Net ecosystem carbon exchange for Bermuda grass growing in mesocosms as affected by irrigation frequency
2022-04-16YuanLIGabrielMOINETTimothyCLOUGHJohnHUNTandDavidWHITEHEAD
Yuan LI∗Gabriel Y.K.MOINETTimothy J.CLOUGHJohn E.HUNT and David WHITEHEAD
1Department of Soil and Physical Sciences,P.O.Box85084,Lincoln University,Lincoln 7647(New Zealand)
2Manaaki Whenua-Landcare Research,P.O.Box69040,Lincoln 7640(New Zealand)
3Present address:State KeyLaboratoryof Grassland Agro-ecosystems,College of Pastoral Agriculture Science and Technology,Lanzhou University,Lanzhou 730020(China)
4Present address:Soil BiologyGroup,Wageningen Universityand Research,P.O.Box47,Wageningen 6700 AA(The Netherlands)
ABSTRACT Intensification of grazed grasslands following conversion from dryland to irrigated farming has the potential to alter ecosystem carbon(C)cycling and affect components of carbon dioxide(CO2)exchange that could lead to either net accumulation or loss of soil C.While there are many studies on the effect of water availability on biomass production and soil C stocks,much less is known about the effect of the frequency of water inputs on the components of CO2 exchange.We grew Bermuda grass(Cynodon dactylon L.)in mesocosms under irrigation frequencies of every day(I1 treatment,30 d),every two days(I2 treatment,12 d),every three days(I3 treatment,30 d),and every six days(I6 treatment,18 d,after I2 treatment).Rates of CO2 exchange for estimating net ecosystem CO2 exchange(FN),ecosystem respiration(RE),and soil respiration(RS)were measured,and gross C uptake by plants(FG)and respiration from leaves(RL)were calculated during two periods,1–12 and 13–30 d,of the 30-d experiment.During the first 12 d,there were no significant differences in cumulative FN (mean±standard deviation,61±30 g C m−2,n=4).During the subsequent 18 d,cumulative FN decreased with decreasing irrigation frequency and increasing cumulative soil water deficit(W),with values of 70±22,60±16,and 18±12 g C m−2 for the I1,I3,and I6 treatments,respectively.There were similar decreases in FG,RE, and RL with increasing W,but differences in RS were not significant.Use of the C4 grass growing in a C3-derived soil enabled partitioning of RS into its autotrophic(RA)and heterotrophic(RH)components using a 13C natural abundance isotopic technique at the end of the experiment when differences in cumulative W between the treatments were the greatest.The values of RH and its percentage contributions to RS (43%±8%,42%±8%,and 8%±5%for the I1,I3,and I6 treatments,respectively)suggested that RH remained unaffected across a wide range of W and then decreased under extreme W.There were no significant differences in aboveground biomass between the treatments.Nitrous oxide(N2O)emission was measured to determine if there was a trade-offeffect between irrigation frequency and increasing W on net greenhouse gas emission,but no significant differences were found between the treatments.These findings suggest that over short periods in well-drained soil,irrigation frequency could be managed to manipulate soil water deficit in order to reduce net belowground respiratory C losses,particularly those from the microbial decomposition of soil organic matter,with no significant effect on biomass production and N2O emission.
KeyWords: 13C natural abundance,CO2 exchange,N2O emission,soil heterotrophic respiration,water deficit
INTRODUCTION
Conversion of non-irrigated grasslands to high-intensity grazing systems with irrigation is a major land-use change in dryland areas of New Zealand to increase feed supply for cattle during periods with low rainfall(MacLeod and Moller,2006).However,despite increased aboveground production(Condronet al., 2014), there is increasing evidence that irrigation in grazed grasslands of New Zealand leads to a decrease(Houlbrookeet al.,2008;Mudgeet al.,2017)or no change(Condronet al.,2014;Kelliheret al.,2015)in soil carbon(C)stocks when compared with stocks at adjacent non-irrigated sites.Conversely,in humid environments similar to those in New Zealand,the effects of irrigation on soil C stocks are inconsistent(Trostet al.,2013).Recognition of the need to increase soil C stocks to improve soil quality and offset greenhouse gas emissions(Rumpelet al.,2018)means that it has become urgent to investigate the processes regulating inputs and losses of C in irrigated grasslands to inform managers about the most effective practices to minimise C losses.
Irrigation increases water content in root zone, which alters the rates of photosynthesis and respiration in grasslands (Scottet al., 2009; Hussainet al., 2015).Irrigation also modifies the microbial processes that regulate C cycling(Entryet al.,2008;Trostet al.,2013;Karlowskyet al.,2018)and thus changes the components of C balance(Moinetet al., 2017; Whiteheadet al., 2018).The magnitude of theproportional changes in ecosystem respiration(RE)and photosynthesis in response to changes in soil water availability may not be the same,leading to changes in net ecosystem CO2exchange (FN).Studies at the field scale using eddy covariance estimates of net CO2exchange have shown that gross photosynthesis(FG)is more sensitive to water availability thanRE(Schwalmet al.,2010;Huntet al.,2016).This,in part,may be due to differences in the allocation of photosynthates to roots and shoots,subsequently affecting the relative proportions of aboveground respiration from leaves(RL)and root respiration(RA).Therefore,the ratio ofRLtoRAmay not be linear with changes in soil water availability(Mokanyet al., 2006).Water availability also has proportionally different effects on autotrophic respiration from roots(i.e.,RA), which depend on the supply of carbohydrates from photosynthesis(Huxmanet al.,2004)and heterotrophic respiration(RH)from the decomposition of soil organic matter(SOM)(Moinetet al.,2016a;Zhouet al.,2016;Zhanget al.,2019),two components that comprise soil respiration(RS).To address this,it is important to partition the effects of treatments onRAandRH.Partitioning can be done by calculating the difference between the isotopic signature of13CO2respired from roots of C3plants and that from SOM after long-term growth of C4plants(Uchidaet al., 2010) using a natural abundance C isotope technique(Moinetet al.,2016b).
A meta-analysis of global data showed that droughtinduced soil water deficits resulted in decreases in aboveground and belowground net primary production,plant C pools,andRSand its components,while irrigation induced increases in these variables(Zhouet al.,2016).Photosynthesis and plant respiration are strongly regulated by stomatal and enzymatic processes,and a broken-stick model has long been used to describe a limit for soil water deficit, below which there is no change inFG,RL,RE,and thusFNand above which there is a linear decrease(Periet al.,2003).
Since the interacting microbial processes that regulate C cycling are sensitive to soil water content,there is an opportunity to schedule the frequency of irrigation to minimise microbially driven SOM decomposition rate.Increased water availability under irrigation could lead to increased input of C into the soil(Kochsieket al.,2009;Whiteheadet al.,2018).However,high soil water content also enhances SOM decomposition rate(Schipperet al.,2013;Condronet al.,2014)by increasing soil C availability for microbial activity(Trostet al., 2013; Fuchsluegeret al., 2014), especially during periods when soil temperature is high(Mudgeet al.,2017).Studies at irrigated sites have shown increases in nitrous oxide(N2O)emission following nitrogen(N)fertiliser application, which can account for up to 90% of annual N2O loss when denitrification occurs in soil where high water content results in anaerobic conditions(Scheeret al.,2008;Mumfordet al.,2019),especially following irrigation(Owenset al.,2016).As soils dry and become aerobic,N2O emission gradually declines(Kirkmanet al.,2001;Balaineet al.,2016).Therefore,it is important to measure both net CO2and N2O exchanges in response to irrigation to quantify the effect on all greenhouse gas emissions and ensure that there is no net trade-offeffect.
This study investigated the effect of irrigation frequency on the components of CO2exchange using a model grassland system growing in mesocosms under controlled conditions.Estimates of the CO2exchange components for plants and soil were made from measurements taken over a period of 30 d.The use of growing C4Bermuda grass(Cynodon dactylonL.)on soil from a site of continuous C3grassland in mesocosms allowed partitioning of belowground respiration sources from measurements of the natural abundance of the13C stable isotope at the end of the experiment.Concurrent measurements of N2O emission from soil in the same mesocosms were made.It was hypothesised that increasing cumulative soil water deficit(W)resulting from decreasing irrigation frequency would lead to:i)decreasedFNbecause of reduced photosynthesis and a less sensitive decrease in plant and soil respiration, ii) an increased ratio of root to soil heterotrophic respiration(RA/RH)comprisingRS,and iii)decreased soil N2O emission because of increased soil aerobic conditions.
MATERIALS AND METHODS
Mesocosm preparation and experimental design
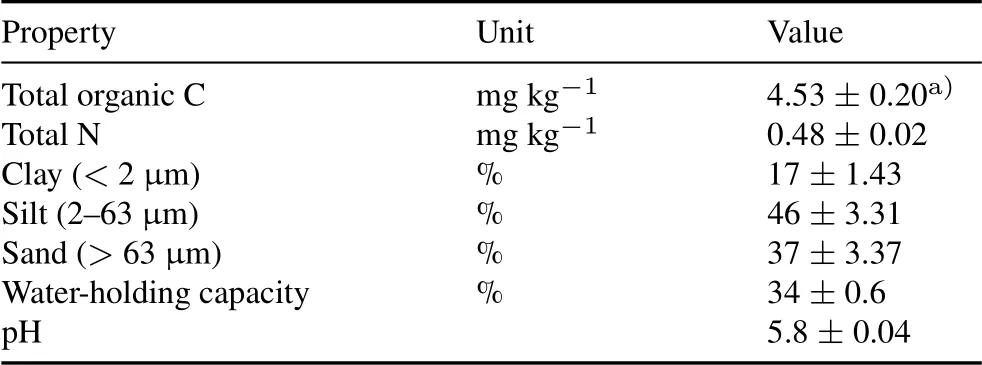
TABLE ISelected physical and chemical properties of the soil used
Fifteen Bermuda grass seeds(10 g m−2,PGG Wrightson Seeds,Christchurch,New Zealand)were sown outside each PVC collar.The mesocosms were then placed in a growth cabinet(Model HGC 1514,Weiss Gallenkamp,UK)set at constant conditions of air temperature of 25°C,photoperiod of 16h at an irradiance (400–700 nm) of 600–650 µmol m−2s−1,ad relative humidity of 70%.The grass was left to establish and grow with a plentiful water supply for 12 weeks.At the start of the experimental period,the grass was clipped to a height of 30 mm above the soil surface,and ammonium sulphate solution (1 mol L−1) was applied to supply N equivalent to 50 kg N ha−1After a further six days,the mesocosms were watered to ensure that the soil water content remained constant at field capacity.The mesocosms were then weighed to measure water loss from evaporation,and this amount of water was supplemented to return the soil water content to field capacity(mean±standard deviation,34%±0.6%).In the first 12 d,the irrigation frequency was either each day(I1treatment),every two days(I2treatment),or every three days(I3treatment).After day 12,there were no significant differences in the components of CO2exchange among the three treatments,so the I2treatment was modified to a lower irrigation frequency of every 6d(I6treatment),and all treatments were continued for a further 18 d.Over the 30-d experiment,the growth rate of the Bermuda grass was high, typical of periods between grazing under field conditions.There were four replicate mesocosms for each treatment, and the mesocosms were placed on saucers to allow the plants to access all water supplied with no losses from drainage.
Soil volumetric water content(θv)was measured every 15 min in each mesocosm using sensors(a CS 616reflectometer,Campbell Scientific,Logan,USA)connected to a data logger and multiplexer(Model CR 1000,AM416,Campbell Scientific,Logan,USA)installed diagonally across the soil profile in two replicate mesocosms for each treatment.A three-point linear calibration(0.1,0.25,and 0.4 m3m−3)with accuracy ranging from−4%to 6%was used to calibrate the soil water content sensors.Daily soil water deficit(W)was calculated from the difference between the mass of each mesocosm for soil at field capacity and the actual mass measured on any given day.This difference in mass was due to water loss,and the mass of water lost was converted into a depth of water using the mesocosm soil surface area and summed to give values of cumulativeW.At the end of the experiment,the aboveground biomass was cut to 30 mm above the soil surface,dried at 60°C,and weighed to determine the total aboveground dry matter production during the experimental period.
Measurements of FN,RS,and N2O emission
Net ecosystem CO2exchange is the difference betweenFGandRE,REcomprisedRLandRS,andRScomprisedRAandRH:

Net ecosystem CO2exchange was determined on each mesocosm under full irradiance (400–700 nm) of 600–650 µmol m−2s−1.The rate of change in CO2partial pressure was measured within a purpose-built cylindrical polycarbonate chamber(200 mm diameter,210 mm height)placed on the top of each mesocosm over a period of 2 min as described by Moinetet al.(2016b).We adopted the terminology that positive values ofFNindicate net uptake of CO2.The measurement was repeated to estimateREby excluding light using a dark cloth placed over the mesocosm and chamber.Then,RSwas measured by placing a chamber from an automatic soil respiration system(Model LI-8100,LI-COR Inc.,Lincoln,USA)on the central collar in each mesocosm.Estimates ofFGandRLwere calculated from Eqs.1 and 2.Subsequently, the central collar was sealed with a gas-tight lid fitted with a two-way stopcock, and a 25G hypodermic needle was used to remove gas samples(10 mL)for measurements of N2O partial pressure at 0,30,and 60-min intervals after the lid was sealed.These samples were injected into previously evacuated 6mL Exetainer®vials (Labco Ltd., High Wycombe, UK) for analysis with gas chromatography(SRI-8610,Torrance,USA)equipped with a63Ni electron capture detector.The rate of increase in N2O partial pressure was used to calculate N2O emission following Hutchinson and Mosier (1981).Measurements of net ecosystem CO2exchange,RS, and N2O emission were made each day over 30 d and summed to obtain their cumulative values.
Partitioning the sources of soil respiration
At the end of the experimental period when differences inWbetween the treatments were the greatest, the natural abundance of13C (δ13C) isotope technique was usedto partitionRSintoRHandRA.The technique requires the measurement of13C isotopic signatures of the CO2respired from the undisturbed soil(δ13CRS)and the isotopic signatures of roots(δ13CRA)and root-free soil(δ13CRH).Measurement ofδ13CRSfrom each treatment was made by collecting air respired from the soil surface using a partially automated open chamber system described in detail by Midwoodet al.(2008).The chambers were placed on the central collar in each mesocosm,and CO2-free air was supplied at a variable rate to maintain the CO2partial pressure inside the chamber constant at 500µmol mol−1.After an equilibration period of about 90 min,500-mL respired air was collected into gas-tight sample bags(Tedlar®Keika Ventures,Chapel Hill, USA)that were flushed twice with CO2-free air and evacuated before use.The gas was analysed forδ13C values with a cavity ringdown spectrometer(model G2121-I,Picarro Inc.,Santa Clara,USA).Theδ13C signature for the reference gas was calibrated using Pee Dee Belemnite(PDB)as the certified standard.
Immediately after the measurement ofδ13CRS, the mesocosms were sampled destructively for collection of roots and soil samples.Plant roots were separated from the soil, washed with deionised water, and dried in an oven at 65°C.One composite soil sample was taken from each mesocosm,and a subsample was freed from root material and dried in an oven at 105°C.All oven-dried materials were ground in a ball mill,andδ13C composition was measured with a continuous-flow isotope ratio mass spectrometer(Model CFIRMS, Sercon 20-22, Sercon, Cheshire, UK)interfaced with a TGII cryofocusing unit(Sercon,Cheshire,UK)to determine the values ofδ13CRAandδ13CRH.
The proportion of respiration derived fromRH, fRH,andRHwere calculated using a mass balance approach(Millardet al.,2010;Moinetet al.,2016a,b):

Data analyses
The effects of irrigation frequency on cumulativeFN,FG,RE,RS,RL,and N2O emission and values ofRAandRHwere tested in a one-way analysis of variance(ANOVA).Differences between treatments were then compared using Tukey’s honestly significant difference(HSD)test in the agricolae package of R(De Mendiburu,2014).Relationships ofFN,FG,andREwith increasingWwere fitted using a broken-stick linear regression model with a threshold value forWbelow which the variables remained constant and above which the values began to decrease(Sadras and Milroy, 1996).Analyses were done using the segmented package for R that estimates the threshold value and regression slopes(Muggeo,2008).The response ofRStoWis a complex process involving bothRAandRHthat cannot be explained simply by linear regression,and a quadratic model was found to provide an appropriate fit to these data.
12. Fiddler: Usually to dream of a fiddle8 foretells harmony in the home (Miller 234). Marie-Louise von Franz writes, the animus appears to be poor and often never revels54 the great treasures of the unconscious which are at his disposal (173). The fiddler (Thrushbeard) being the animus here.Return to place in story.
RESULTS
Soil water content and cumulative W
As anticipated,soilθvvalues decreased gradually during the period when no water was applied and then returned to a value between 0.32 and 0.35 m3m−3immediately after water was applied(Fig.1).The minimum values reached forθvdecreased with decreasing irrigation frequency to 0.23,0.20,0.18,and 0.08 m3m−3in the I1,I2,I3,and I6treatments,respectively.The pronounced effects of the I6treatment on CO2exchange components following the lack of response in the I2treatment after the first 12 d supported the decision to introduce the new treatment.As a further indicator of treatment effects and associated differences in soil water content and soil anaerobic conditions,calculations of water filled-pore space showed a decreasing range from 54%to36%with decreasing irrigation frequency.Mean values of relative gas diffusivity calculated following Moldrupet al.(2013)were 0.027,0.033,0.041,and 0.066in the I1,I2,and I6treatments,respectively.
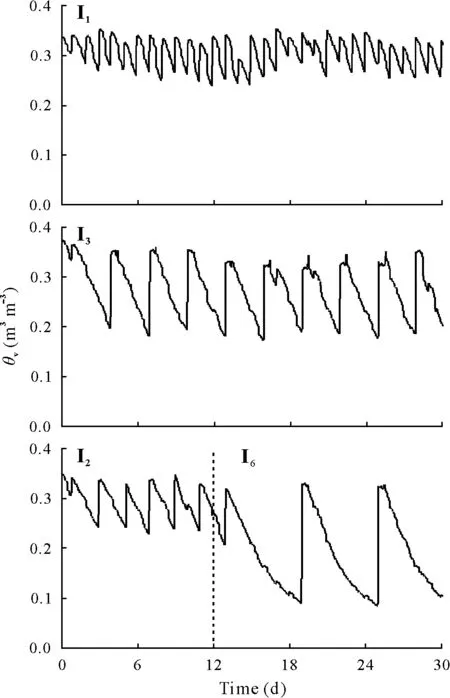
Fig.1 Soil volumetric water content(θv)in the 30-d experiment in mesocosms under irrigation frequencies of every day (I1 treatment, 30 d),every two days(I2 treatment,12 d),every three days(I3 treatment,30 d),and every six days(I6 treatment,18 d,after I2 treatment)(n=4).
Over the course of the two periods of measurement(12 and 18 d), values of cumulativeWcalculated from summing daily values ofθvincreased with decreasing irrigation frequency(Table II).At the end of the 30-d experiment,the cumulativeWin the I1treatment was 3–4-fold that of the I6treatment.
Aboveground biomass production
There were no significant changes in aboveground dry matter biomass production with irrigation frequency over the 30-d experimental period.The mean±standard deviation values of the aboveground dry matter biomass production were 660.4±71.9,598.9±18.0,and 571.7±34.7 g m−2for the I1,I3,and I6treatments,respectively.
Responses of CO2 exchange components to W
Preliminary analysis of the data indicated that the responses of the CO2exchange components to increasingWusing the broken-stick model estimated the threshold values below which the components remained constant to be 29,28,and 29 mm forFN,FG,andRE,respectively(Fig.2a).AsWincreased beyond these critical values,the values ofFNdeclined from 5 to−9µmol m−2s−1,explaining 31%of the variation(P <0.001)(Fig.2).CumulativeFNvalues of 69±33, 57±31, and 57±27 g C m−2for the I1,I2, and I3treatments, respectively, were not significantly different during the first 12 d,but were significantly different between the I1, I3, and I6treatments (70±22, 60±16,and 18±12 g C m−2,respectively)for the subsequent 18 d(Table II).Values ofFGalso declined with increasingW,with the model explaining 35%of the variation(P <0.001)(Fig.2b).CumulativeFGvalues were not significantly different between the I1,I2,and I3treatments up to day 12(365±50,345±62,and 329±40 g C m−2,respectively),but the differences between the I1,I3,and I6treatments(443±39,405±32,and 299±13 g C m−2,respectively)were significant(P <0.05)during the subsequent 18 d(Table II).
The values ofRLdeclined linearly with increasingW, with the threshold in the broken-stick model atW=17 mm,and 50%of the variation was explained by the model(Fig.3b).CumulativeRLvalues decreased with increasingWand were significantly different between treatments during days 1 to 12,with the differences increasing from days 13 to 30(P <0.05)(Table II).The values ofREalso declined with increasingW(Fig.2c)after day 12,and the cumulativeREvalues are significantly different(P <0.05)between the treatments(Table II).

Fig.2 Relationships of net ecosystem CO2 exchange(FN)(a),gross C uptake by plants(FG)(b),and ecosystem respiration(RE)(c)with water deficit in the 30-d experiment in mesocosms under irrigation frequencies of every day(I1 treatment,30 d),every two days(I2 treatment,12 d),every three days(I3 treatment,30 d),and every six days(I6 treatment,18 d,after I2 treatment)(n=4).The threshold values for water deficit between the two lines for FN,FG,and RE are 29,28,and 29 mm,respectively.
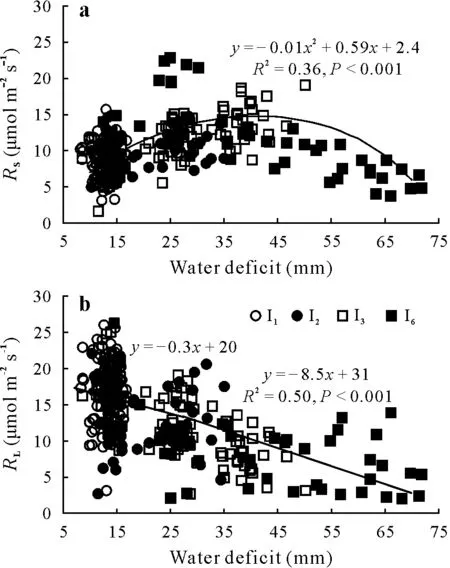
Fig.3 Relationships of soil respiration(RS)(a)and leaf respiration(RL)(b)with water deficit in the 30-d experiment in mesocosms under irrigation frequencies of every day(I1 treatment,30 d),every two days(I2 treatment,12 d),every three days(I3 treatment,30 d),and every six days(I6 treatment,18 d,after I2 treatment).The threshold value for water deficit between the two lines for RL is 17.1 mm.
The quadratic fit toRS(R2=0.36,P <0.001)showed a maximumRSvalue atW=37 mm.CumulativeRSvalues for the I3and the I6treatments were significantly higher(P <0.05)than those for the I1treatment during the second period(18 d) (Table II).At the end of the experimental period,differences in isotopic signatures of air respired at the soil surface and root and soil components allowed partitioning ofRAandRH(Table SI,see Supplementary Material for Table SI).Despite the increase in cumulativeRSover the experimental period(Table II),measurements at the end of the period,when the difference inWwas most pronounced,showed that bothRAandRHwere lower for the I6treatment than for the other treatments(Fig.4).There was a marked decrease in the proportional contribution ofRHtoRSat the end of the experiment in the I6treatment(Fig.4).

Fig.4 Soil heterotrophic respiration(RH)(a)and autotrophic respiration(RA)(b)and their proportional contributions to soil respiration at the end of the 30-d experiment in mesocosms under irrigation frequencies of every day(I1 treatment,30 d),every two days(I2 treatment,12 d),every three days(I3 treatment,30 d),and every six days(I6 treatment,18 d,after I2 treatment).Values are means with standard deviations shown by the vertical bars(n =4).Bars with upper-and lowercase letters indicate significant differences in RA and RH,respectively.
Soil N2O emission
The cumulative N2O emission ranged from 0.1 to 6.9 mg N m−2h−1(Table II).There were no significant differences in cumulative N2O emission between the treatments.

TABLE IICumulative water deficit(W),net ecosystem CO2 exchange(FN)and its components,gross C uptake by plants(FG),ecosystem respiration(RE),leaf respiration(RL), and soil respiration(RS), and soil N2O emission over the two periods of measurements in the 30-d experiment in mesocosms under irrigation frequencies of every day(I1 treatment,30 d),every two days(I2 treatment,12 d),every three days(I3 treatment,30 d),and every six days(I6 treatment,18 d,after I2 treatment)
DISCUSSION
Responses of CO2 exchange components to irrigation frequency
The threshold values ofWfor the broken-stick model below whichFN,FG, andREremained constant at the maximum values were similar regardless of treatment and ranged from 47 to 57 mm, suggesting similar threshold values for photosynthesis and ecosystem respiration.The lack of any treatment differences in cumulativeFN,FG,andREduring the first 12 d demonstrated that these processes were insensitive to mild increases inWup to 30 mm.This coincides closely with the breakpoints between no effects and decreases in both photosynthesis and leaf expansion with increasingWfor a range of crops and soil types(Sadras and Milroy, 1996).Such an approach with a decrease in plant growth atWabove a threshold value has long been used to manage irrigation scheduling for field crops(Monteith,1986).
Based on a meta-analysis of data across biomes,Zhouet al.(2016)concluded that drought resulted in decreasednet photosynthesis by 25%and decreasedREby 18%and irrigation increased plant net photosynthesis by 34% and increasedREby 26%.In this study,REshowed less sensitivity to drying thanFG,andFNbecame negative at higher values ofW.However,in contrast to the study by Zhanget al.(2019),we did not find a significant difference in aboveground production with increasingW.This may be explained by the characteristically low sensitivity of the C4Bermuda grass to drought.The C allocated to the aboveground plant component must have remained unchanged with increasingW,despite decreasing net ecosystem C uptake.Confirmation for this comes from the observation that reductions inFGandRLwere similar in response to increasingW,with decreases in their cumulative values between the I1and I6treatments being 67%and 60%forFGandRL,respectively.
The decline inREwith increasingWresulted from different responses ofRLandRS(Fig.3, Table II).The differences in the responses ofRLandRSwere attributed to a complex interaction between autotrophic and heterotrophic components of the soil, leading to the observed quadratic response ofRS.From comparative measurements at 19 temperate grassland sites, Burriet al.(2018) concluded that the response of decreasingRSto drought was independent of aboveground productivity,but was strongly linked with belowground C allocation.The increasing relative C allocation to promote root growth(van Wijk,2011)and associated respiration rates(Meier and Leuschner,2008)could explain our observations thatRAincreased with moderate increases inWand then decreased with further increases inW(Fig.4).
The decrease inRH(23%) with decreasing irrigation frequency and increasingWacross the treatment extremes was greater than the change inRA(10%),leading to a decrease in the proportional contribution ofRHtoRS(Fig.4).This result is in contrast with findings from a meta-analysis of grassland studies in field conditions showing that there were no differences in the ratio ofRAtoRHwith increasing drought(Zhouet al.,2016).However,our result is consistent with other observations.For example, Heinemeyeret al.(2012)reported thatRAwas relatively stable throughout a 3-month period in a temperate grassland during summer,and Moinetet al.(2019)showed that the13C isotopic signatures of CO2emitted from different grassland roots remained constant through a severe drought,suggesting high drought tolerance.Other studies have shown thatRHis sensitive to increasingW,particularly in semi-arid systems(Chenet al.,2009)when the balance between water content and oxygen availability becomes suboptimal for microbial activity.These results and our findings suggest that increased frequency of summer droughts could lead to reduction inRSresulting from a mild effect onRA,but strong reduction inRHand SOM decomposition rate.We were not able to quantify the relative significance of respired CO2pulses that may have occurred rapidly after soil rewetting.Although pulse emissions have been measured from soils that have been rewetted following air drying to low water content lasting several days,resulting in strong reduction in microbial activity prior to further rewatering(Meisneret al.,2015),pulses are short lived(Moinetet al.,2019).In this study,the lack of differences in plant aboveground biomass production suggests that water contents in soil were unlikely to be sufficiently low for pulse emissions to be significant.
Soil N2O emission
Soil N2O emission measured in this study ranged from 0 to 0.15 mg N m−2d−1and was lower than that typically measured in field conditions in irrigated,ungrazed grasslands(Saggaret al.,2010).While the low N supply would have constrained N2O emission, oxygen availability is a main driver of denitrification and is strongly regulated by soil water content(Linn and Doran,1984;Owenset al.,2016;Liet al.,2021).The conditions of low N availability,low values of water-filled pore space,and high soil relative diffusivity suggest that N2O emission was dominated by nitrification and that oxygen supply was not sufficiently low to support denitrification to N2O or full denitrification to N2(Balaineet al.,2016).The lack of differences in N2O emission between the irrigation treatments is consistent with earlier findings for freely draining soils in field conditions (Owenset al.,2016).
CONCLUSIONS
The findings from growing Bermuda grass in mesocosms showed that decreases in net ecosystem CO2exchange(FN)with increasing cumulative soil water deficit(W)were moderated by the offset between a strong decrease in gross C uptake by plants (FG) and a less sensitive response in ecosystem respiration(RE).However,this did not result in changes in aboveground biomass production with increasingW.Although cumulativeRSdid not increase with increasingW,the use of a13C natural abundance stable isotope technique showed that while root respiration(RA)was not different between the treatments, there was a marked decrease in the proportional contribution ofRHtoRS.This suggests that decreasing the irrigation frequency could lead to reduction in SOM decomposition rate while keeping C inputs to the soil constant(with supporting evidence from small changes inRA)and with no change in aboveground biomass for the Bermuda grass ecosystem.These findings highlight the importance of understanding and incorporating the relative responses of the components of CO2exchange to increasingWin models that predict C losses from soil.The lack of differences in soil N2O emission in the absence of high inputs of N in this well-drained,aerobic soil providesevidence that there would be no beneficial or trade-offeffect for N2O emission.It could be concluded that changes to irrigation scheduling could be used to minimise soil C losses with no effect on N2O emission at sites with well-drained soils and low N inputs;however,further research is needed to address differences between soil types and conditions.
ACKNOWLEDGEMENTS
This work was funded by the New Zealand Agricultural Greenhouse Gas Research Centre(NZAGRC)and National Natural Science Foundation of China(No.32101431)to support Yuan Li.The authors especially thank Roger Cresswell,Kethsiri Alwis, Emily Huang at Lincoln University, New Zealand for laboratory analyses.We thank Alan Stewart at PGG Wrightson Seeds, New Zealand for providing grass seeds.We are grateful to Neil Smith,Graeme Rogers,Trevor Hendry, Brent Richards, Andrea Leptin, David Rex, and Zach Simpson at Lincoln University,New Zealand for their help with laboratory and statistical analyses.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Variability in pattern and hydrogen isotope composition(δ2H)of long-chain n-alkanes of surface soils and its relations to climate and vegetation characteristics:A meta-analysis
- Synergy of remotely sensed data in spatiotemporal dynamic modeling of the crop and cover management factor
- Heterotrophy-coordinated diazotrophy is associated with significant changes of rare taxa in soil microbiome
- Impact of drilling waste pollution on land cover in a high subarctic forest-tundra zone
- Short-term biochar effect on soil physicochemical and microbiological properties of a degraded alpine grassland
- Co-inoculation of indigenous Pseudomonas oryzihabitans and Bradyrhizobium sp.modulates the growth,symbiotic efficacy,nutrient acquisition,and grain yield of soybean
