350007, China)
b (State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China)
c (University of the Chinese Academy of Sciences, Beijing 100049, China)
ABSTRACT A metal-organic framework (MOF)-conductive polymer composite film was constructed from PCN-222(Fe) nanoparticles and PEDOT:PSS solution by simple drop-casting approach. The composite film was tested as an electrocatalytic device for oxygen reduction reaction (ORR). The combination of PCN-222(Fe) MOF particles and conductive PEDOT matrix facilitates electron transfer in the composite material and improves the ORR performance of PCN-222(Fe). Levich plot and H2O2 quantification experiment show that PCN-222(Fe)/PEDOT:PSS film mainly catalyzes two-electron oxygen reduction and produces H2O2.
Keywords: metal-organic frameworks (MOFs), conductive polymer, porphyrin, oxygen reduction reaction(ORR), electrocatalysis; DOI: 10.14102/j.cnki.0254-5861.2011-3350
1 INTRODUCTION
Metal-organic frameworks (MOFs) are a class of 3Dporous materials composed of metal ions or clusters and organic linkers[1-7]. Owing to their high surface areas, tunable pore sizes and capability for functionalization, MOFs have found potential applications on gas adsorption[8,9], separation[10-12],chemical sensing[13,14], catalysis[15-18], drug delivery[19-22]and many other fields[23,24]in the past 20 years. Recently, electrochemical applications of MOFs have been also intensively explored[25-32]. MOFs constructed from redox-active metal nodes or redox-active linkers or decorated with redox-active functional groups have been reported as electrocatalysts for oxygen reduction, oxygen evolution or organic electrochemical transformations[33-36]. However, one major drawback of MOFs in electrochemical applications is the insulating nature of most MOF materials, which hinders electron transfer between MOF particles and electrodes[37-42]. Three strategies have been proposed to improve the conductivity of MOFs and MOF-based materials: (i) developing conductive MOFs[37];(ii) converting MOFs to MOF-derived carbon materials at high temperature[43]; (iii) constructing composite materials with MOFs and conductive matrix such as carbon[44-48], metal nanoparticles[41,49]or conductive polymers[50-53]. Among these strategies, fabrication of MOF-conductive polymer composites is of particular interest as it allows conductivity improvement as well as the retention of porous MOF structures and well-defined electroactive sites in MOF pores/channels.Moreover, the interaction between MOF particles and conductive polymer matrix can be readily tuned by introducing pendant groups on polymer backbones and MOF particle surfaces, and thus enhanced electron transfer efficiencies can be expected. Although there have been several reports on electrochemical applications of MOF-conductive polymer composites, not much efforts have been put on making such a composite film or membrane device. Such MOF-based, porous and conductive films/membranes will potentially be applied as gas-diffusive or selective electrodes. As most MOFs are ready for inner-surface functionalization, it will open up possibilities for highly selective electrochemical sensing or electrocatalysis.
In this work, we report the fabrication and oxygen reduction reaction (ORR) catalytic activity of PCN-222(Fe)/PEDOT:PSS (PEDOT = poly(3,4-ethylenedioxythiophene),PSS = polystyrenesulfonate) film. PCN-222(Fe) is built from redox-active Fe-porphyrin-derived linkers and reported to be active for electrochemical sensing applications[50]. The selfdoping PEDOT:PSS matrix can exhibit conductivity up to 3000 S⋅cm-1upon acid treatment[54,55], which facilitates electron transfer between the electrodes and MOF particles. As a result, the MOF-conductive polymer composite film device showed enhanced electrocatalytic activities compared with the bare PCN-222(Fe) particles.
2 EXPERIMENTAL
2. 1 Materials
PEDOT:PSS (Clevios PH 1000, 1.3 wt.% in water) solution was purchased from H. C. Starck. Nafion® (5 wt.% in lower aliphatic alcohols and water) solution was purchased from Sigma-Aldrich. All other reagents were used as received from multiple suppliers (Adamas, Sinopharm, Strem, etc.)without further purification.
2. 2 Preparation and activation of PCN-222(Fe)
PCN-222(Fe) nanoparticles were synthesized via a solvothermal method with modified literature procedure[56,57]. In a typical procedure, 20 mg (0.86 mmol) of ZrCl4was dissolved in 4 mL DMF in a 20 mL Pyrex vial, to which a mixture of 100µL H2O, 20 mg FeTCPPCl and 240 µL (4.2 mmol) acetic acid was added. The resulting solution was then put in a preheated oven at 120 °C for 15 min. After the reaction mixture was cooled to room temperature, PCN-222(Fe) nanoparticles were separated by centrifugation and subsequently washed with fresh DMF and acetone for several times. The obtained solid was then heated in a DMF/8M HCl mixture at 120 °C for 12 hours to completely remove the unreacted ligands and zirconium species, followed by guest exchange with acetone and vacuum-drying to give activated PCN-222(Fe).
2. 3 Preparation of the working electrodes
In a typical procedure, 7 mg of PCN-222(Fe) nanoparticles and 40µL Nafion® solution were added to 1 mL of PEDOT:PSS aqueous solution and sonicated for 1 h to prepare the PCN-222(Fe)/PEDOT:PSS ink (referred to as the “ink” below). 2.2 µL of the ink (7 mg⋅mL-1) was drop-cast onto a polished 3-mm glassy carbon electrode (S = 0.07 cm2), dried under ambient conditions and treated with 50% H2SO4for 10 min. Subsequently, the electrode was washed with deionized water to remove excess H2SO4and dried under room temperature. For RDE measurements, similar procedure was applied except that 7.4 µL of the ink was drop-cast onto the 5.6-mm glassy carbon disk electrode (S = 0.25 cm2).
2. 4 Electrochemical measurements
Cyclic voltammograms (CVs) were recorded on an electrochemical workstation (CHI1140C, CH Instrument). Aqueous solution of H2SO4(0.5 M, pH= 0.52) was used as the electrolyte in all LSV and CV measurements. Standard threeelectrode setup was applied with a carbon rod counter electrode and a Ag/AgCl/KCl(sat.) reference electrode. Modified glass carbon electrodes (see above) were used as the working electrode. Rotating disk electrode (RDE) measurements were carried out on an electrode rotator (Pine Instrument). Linear sweep voltammograms (LSVs) of RDE measurements were recorded at different rotation rates with a potential scan rate of 10 mV⋅s-1.
In RDE experiments,nwas calculated by Levich equation:
jL= 0.20nFCO(DO)2/3ω1/2ν-1/6
Here,jLrepresents the limiting current density; n is the average electron transfer number in ORR; ω is the rotation rate in the unit of rpm;Fis the Faraday constant (96485 C⋅mol-1);CO, DOand νrepresent the concentration of O2(1.1 × 10-6mol⋅cm-3), the diffusion constant of O2(1.9 × 10-5cm2⋅s-1)and the kinematics viscosity of the solution (0.01 cm2⋅s-1),respectively.
2. 5 Quantification of hydrogen peroxide
Control potential electrolysis was performed in 0.5 M H2SO4(aq) using a PCN-222(Fe)/PEDOT:PSS film-coated glassy carbon working electrode. Catholyte and anolyte (25 mL each) regions were separated by a Nafion 117 film. After one-hour electrolysis at -0.25 Vvs. RHE, 1.0 mL of catholyte was mixed with excess Ce(IV) solution (2.0 mL, 140µM),while the concentration of residue Ce(IV) was determined by UV-vis spectrometry at 320 nm.
The amount of hydrogen peroxide produced in electrolysis was calculated according to the consumption of cerium sulfate:
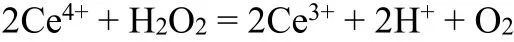
3 RESULTS AND DISCUSSION
3. 1 Fabrication and characterization of the composite film
The formation of pure PCN-222(Fe) phase was confirmed by powder X-ray diffraction (PXRD) patterns (Fig. 1a). Scan electron microscopic (SEM) images (Fig. 2a) showed that the obtained nanoparticles are in rugby shape with an average diameter of 174 nm and length of 348 nm. Inclusion of PCN-222(Fe) particles in the composite film was confirmed by multiple characterization. Inductively coupled plasma optical emission spectroscopic (ICP-OES) analysis (Table S1)gave a Fe content of 1.4% and a Zr content of 6.54% for PCN-222(Fe)/PEDOT:PSS film. Assuming all Fe and Zr elements in the composite film come from PCN-222(Fe), it is calculated that PCN-222(Fe) particles weigh 44 wt.% of the composite film, consistent with the calculation based on material feed (36~43 wt.%). While PEDOT:PSS-only film showed no significant XRD signal, the PCN-222(Fe)/PEDOT:PSS film exhibits characteristic XRD peaks for PCN-222(Fe), showing that the MOF nanoparticles are successfully included in the film and stable under acidic conditions (Fig. 1a). FT-IR spectrum of the composite film (Fig. 1b)displays characteristic 2927 and 999 cm-1peaks for PCN-222(Fe), which corresponds to C-H and Fe-N stretching vibration, respectively[50,58,59]. The morphology of PCN-222(Fe)/PEDOT:PSS film was also explored by scanning electron microscopy (SEM). As shown in Fig. 2b, PCN-222(Fe) nanoparticles distribute evenly in the composite film.The particle distribution was also confirmed by EDS elemental mapping measurements (Fig. S2). Cross-section SEM images shows that the thickness of PCN-222(Fe)/PEDOT:PSS film is approximately 3.5 µm (Fig. 2d), slightly thicker than pure PEDOT:PSS film, which is about 2 µm (Fig. 2c).

Fig. 1. (a) XRD patterns of simulated PCN-222(Fe), as-prepared PCN-222(Fe), PEDOT:PSS film and PCN-222(Fe)/PEDOT:PSS film.(b) FTIR spectra of PCN-222(Fe), PEDOT:PSS film and PCN-222(Fe)/PEDOT:PSS film

Fig. 2. (a, b) SEM images of (a) PCN-222(Fe) and (b) PCN-222(Fe)/PEDOT:PSS film. Inset: distribution of particle size.(c, d) Cross-section SEM images of (c) PEDOT:PSS film and (d) PCN-222/PEDOT:PSS film
3. 2 Electrochemical oxygen reduction on the composite film
The electrochemical performance of PCN-222(Fe)/PEDOT:PSS film was investigated in nitrogen or oxygen saturated 0.5 M H2SO4with a three-electrode system. Under nitrogen atmosphere, PEDOT:PSS film showed a broad reduction signal down to -0.142 Vvs.reversible hydrogen electrode (RHE) and the corresponding oxidation at -0.122 Vvs.RHE, which are attributed to the reduction and oxidation of PEDOT backbones (Fig. 3a). PCN-222(Fe)/PEDOT:PSS film showed a reduction peak at 0.078 Vvs.RHE, which corresponds to Fe(III)TCPP/Fe(II)TCPP reduction[60-62](Fig. 3a). In anodic scan, the oxidation peak appears at 0.048 Vvs.RHE due to the coexistence of Fe(II)TCPP/Fe(III)TCPP and PEDOT backbone oxidation processes. Both reduction and oxidation peaks on PCN-222(Fe)/PEDOT:PSS film showed linear dependence with CV scan rates (Figs. 3d and S3), consistent with the presence of surface-bounded electroactive Feporphyrin species. When PCN-222(Fe) nanoparticles were deposited directly on glassy carbon electrode without making the composite film, no obvious oxidation or reduction peaks were observed (Fig. 3a). In oxygen-saturated 0.5 M H2SO4electrolyte (Fig. 3b), the oxygen reduction peak on PCN-222(Fe)/PEDOT:PSS film electrode appears at 0.156 Vvs.RHE with a peak current density of 2.18 mA·cm-2. The ORR peak currents showed typical diffusive behavior with linear dependence with the square root of CV scan rates (Fig. 3f),showing that the final electroactive species is dissolved oxygen. In this case, the FeIII/IIreduction peak cannot be clearly differentiated due to the rapid reaction between the generated Fe(II)TCPP and O2[60]. A well-defined adsorptive Fe(II)TCPPFe(III)TCPP oxidation peak is found at 0.083 Vvs.RHE(Fig. 3b) during anodic scans, indicating good reversibility of the FeIII/IIreduction process. Remarkably, oxygen reduction on PCN-222(Fe)/PEDOT:PSS film takes place at more positive potential and exhibits a higher peak current density than those of PCN-222(Fe) nanoparticles (0.043 Vvs.RHE; 0.64 mA·cm-2). These results clearly indicate that the ORR performance of PCN-222(Fe) is significantly improved through inclusion in conductive PEDOT:PSS matrix. Such enhanced electrochemical performance of MOF-conductive polymer composite can be attributed to better electron transfer rate between the MOF nanoparticles and electrode through conductive polymer matrix.H2O2yield of 8.6% at the potential range below 0.14Vvs.RHE (Fig. S6). This result is inconsistent with the above RDE results, which suggests a two-electron process that dominates the ORR process on PCN-222(Fe)/PEDOT:PSS film electrode.Therefore, we next quantified the production of H2O2by Ce(IV) spectrometric titration. Electrolysis at -0.25 Vvs.RHE gave a current density of about 0.5 mA⋅cm-2(Fig. 4d).After one-hour electrolysis, the catholyte was collected and analyzed by UV-vis spectrum. The production rate and Faraday efficiency of hydrogen peroxide production are calculated to be 57.4 mmol⋅g-1⋅h-1and 76%, respectively. The UV-vis quantification results correspond to annvalue of 2.5,slightly higher than that from Levich plot but inconsistent with RRDE. We thus conclude that RRDE is not an accurate technique for hydrogen peroxide detection in our system,probably because hydrogen peroxide can be trapped in the MOF pores and fails to diffuse rapidly into the electrolyte. In this scenario, the apparent ring collection efficiency is lower,and hydrogen peroxide cannot be detected quantitatively by the ring electrode. This H2O2trapping effect also accounts for the lower H2O2production efficiency as detected in UV-vis experiment than in Levich plot. In a word, it can be concluded that PCN-222(Fe)/PEDOT:PSS film is a two-electron ORR catalyst in acidic media. The absence of a strong interaction between MOF and PEDOT matrix may limit the rate of electron transfer and the reduction of H2O2.Introduction of pendant carboxylic groups on polymer backbones could possibily enhance the interaction of polymer and MOF particles, and thus improve the performance of composite material.
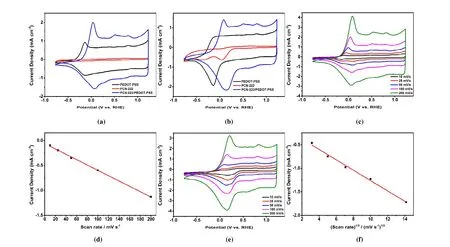
Fig. 3. (a, b) Cyclic voltammograms (CVs) of PCN-222(Fe), PEDOT:PSS film and PCN-222(Fe)/PEDOT:PSS film in (a) N2 or (b) O2-saturated 0.5 M H2SO4 electrolyte at a scan rate of 100 mV·s-1. (c, e) The CVs of the PCN-222(Fe)/PEDOT:PSS film in (c) N2 or (e) O2-saturated 0.5 M H2SO4 electrolyte at different scan rates (10~200 mV·s-1). (d) The plot of reduction peak current density (jp) vs.scan rate (v) in N2-saturated 0.5 M H2SO4 electrolyte. (f) The plot of reduction peak current density (jp) vs.the square root of scan rate (v 1/2) in O2-saturated 0.5 M H2SO4 electrolyte

Fig. 4. (a) RDE linear sweep voltammograms (LSVs) of PCN-222(Fe)/PEDOT:PSS film, PEDOT:PSS film, PCN-222(Fe) and Pt/C in O2-saturated 0.5 M H2SO4 electrolyte at a rotation rate of 1600 rpm. (b) RDE LSVs of PCN-222(Fe)/PEDOT:PSS film in O2-saturated 0.5 M H2SO4 electrolyte at various rotation rates. (c) Levich plots (jL vs. ω1/2) of PCN-222(Fe)/PEDOT:PSS film.(d) I-t curves of PCN-222(Fe)/PEDOT:PSS at -0.25 V vs RHE
4 CONCLUSION
In this study, we constructed a MOF-conductive polymer composite film by combining PCN-222(Fe) particles and conductive PEDOT:PSS polymer. The conductive PEDOT:PSS matrix is expected to facilitate electron transfer between electrode and PCN-222(Fe) particles and lead to better electrocatalytic performance. As a proof-of-concept study, we tested the ORR activity of the composite film, which showed enhanced performance compared with PCN-222(Fe) particles or PEDOT:PSS only film. This study thus provides an example how conductive polymer matrix can improve the electrochemical performance of intrinsically non-conductive MOFs.We believe that the fabrication of MOF-conductive polymer composite materials will open up the possibilities for MOFs in multiple electrochemical applications such as electrocatalysis, energy storage and electrochemical sensing.
杂志排行
结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①
