Analgesic-like activity of perillyl acetate: In vivo and in silico studies
2022-04-12RenanBragaHumbertoAndradeRyldeneCruzMayaraMaiaCarolinaLimaAndersonSantosAndrMirandaAllanaDuarteMarcusScottiReinaldoAlmeidaDamiSousa
Renan Braga, Humberto Andrade, Ryldene Cruz, Mayara Maia, Carolina Lima, Anderson Santos, André Miranda, Allana Duarte, Marcus Scotti, Reinaldo Almeida,3, Damião Sousa,3✉
1Postgraduate Program in Natural and Synthetic Bioactive Products, Federal University of Paraíba, 58051-970, João Pessoa, PB, Brazil
2Research Institute for Drugs and Medicines, Federal University of Paraíba, CP 5009, João Pessoa, 58051-900, PB, Brazil
3Department of Pharmaceutical Sciences, Federal University of Paraíba, 58051-970, João Pessoa, PB, Brazil
ABSTRACT
Objective:To evaluate the antinociceptive activity of perillyl acetate in mice and in silico simulations.
Methods:The vehicle, perillyl acetate (100, 150 and/or 200 mg/kg, i.p.), diazepam (2 mg/kg, i.p.) or morphine (6 mg/kg, i.p.)was administered to mice, respectively. Rotarod test, acetic acidinduced abdominal writhing, formalin-induced nociception, hot plate test, and tail-flick test were performed. Opioid receptorsinvolvement in perillyl acetate antinociceptive effect was also investigated.
Results:Perillyl acetate did not affect the motor coordination of mice. However, it reduced the number of acetic acid-induced abdominal twitches and licking times in the formalin test. There was an increase of latency time in the tail-flick test of 30 and 60 minutes. Pretreatment with naloxone reversed the antinociceptive effect of perillyl acetate (200 mg/kg). In silico analysis demonstrated that perillyl acetate could bind to μ-opioid receptors.
Conclusions:Perillyl acetate has antinociceptive effect at the spinal level in animal nociception models, without affecting the locomotor integrity and possibly through μ-opioid receptors. In silico studies have suggested that perillyl acetate can act as a μ-opioid receptor agonist.
KEYWORDS: Analgesic; Biological products; Opioid; Essential oil; Pain; Monoterpene; Perillyl acetate
Significance
Perillyl acetate is a monoterpene in essential oils of plants and is synthesized from perillyl alcohol, which presents several applications in oncology and pain. Therefore, in the present study, the antinociceptive potential of perillyl acetate and its possible mechanism of action were investigated. Perillyl acetate has antinociceptive effect in animal models, as μ-opioid receptors agonist and may become a new option for the pharmacological treatment of pain.
1. Introduction
Pain is defined as an unpleasant sensory and emotional experience associated with or similar to that associated with actual or potential tissue damage[1]. As one of the most relevant causes of human suffering, pain causes disability and impairment of life quality[2].Pain is considered a complex experience, which beyond transduction of nociceptive stimulus, involves both cognitive and emotional processing, and differential behavioral responses are also processed by the brain[3]. Pain is present in some moments of human life,especially when affected by several pathologies. For example, the world population has been suffering the consequences of the serious pandemic of COVID-19, including different strains of SARS-CoV-2 and the risk of reinfection[4]. Pain is one of the symptoms present in SARS-CoV-2 infection, such as headache, muscle pain, pain in the pectoral region, and sore throat[5].
Vegetable products are an important source of pharmacologically active compounds with an analgesic profile, such as morphine[6].Studies using animal models of pain show that several chemical classes found in nature are good candidates for antinociceptive drugs[7]. Some of these compounds have other biological activities,including anti-coronavirus and antioxidant potential[8]. Many essential oils and their chemical constituents play a promising antinociceptive activity via various mechanisms of action[9]. The chemical structures of some of these components have been used as prototypes for the development of synthetic derivatives with antinociceptive potential[10,11].
Perillyl acetate (PAC) is a monoterpene found in essential oils of plants, such as Fortunella japonica Swingle and Citrus natsudaidai Hayata[12,13], and is synthesized from perillyl alcohol[14]. This precursor presents several applications in oncology[15]and other disorders, especially those involving pain[16,17]. PAC is a structurally similar monoterpene and more lipophilic than perillyl alcohol.Therefore, in the present study, the antinociceptive potential of PAC and its possible mechanism of action were investigated.
2. Materials and methods
2.1. Drugs, reagents, and equipment
PAC was prepared via acetylation of perillyl alcohol using acetic anhydride and pyridine at reflux temperature as previously published[14]. Main reagents included polyoxyethylene sorbitan monooleate (Tween 80, Vetec, Brazil), acetic acid (Merck, Brasil),diazepam (DZP) (Merck, Brazil), naloxone (Sigma-Aldrich, Brazil),and morphine (Merck, Brazil). Agents were intraperitoneally (i.p.)administered at 0.1 mL/10 g. The choice of doses of 100, 150, and 200 mg/kg of PAC was based on a pharmacological behavioral screening, where these doses promoted antinociception in a doseresponse curve. The equipment used in the tail-flick test, rotarod test,and tail-flick test was from the company Insight-Brazil.
2.2. Animals
The animals were male Swiss mice (Mus musculus), 2-3 months old, weighing between 25-30 g, randomly housed in appropriate cages, following a 12-hour light-dark cycle and free access to water and food (Purine). Each experimental group contained 6 animals.
2.3. Locomotor activity-rotarod test
The rotarod test checks motor coordination and muscle relaxation produced by central nervous system (CNS) depressant drugs in animals[18]. The animals were preselected 24 h before the experiments, and those who were unable to remain on the rotating bar (2.5 cm in diameter, with a frequency of 7 rpm) for 1 min were excluded. Three groups were formed (n=6): PAC (200 mg/kg; i.p.);vehicle (5% Tween 80; i.p.) or DZP (2 mg/kg; i.p.). The animals were individually submitted to the rotarod test at 30, 60, and 120 min after treatments and the time (s) they remained on the bar, up to a maximum of 180 s was recorded.
2.4. Acetic acid-induced abdominal writhing
Acetic acid-induced writhing test is considered a classic model of visceral inflammatory nociception. An acetic acid solution (1%, v/v) is administered intraperitoneally, promoting an irritation of the peritoneum, causing abdominal contractions known as contortions(extension of the abdomen and stretching of the hind limbs)[19].Five groups were formed (n=6): PAC (100, 150, and 200 mg/kg),vehicle (5% Tween 80), or morphine (6 mg/kg). Acetic acid was injected intraperitoneally into each animal 30 min after treatments.The number of writhes was counted for 20 min after acetic acid injection[20].
2.5. Formalin-induced nociception
A 2.5% formalin solution was injected (20 μL) in the subplantar region of the right hind paw of the animal. After this noxious stimulus, the mice were placed in a triangular apparatus composed of two mirrored walls and clear glass, the licking time on the injected paw was considered indicative of nociceptive during two phases: an initial acute phase (0-5 min) and a phase late (15-30 min after formalin injection)[21]. The animals were treated in the writhing test 1 h before formalin injection.
2.6. Hot plate test
The mice were submitted to a hot plate [(55 ± 1) ℃]. The latency to jump off the hot plate surface or lick a hind paw was measured.The maximum time the animals remained on the hot plate was 30 s, after which the animals were removed to avoid tissue damage[22].One day before the test, the mice were pre-selected. Only animals with latency times < 10 s were used. Latencies (s) were measured at 0, 30, 60, and 120 min after intraperitoneal administration of vehicle (5% Tween 80), PAC (200 mg/kg), or morphine (6 mg/kg).
2.7. Tail-flick test
A radiant source of heat was focused on the animal’s tail as a thermal nociceptive stimulant, causing its withdrawal/movement(flick). A maximum cut-off of 30 s was used to avoid tissue damage[23]. The animals were treated with PAC (200 mg/kg),vehicle (5% Tween 80), or morphine (6 mg/kg). Withdrawal latencies (s) were measured at 0, 30, 60, 120, and 240 min after treatment.
2.8. Opioid receptors-involvement in PAC antinociceptive effect
To identify possible opioid receptor involvement in the antinociceptive effect of PAC, six groups were used: the control, PAC (200 mg/kg,i.p.), morphine (6 mg/kg, i.p.), and the remaining groups received the same prior treatments. However, 15 min earlier, they were given naloxone (5 mg/kg, s.c.), a non-selective opioid antagonist.The writhing test was then performed[24].
2.9. Molecular docking
The structure of the protein μ-opioid receptor bound to the BU72 agonist (PDB ID: 5C1M)[25]was downloaded from the RCSB Protein DataBank (PDB). The PAC structure was then submitted to molecular docking using the Molegro Virtual Docker, v. 6.0.1(MVD)[26]. All water molecules were deleted from the enzyme structure, and the enzyme and compound structures were prepared using the default parameter settings in the MOLE software package(Score function: MolDock Score; ligand evaluation: internal hydrogen bond, Sp2-Sp2 torsions, internal ES, all checked; number of runs: 10 runs; algorithm: MolDock SE; maximum inter-actions:1 500; maximum population size: 50; maximum number of steps:300; neighbor distance factor: 1.00; and the maximum number of conformations returned: 5). The docking procedure was performed using a GRID with a radius of 15 Å, and a resolution of 0.30 a to cover the ligand-binding site in the structure of the enzyme.
2.10. Statistical analysis
Results were analyzed using Kruskal-Wallis followed by Dunn's test or Mann Whitney test for non-parametric data. Results were expressed as median (IQR). Differences were considered statistically significant when P<0.05. The data were analyzed using the GraphPad Prism program version 7.00 (GraphPad Software Incorporated, San Diego, CA, USA).
2.11. Ethical statement
All experimental procedures were approved by the Ethics Committee on the Use of Animals of UFPB, under Certificate No.015/2016.
3. Results
3.1. Effect of PAC on motor coordination
There was no significant difference in the time spent on the rotating bar between the PAC group (200 mg/kg) and the control group at 30, 60, and 120 min after treatment. DZP (2 mg/kg, i.p.)significantly reduced the time spent on the rotating bar at 30 and 60 min after treatment (P<0.001) (Figure 1).

Figure 1. Effect of perillyl acetate (PAC) (200 mg/kg) on rotarod test in mice. The data are expressed in median (IQR) (n=6). Data were analyzed by Mann Whitney’s test. ***P<0.001 vs. the control. DZP: diazepam (2 mg/kg). A: 30'; B: 60'; C: 120'.
3.2. Effect of PAC on acetic acid-induced writhing
PAC at 150 and 200 mg/kg markedly reduced the number of abdominal contortions compared to the control group (22.1 ± 3.1)(P<0.01). Morphine also significantly reduced the number of writhes (P<0.01).
3.3. Effect of PAC on formalin-induced nociception
PAC at 100 and 150 mg/kg did not significantly decrease the paw licking time in the first phase of the formalin test, in comparison to the control group. However, PAC at the dose of 200 mg/kg significantly reduced licking time (P<0.01) (Figure 2A). PAC at 150 and 200 mg/kg significantly decreased the paw licking time in the second phase compared to the control group (P<0.01) (Figure 2B). Morphine significantly decreased paw licking time in the first phase (P<0.001) and the second phase (P<0.01).
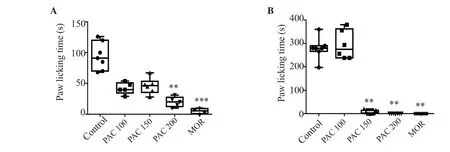
Figure 2. Effect of PAC (100, 150, and 200 mg/kg) in phase 1 (A) and phase 2 (B) of the formalin test. The data are expressed in median (IQR) (n=6). Data were analyzed using Kruskal-Wallis (phase 1, H=23.04; phase 2, H= 21.5) followed by Dunn’s test; **P<0.01, ***P<0.001 vs. the control. MOR: morphine.
3.4. Hot plate test
There was no significant difference in the latency times between the PAC 200 mg/kg group and the control group at 30, 60, and 120 min after administration. Morphine significantly increased the latency times (P<0.01) (Figure 3).
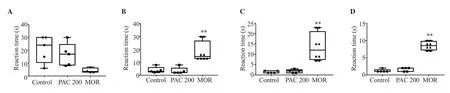
Figure 3. Effect of PAC (200 mg/kg) in the hot plate test in mice. The data are expressed in median (IQR) (n=6). Data were analyzed using Mann Whitney test(0’, H=7.39; 30’, H=13.29; 60’, H=13.37; 120’, H=13.67) followed by Dunn’s test; **P<0.01 vs. the control. A: 0'; B: 30'; C: 60'; D: 120'.
3.5. Tail-flick test
PAC at 200 mg/kg significantly increased latency times at 30 and 60 min after administration when compared to the control group(P<0.05). After 120 min of PAC treatment, the effect was not significant. Morphine also increased the latency at 30 (P<0.01) and 60 (P<0.05) min after treatment significantly (Figure 4).
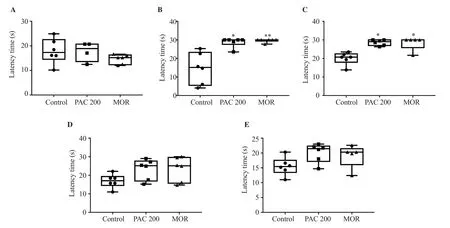
Figure 4. Effect of PAC (200 mg/kg) in the tail-flick test in mice. The data are expressed in median (IQR) (n=6). Data were analyzed using Mann Whitney test(0’, H=3.74; 30’, H=17.80; 60’, H=14.50; 120’, H=5.21; 240’, H=6.50) followed by Dunn’s test; *P<0.05, **P<0.01 vs. the control. A: 0'; B: 30'; C: 60'; D: 120';E: 240'.
3.6. Acetic acid-induced writhing test using an opioid antagonist
According to Figure 5, PAC at 200 mg/kg significantly reduced the number of writhes compared to the control group (P<0.01).However, the reduction in the number of writhes induced by PAC was significantly reversed with naloxone (5 mg/kg) pretreatment when compared to PAC alone (P<0.05). Naloxone also reverted the effect of morphine (6 mg/kg) when compared to the group receiving morphine alone (P<0.001).
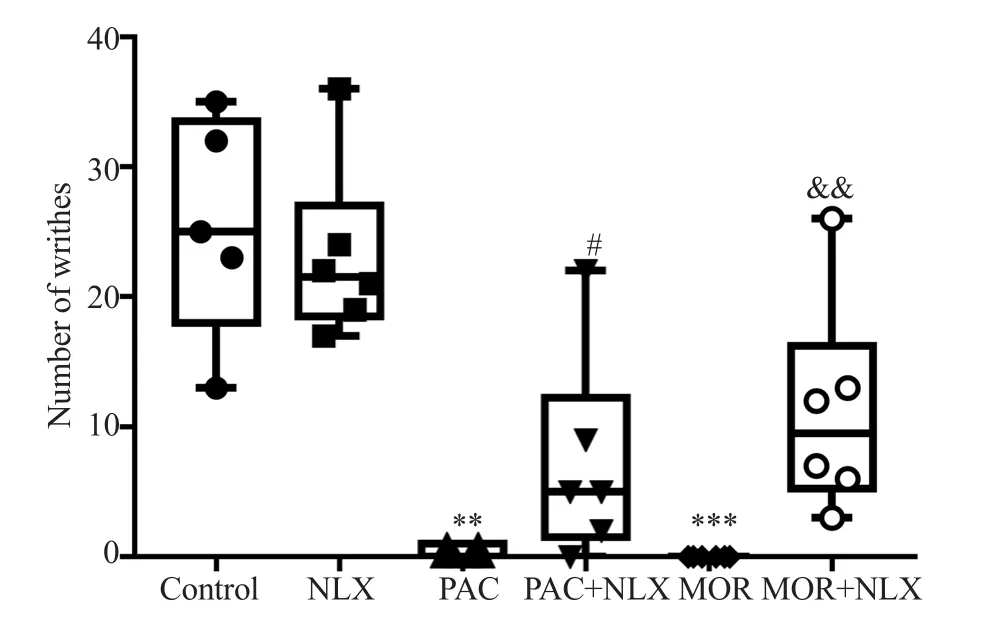
Figure 5. Effect of naloxone (NLX) on the antinociceptive effect of PAC and MOR on acetic acid-induced writhing in mice. The data are expressed in median (IQR) (n=6). Data were analyzed using Kruskal-Wallis test (H=27.68)followed by Dunn’s test; **P<0.01, ***P<0.001 vs. the control. #P<0.05 vs. PAC;&&P<0.01 vs. MOR.
3.7. Molecular docking
Docking was validated by redocking the original ligand BU72 in the active site of the μ-opioid receptor. The superposition of poses is represented in Figure 6 and reveals a perfect match. We evaluated the potential of using PAC as an μ-opioid receptor agonist and the Re-rank Score of both compounds indicated that PAC (-70.10 kJ/mol) exhibited potential agonist activity in μ-opioid as BU72 (-68.26 kJ/mol) (Figure 7).
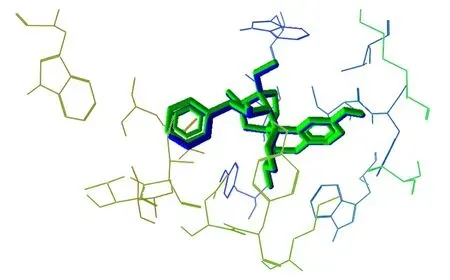
Figure 6. Superposition of crystal pose (green) and docking pose (blue),validating the methodology.
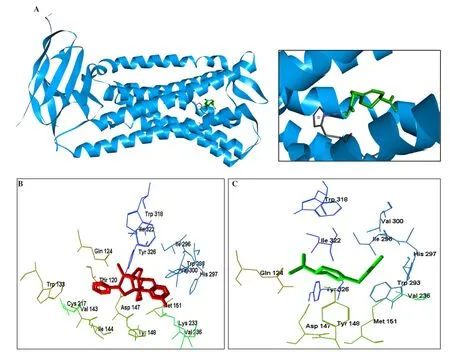
Figure 7. Structure of the μ-opioid receptor (A) and 3D interactions with compounds. B: structure of BU72 and C: structure of perilyl acetate.
4. Discussion
Perillyl alcohol has several applications in treatment of oncology[15]and other diseases, many of which involve pain[16,17]. In this paper, we studied the phenylpropanoid PAC, a synthetic derivative of perillyl alcohol, which has an antinociceptive central effect mediated by the opioid pathway.
Aiming to estimate PAC antinociceptive property, mice were used for nociceptive behavioral tests with different stimuli and types of nociception: visceral nociception (writhing test), neurogenic and inflammatory nociception (formalin-induced nociception), and thermal nociception (hot plate and tail-flick test)[24,27].
It's already known that compounds capable of altering locomotor activity can impair the interpretation of the antinociceptive effect[28].Rotarod test evaluates the effects of drugs on muscle relaxation or neurotoxicity. Excluding a possible false-positive response in the nociceptive behavior, PAC did not affect the locomotor integrity of treated animals. However, there are still no acute and chronic toxicity studies on PAC.
The present study evaluated PAC antinociceptive effect with acetic acid-induced abdominal writhing test[19], which is used to screen new drugs for the treatment of pain and inflammation[27].The injection of acetic acid into the peritoneal cavity promotes activation of acid-sensing ionic channels and proton-gated cation channels in peripheral sensory neurons[29]. Acetic acid also stimulates the release of cytokines, such as prostaglandin E2and prostaglandin F2[30]. According to the results, PAC reduced the number of writhes. However, due to the low specificity of the method, we can’t affirm if antinociception promoted by PAC was related to a reduction in inflammatory events or direct inhibition of nociceptors. So, the formalin-induced nociception test was employed, since it is a more specific model that can differentiate pain into two phases: central and peripheral[31].
Formalin into the mice paw promotes two phases of nociceptive response (paw licking time) which consists of early neurogenic and inflammatory phases. The first phase occurs 0-5 min after intraplantar administration of formalin and reflects intense stimulation of sensory C-fibers through activation of transient receptor potential ankyrin 1 receptors[32]; it is also reported that calcitonin generelated, substance P and mediators like bradykinin and glutamate exert an effect on neurogenic pain response[21,33]. The interphase period (5-15 min) is observed, where the response of the animal to the pain stimulus is reduced, which is due to not fully understood inhibitory processes[34]. Some authors suggest certain mechanisms that may be involved, such as activation of the opioid system[35], or release of gamma-aminobutyric acid neurotransmitters that target the gamma-amino butyric acid A receptor in the spinal cord and result in decreased C-fiber activity[36]. In the second phase (15-30 min) we can see an inflammatory process, increasing mediators like prostaglandin, histamine, serotonin, bradykinin[37], and nitric oxide[38]. Drugs such as opioid analgesics act by inhibiting nociception in the neurogenic and inflammatory phases. While non-steroidal anti-inflammatory drugs only inhibit the second phase[21]. PAC (200 mg/kg) decreases the paw licking time in the first and second phases. This is a characteristic of analgesic central drugs, such as opioids. Similar results were reported for perillyl alcohol which also reduced paw licking times in both phases of the test[16]. The literature reports that transient receptor potential vanilloid1 (TRPV1) and TRPA1 antagonists reduce the pain response stimulated by formalin[32]. According to Endres-Becker et al.[39], μ-opioid receptor activation can inhibit TRPV1 activity and cAMP pathway via Gi/o proteins[40]. Thus, our results demonstrated that PAC inhibited pain in both phases of the formalin test, we can suggest a possible opioid, TRPV1 and TRPA1 receptor involvement in the antinociceptive effect.
To verify the central antinociceptive involvement of PAC, a hot plate test was performed[27]. The plate heated at a constant temperature [(55 ± 1) ℃]causes behavioral responses such as hind paw licking and jumping[41], which represent supra-spinal sensory activation, once thermal stimulation can activate VR-1 type receptors (activation threshold = 43 ℃), and type VRL-1 receptor(activation threshold = 52 ℃), stimulating Aδ and C fibers[42].These fibers carry the impulse through the dorsal horn of the spinal cord to the somatosensory cortex where they are interpreted[37].In contrast to perillyl alcohol, which reported a higher latency of response to the hot plate thermal stimulation[16], PAC presented no significant changes. This indicates non-participation of supra-spinal mechanisms in the central antinociception mediated by PAC.
The tail-flick test evaluates nociception at the central spinal level[22].Tail withdrawal reflex in the tail- flick assay is a nociceptive parameter that represents spinal sensory integration, where an increase in latency time is relevant for evaluating central antinociceptive action. Central analgesics, such as opioids, are capable of suppressing spinal neuron response to thermal stimuli in the tail, increasing the latency time[43].The effectiveness of PAC (200 mg/kg) in the tail-flick test suggested antinociceptive effect through reduction of painful stimulation in the spinal cord[23]. This result is different from perillyl alcohol which acts at supra-spinal levels. The results highlight the importance of structural drug modifications where structurally similar drugs can reveal different routes for promoting analgesic effects.
Since the tail-flick test can identify the activity of compounds whose mechanisms are similar to opioid analgesics, the PAC mechanism of action was investigated. To explore the PAC antinociceptive mechanism, the opioid receptor pathway was investigated in the abdominal writhing test, which is considered useful in investigating opioid analgesic drugs[44]. μ, κ, and Ω opioid pathway receptors are found both in the central nervous system and in peripheral tissues. μ-opioid receptor is the main target for analgesic opioids.Naloxone, a nonspecific antagonist of the opioid receptors, more selective for the μ-opioid receptor, is frequently used in researching the involvement of opioid signaling in pain control[45]. PAC appears to promote its antinociceptive effects by the opioidergic pathway since its effects were partially reversed by pretreatment with naloxone. These findings are in accordance with previous reports where naloxone partially reverted perillyl alcohol’s antinociceptive effect[16], thus reinforcing the possible participation of opioid signaling in the antinociceptive action.
Since naloxone presents a high affinity for the μ-opioid receptor, it suggests that PAC exerts its antinociceptive effects via the μ-opioid receptor pathway. In this context, to evaluate the interaction between PAC and the μ-opioid receptor, we decided to perform a docking study. In the docking results, the PAC was assigned a similar rerank score to BU72, an agonist of μ-opioid receptors, presenting the same types of interactions with certain critical residues. Binding energy values and in vivo analysis suggest that PAC promotes antinociceptive effect via the μ-opioid receptor. The activity of PAC was not completely reversed by naloxone, an indication of activity with undesignated antinociception mechanisms.
The main limitations of this work were that we are not yet able to proceed with clinical trials, once the acute and chronic toxicity of PAC has not yet been determined, and in addition, to better understand its mechanism of action, electrophysiological methods should be done.
Taken together, the results of this study proved that PAC has an antinociceptive effect at the spinal level in animal nociception models, without affecting the locomotor integrity and possibly through μ-opioid receptors. In silico studies have suggested that PAC can act as a μ-opioid receptor agonist.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This study was supported by funds from the Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development(CNPq).
Authors’ contributions
All authors contributed to the development of the article. BRM,AHHN, and CRMD involved in the development of the research and the writing of the manuscript. LCUGB, SAF, DABS, and MAM participated in the process of data collection, analysis and interpretation of the data. SMT and MMS contributed to in silico analysis. SDP and ARN contributed to the critical review and final writing of the manuscript.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cardiovascular protective properties of gastrodin
- Lipid-lowering effect of Oroxylum indicum (L.) Kurz extract in hyperlipidemic mice
- Anti-obesity effect and UHPLC-QTOF-MS/MS based metabolite profiling of Solanum nigrum leaf extract
- Methyl gallate isolated from Mangifera pajang kernel induces proliferation inhibition and apoptosis in MCF-7 breast cancer cells via oxidative stress
