MTBSTFA derivatization-LC-MS/MS approach for the quantitative analysis of endogenous nucleotides in human colorectal carcinoma cells
2022-04-07HuixiaZhangYanLiZhengLiChristopherWaiKeiLamPengZhuCaiyunWangHuaZhouWeiZhang
Huixia Zhang,Yan Li,Zheng Li,Christopher Wai-Kei Lam,Peng Zhu,Caiyun Wang,Hua Zhou,Wei Zhang
State Key Laboratory of Quality Research in Chinese Medicines,Macau Institute for Applied Research in Medicine and Health,Macau University of Science and Technology,Taipa,Macau,China
Keywords:
Ribonucleotides
Deoxyribonucleotides
Derivatization
N-(t-butyldimethylsilyl)-N-
methyltrifluoroacetamide
High-performance liquid chromatography
tandem mass spectrometry
A B S T R A C T
Endogenous ribonucleotides(RNs)and deoxyribonucleotides(dRNs)are important metabolites related to the pathogenesis of many diseases.In light of their physiological and pathological significances,a novel and sensitive pre-column derivatization method with N-(t-butyldimethylsilyl)-N-methyltrifluoroacetamide(MTBSTFA)was developed to determine RNs and dRNs in human cells using highperformance liquid chromatography tandem mass spectrometry(HPLC-MS/MS).A one-step extraction of cells with 85% methanol followed by a simple derivatization reaction within 5 minat room temperature contributed to shortened analysis time.The derivatives of 22 nucleoside mono-,di-and triphosphates were retained on the typical C18column and eluted by ammonium acetate and acetonitrile in 9 min.Under these optimal conditions,good linearity was achieved in the tested calibration ranges.The lower limit of quantitation(LLOQ)was determined to be 0.1-0.4μM for the tested RNs and 0.001-0.1μM for dRNs.In addition,the precision(CV)was<15% and the RSD of stability was lower than 10.4%.Furthermore,this method was applied to quantify the endogenous nucleotides in human colorectal carcinoma cell lines HCT 116 exposed to 10-hydroxycamptothecin.In conclusion,our method has proven to be simple,rapid,sensitive,and reliable.It may be used for specific expanded studies on intracellular pharmacology in vitro.
1.Introduction
Endogenous ribonucleotides(RNs)and deoxyribonucleotides(dRNs)are essential for life process and related to the pathogenesis of many diseases such as neurodegeneration[1],cancer[2],and mitochondrial depletion syndrome[3,4].Deoxy-and ribonucleoside triphosphates(dNTPs and NTPs)are the precursors for DNA and RNA synthesis,and source energy providers such as adenosine triphosphate(ATP)as well as guanosine triphosphate(GTP).Nucleoside mono-and diphosphates are also involved in several physiological processes including signal transduction[5]and nutrient metabolism.Adenosine monophosphate(AMP)can initiate the AMP-activated protein kinase(AMPK)which plays a role in cellular energy homeostasis to activate glucose and fatty acid uptake and oxidation when cellular energy is low[6,7].Perturbations of RNs and dRNs pools can cause serious diseases either by decreased level of one of the nucleotides or by an imbalance among them.For instance,the balance of nucleotides pool is critical for the high fidelity of DNA replication as the imbalance of the dNTP/NTP ratios will increase the possibility of incorrectly incorporating NTPs into DNA and result in DNA instability[8].These considerations underscore the potential utility of a rapid and convenient assay for quantification of different phosphorylated forms of intracellular RNs and dRNs.
Several analytical methods have been established to quantify nucleotides,including radioimmunoassay[9],aptamer-modified gold nanoparticles[10,11],and high-performance liquid chromatography(HPLC)or capillary electrophoresis[12]with tandem mass spectrometry(MS/MS)detection.Although the sensitivity of radioimmunoassay and aptamer-modified gold nanoparticles for the determination of RNs and dRNs has been reported previously,these approaches are hindered by the lack of specific antibodies or aptamers directed against the monophosphate and diphosphate nucleotides.Capillary zone electrophoresis(CZE)[13]and micellar electrokinetic chromatography(MEKC)[14]have a unique advantage in separating high-charged analytes.However,the incompatibility of the mobile phase containing large amounts of nonvolatile buffers with mass spectrometry limits the application of MEKC.The poor precision resulting from poor injection repeatability is also a major obstacle for the widespread use of CZE in routine analysis.The addition of ion-pair reagents into mobile phase is another conventional strategy to enhance the separation of RNs and dRNs in reversed-phase liquid chromatography(RPLC)because these molecules are extremely polar due to their polyanionic phosphate groups[15-17].However,there is no doubt that ion-pair reagents can cause ionization suppression of the analytes and contaminate the ion source as well as the HPLC systems.During the last decade,the analysis of nucleotides has evolved from ionpairing chromatography to a novel,more compatible hydrophilic interaction chromatography(HILIC),which has a good separation performance against highly hydrophilic analytes and is compatible with MS using ion-pair free agents[8,18-20].Although HILIC has been broadly used to quantify polar compounds,severe peak tailing and poor peak shape have always been problematic.Kong et al.[8]developed a ZIC-cHILIC method to quantify eight NTPs and dNTPs.However,it remains uncertain whether this strategy can be used to accurately measure deoxyribonucleoside monophosphates and diphosphates levels because their amounts were much lower than those of the respective triphosphate metabolites.Moreover,positive electrospray ionization was employed in this methodology,which would significantly decrease its sensitivity due to numerous hydroxyl groups of RNs and dRNs.So far,the simultaneous separation of RNs and dRNs is still a highly complex issue.
Derivatization is a powerful tool used to increase the volatility and chromatographic mobility of polar and unstable organic compounds [21].Several derivatization methods using chloroacetaldehyde, propionyl or benzoyl acid anhydride,hexamethyleneimine,etc.have been reported in the literature for the quantification of nucleotides in biological samples with the aid of LC-MS/MS.Zhang et al.[22]synthesized chloroacetaldehyde derivatives of adenosine 3′,5′-cyclic monophosphate(cAMP)for LC fluorescence analysis by derivatizing the pyrimidine base with 2-chloroacetaldehye at 80°C for 30 min,which is likely to result in degradation of nucleoside di-and triphosphate.Nordstro¨m et al.[23]used propionyl or benzoyl derivatives to increase hydrophobicity and electrospray ionozation(ESI)response of AMP,ADP and ATP.The derivatization was performed in acetonitrile and N-methylimidazole at 37°C for 30 min.However,these methods are useable only for the trace amount of NTPs and dNTPs because of their poor solubility in these relatively hydrophobic solvents.Flarakos et al.[24]then developed a method for the synthesis of dGMP derivatives utilizing coupling reagents typically employed in peptide synthesis.The hydrophobic derivatives showed increased ionization efficiency and improved peak shape in an LC-MS/MS method.Unfortunately, this method is complex and timeconsuming.Silylation is another derivatization reagent implemented to address the lack of volatility and chromatographic retention.For this method,N-(t-butyldimethylsilyl)-N-methyltrifluoroacetamide(MTBSTFA)is preferred for polar and unstable organic compounds according to substituting the labile hydrogen with t-butyldimethylsilyl on the polar function groups such as-COOH,-OH,and-NH[25].The derivatives could possess higher molecular weights and slightly stronger resistance to moisture.Up to date,it is mainly used in gas chromatography and few approaches are reported to determine nucleotides with liquid chromatography.
In this study,a simple,rapid and sensitive MTBSTFA derivatization method has been developed to quantify 22 nucleotides in human cells.Moreover,a detailed study containing optimization of derivatization conditions,selectivity of preferred extraction reagents,and the optimal liquid chromatography gradient was carried out.This derivatization approach was also applied to determine the concentrations of RNs and dRNs in HCT 116 cells treated with 10-hydroxycamptothecin in order to prove the versatility for this method.
2.Materials and methods
2.1.Chemicals and reagents
Adenosine monophosphate (AMP), adenosine diphosphate (ADP),adenosine triphosphate (ATP), cytidine monophosphate (CMP), cytidine diphosphate (CDP), cytidine triphosphate (CTP), guanosinemonophosphate (GMP), guanosine diphosphate (GDP), guanosinetriphosphate (GTP), uridine monophosphate (UMP), uridine diphosphate (UDP), uridine triphosphate (UTP), deoxyadenosine monophosphate (dAMP), deoxyadenosine diphosphate (dADP),deoxyadenosine triphosphate (dATP), deoxycytidine monophosphate (dCMP), deoxycytidine diphosphate (dCDP), deoxycytidine triphosphate (dCTP), deoxyguanosine monophosphate(dGMP), thymidine monophosphate (dTMP), thymidine diphosphate(dTDP), thymidine triphosphate(dTTP),stable isotope labeled adenosine-13C10,15N5-triphosphate (ATP-13C10,15N5),adenosine-13C10,15N5-monophosphate(AMP-13C10,15N5),and ammonium acetate(NH4OAc)were purchased from Sigma Aldrich Chemical Co.(St.Louis,MO,USA).HPLC-mass grade methanol and acetonitrile were obtained from J.T.Baker Chemical Co.(Phillipsburg,NJ,USA).N-(t-butyldimethylsilyl)-N-methyltrifluoroacetamide(MTBSTFA,95%)was provided by Dieckmann Chemical Industry Company Ltd.(Hong Kong,China).Ultra-pure water was produced from a deionized water system Milli-Q(Millipore Corporation,Burlington,MA,USA)and used throughout the study.
For cell culture,phosphate buffer saline(PBS),McCoy's 5A medium,penicillin-streptomycin solution and fetal bovine serum(FBS)were obtained from GIBCO Invitrogen Co.(Carlsbad,CA,USA).Human colon cancer epithelial cell lines(HCT 116)were supplied by American Type Culture Collection(ATCC;Rockville,MD,USA).
2.2.Stock solutions,internal standards and calibration curve standards
Initial stock solutions at 400μM(dATP,dCTP and dTTP),500μM(dAMP,dCMP,dGMP dTMP,dADP,dCDP and dTDP),1mM(ADP,CDP,GDP,UDP,ATP,CTP,GTP and UTP),2mM(AMP,CMP,GMP and UMP)in 50% methanol were prepared and stored at-20°C in brown glass bottles until use.The isotope-labeled internal standard(IS)solutions of AMP-13C10,15N5and ATP-13C10,15N5were diluted to a concentration of 20μM in 85% methanol respectively and stored as above.Calibration curve standards and quality control(QC)samples were obtained by mixing and diluting the corresponding stock solutions and IS solutions to appropriate concentrations with analyte-free cell matrix,which was the cell lysate extraction with 85% methanol,and stripped from endogenous interference with activated carbon at 60 mg/mL[26,27].
2.3.Sample preparation and derivatization
HCT 116 cells were maintained with McCoy's 5A medium containing10%(V/V)FBS,100U/mL penicillin and 100μg/mL streptomycin in a 37°C humidified incubator with 5% CO2.After incubation,cells were washed with ice-cold PBS once and then treated with trypsin.Each digested sample was re-suspended in 10 mL of ice-cold PBS,followed by centrifugation for 5 min(300 g,4°C);then the pellets were stored at-80°C until use.Cell pellets were treated with cold 85% methanol(2×106cells/100μL)containing 2μM AMP-13C10,15N5and 2μM ATP-13C10,15N5,then vortexed for 1 min and placed on ice for 10min.After centrifugation(13,000 g)at 4°C for 15min,the supernatant was transferred into another tube.The derivatization reaction was initiated by adding 75μL of derivatization reagent MTBSTFA into 200μL of cell lysis with 85% methanol and completed over 5min with consistent vortex.Then derivatization samples were centrifuged(13,000 g)at 4°C for 10min,and 25μL of the supernatant was injected into LCMS/MS system for analysis.
2.4.LC-MS/MS analysis
2.4.1.Instrumentation
All experiments were carried out on a Thermo Fisher TSQ LCMS/MS system(Thermo Fisher Scientific Co.,San Jose,CA,USA)consisting of an Accela Autosampler,an Accela pump,and a Quantum Access triple quadrupole mass spectrometer.All system control,data acquisition,mass spectral data evaluation,and data processing were performed using XCalibur software version 2.0.7(Thermo Fischer,San Jose,CA,USA)and Thermo LCquan software version 2.5.6(Thermo Fischer).
2.4.2.LC-MS/MS conditions
Chromatographic separation was performed on a Sepax GP-C18column(2.1mm×150 mm,1.8μm;Sepax Technologies,Inc.,Newark,DE,USA)with a mobile phase consisting of 5 mM ammonium acetate aqueous solution(A)and acetonitrile(B).The column was maintained at 35°C and the HPLC system was set up to operate at a flow rate of 0.2mL/min under the following linear gradient conditions:0-2 min,5%-16%B;2-7min,16%-30%B;7-9min,30%-55%B;9-9.2min,55%-5%B;9.2-20 min,5%B.MS conditions were as follows:ESI in negative mode,vaporizer temperature 290°C,capillary temperature 320°C,sheath gas pressure 50 psi,auxiliary gas pressure 20 psi and spray voltage 2600V.Full scan and product ion modes with the range of m/z 100-900 were performed,and the quantitative data were acquired in multiple reactions monitoring(MRM)mode.
2.5.Method development and validation
The method was validated according to the FDA Guidance for Industry:Bioanalytical Method Validation(FDA,2018)[28].In brief,selectivity was assessed by analyzing the analyte-free cell samples that were pre-treated with activated carbon and lower limit of quantification(LLOQs)from six different sources,and comparing their peak areas.The peak areas of co-eluting interfering peaks or background noise should be<20% and<5% that of analytes and IS,respectively,at the LLOQs.Linearity was evaluated by calibration standard assays in duplicate on three consecutive days.The calibration curves consisting of seven calibrator levels were constructed using least-squares linear regression analysis with 1/x2weighting by plotting the peak area ratios of the analyte to the corresponding IS against the concentrations.ATP-13C10,15N5was used as IS for nucleoside triphosphates and AMP-13C10,15N5was used as IS for nucleoside mono-and diphosphates,respectively.Each calibration curve with a correlation coefficient(r2)higher than 0.990 was acceptable.Accuracy and precision were investigated by calculating the relative error(RE)and the coefficients of variation(CV%)of QCs at three concentration levels(low,medium and high),which were assayed in five replicates on three consecutive days.The accuracy and intra-and inter-batch precision were considered acceptable if RE and CV were within±15%.Autosampler stability was evaluated at 4°C for 24h by measuring replicates(n=5)of LQC,MQC,and HQC samples.Analytes were considered stable when the calculated concentration was within±15% of the theoretical value.The matrix effect was evaluated by analyzing two sets of samples at LQC,MQC,and HQC levels:(A)QC samples with analyte-free cell matrix from five lots and(B)neat solution containing the same amount of nucleotides as QC samples,both of which were processed according to the established derivatization procedures.Matrix factor(MF)is the ratio of peak area in the presence of matrix(solution A)to the peak area in absence of matrix(solution B),and was calculated for each lot of matrix.Then,the IS normalized MF(IMF)was calculated by dividing the MF of the analyte by the MF of the IS.The relative standard deviation(RSD)of IMF calculated from the five lots of matrix should be<15%.
2.6.Effect of 10-hydroxycamptothecin on nucleotides and cell cycle distribution analysis
HCT 116cells were also cultured as mentioned above.After the treatment with 10-hydroxycamptothecin for 24h,all the samples were harvested and washed with ice-cold PBS and trypsinized.Then they were divided into two equal parts and centrifuged at 1,500 g for 5 min.One of them was applied to determine the concentrations of RNs and dRNs pools.The other was suspended to be fixed with cold 70%(V/V)ethanol and stored overnight at-20°C for cell cycle distribution analysis.Subsequently,the fixed cells were collected,re-suspended in cold PBS and incubated with propidium iodide(PI,Sigma Aldrich Chemical Co.,St.Louis,MO,USA)containing 0.05% RNase A(Sigma Aldrich)at room temperature for 30 min in the dark.Last,cell cycle distribution profile after the staining treatment was assessed by a flow cytometer(MuseTM cell analyzer,Merck Millipore,Darmstadt,Germany).The percentages of cells in G0/G1,S and G2/M phases were analyzed by using MODFIT software(Verity Sofware House,Topsham,ME,USA).
3.Results and discussion
3.1.MTBSTFA derivatization of nucleotides
Nucleotides are a kind of high polar components with three molecular fragments including a nitrogenous base(either a purine or a pyrimidine),a five carbon sugar and one or more phosphate groups[29].Hence,they are weakly retained on an RPLC column.Silylation is a powerful tool to enhance highly polar compounds retention on RPLC,as well as improving ESI response in the mass spectrometry.In this study,silylation based on MTBSTFA was used to modify the nucleotide compounds for the sake of generating new products with better chromatographic properties.This derivatization reaction was operated under mild reaction conditions and quantitatively completed with good yields.
Fig.1 shows the full scan and product ion mass spectra of silyl derivatives of the representative cytidine phosphates(CMP,CDP and CTP).In the precursor ion full scan spectra,the[M-H]-precursor ions were used for target compounds.From this figure,CMP(m/z 322)could be completely derivatized by MTBSTFA and derivatization product(m/z 436)after the introduction of a tbutyldimethylsilyl with increasing mass of 114 was obviously observed,indicating that the derivatization reaction could fully completed with a good yield.The same results were confirmed in other nucleotide derivatives based on the above pattern.In secondary ion mass spectra,m/z 211 and 273 were the most abundant fragments for MTBSTFA derivatives.The possible fragment pathways are provided in Fig.1B.Based on the secondary mass spectral fragmentation pattern,we confirmed that the silyl group was bonded with the phosphate groups.Also,each of these derivatives presented some other characteristic fragments for the analyzed compounds,such as the ions of base,base combined with pentose or nucleoside combined with a phosphate group,which made identification very reliable.

Fig.1.(A)Full scan and(B)product ion mass spectra of silyl derivatives of the representative cytidine phosphates.
3.2.Optimization of the MTBSTFA derivatization
3.2.1.Effects of time and temperature
It is well known that reaction time can directly affect the derivatization reaction equilibrium and further exerts a great influence on the analysis sensitivity of analytes[30].The reactions were performed at 5,10,20 and 40 min,respectively.The MTBSTFA derivatization reaction could be rapidly completed in 5 min and no extra contribution to the analytical response of the derivatives was observed with increasing reaction time.Finally,5 min was selected as the total reaction time because long reaction time may lead to degradation of RNs and dRNs.
Temperature is another important factor that can affect the MTBSTFA derivatization[31].In order to investigate the impact of temperature on MTBSTFA derivatization,the reaction was conducted at room temperature(about 25°C),37°C,and 45°C.It was found that reaction temperature had a significant effect on the complexity of derivatization products.Briefly,the vice-products with more than one t-butyldimethylsilyl group turned out to be larger after increasing the reaction temperature.However,the ratio was still lower than 50% even at 45°C for 40 min,which demonstrated that it was hard to completely convert more than one silyl group on the analytes due to its potential sterically hindered effect[30].Consequently,reaction at room temperature was optimal for the stability of derivatives with only one silyl group.
3.2.2.Effect of water content
Apart from the reaction time and temperature,the extraction solvent can also influence the derivatization reaction[32].It is known that water has an adverse effect on both the reaction and the stability of the derivatives for silylation[21,30,33].It is ideal to conduct MTBSTFA derivatization reaction without water;however,preparing nucleotides solutions in pure methanol is impossible,especially for nucleoside di-and triphosphates.Therefore,the effect of water was evaluated by preparing standard solutions in methanol containing 5%,10%,15%,20%,and 25%(V/V)of water in order to optimize the suitable reaction solvent.The obtained chromatographic peak areas of RNs in each sample were compared respectively.The peak area of each analyte with methanol at 5% water was used as the standard and the relative response of each analyte with different water content was compared with the standard(Fig.2).The yields for most of the derivatization compounds slightly decreased with increasing water content from5% to 15%.Comparatively,it exhibited a dramatically decreasing trend when the percentage of water in reaction mixture was above 20% and the relative responses were almost lower than half of the initial methanol solution at 5% water especially for di-and triphosphate.This may be caused by more competition between the analytes and water for reacting with MTBSTFA.In short,traces of water up to 15% have similar effects on the derivatization reaction.However,the response declined significantly when the aqueous content was over 15%.Hence,15% water content was finally selected.

Fig.2.Effects of water content on nucleoside mono-(A),di-(B)and tri-(C)phosphates standard solutions.
3.2.3.Sample preparation
Sample preparation was probably the major critical step for the determination of endogenous nucleotides in biological samples that could affect the analysis efficiency and the accuracy of results significantly.According to a former study on determination of intracellular nucleotides[34],the first step of sample pre-treatment was to lyse the cells or organelles and block the metabolism of nucleotides immediately by inactivation of the enzymes.A precipitation step constituted the identical procedure common to all methods previously described.Perchloric acid(PCA),trichloroacetic acid(TCA),methanol,acetonitrile or a mixture of methanol or acetonitrile with different proportions of water were the most commonly used protein precipitation and extraction reagents[35].PCA and TCA deproteinizations were time-consuming for the subsequent requirement of neutralization using basic solutions and the finally extracted samples contained too much water that would deplete the derivatization reagent.Although acetonitrile was commonly used as a reaction solvent in silylation,its poor dissolving capability of nucleotides resulted in the disability of extraction from cell lysis.Therefore,methanol and a series of methanol/water mixtures were investigated to obtain good extraction efficiency and derivatization yield.Methanol was firstly employed to lyse cells and extract nucleotides.However,only nucleoside monophosphates and a part of nucleoside diphosphates could be detected,and there was just extremely low detection signal of nucleoside triphosphates.Considering the low solubility in methanol,we speculated that there were few di-and triphosphates in the cell extraction.So different proportions of water contents were evaluated and optimized:5,10,15,20 and 25%(V/V).Finally,85% methanol(with 15% water)was selected to precipitate the protein which was excellent for interference removal and recovery of all the analytes.
Recently,solid phase extraction(SPE)after protein precipitation has been more and more applied in cellular nucleotides determination[8,16]to enrich analytes and reduce endogenous interference.We also evaluated the effect of SPE.However,the complex elution solution inhibited the derivatization reaction and no expected products were observed.
3.3.Chromatographic separation and mass spectral characteristics of derivatized compounds
Generally,the endogenous RNs and dRNs are too polar to be retained on the RPLC column under conventional chromatographic conditions.Hence,RNs and dRNs including mono-,di-and triphosphate nucleotides were reacted with MTBSTFA.In our study,several elution solvents were tried,including methanol,acetonitrile,water,water containing 0.1% formic acid,and 5mM ammonium acetate(pH 6.8).Acetonitrile and 5 mM ammonium acetate were selected as gradient elution solutions because of their better separation and peak shapes.Finally,an LC-MS/MS based assay was developed for quantitatively profiling these endogenous molecules in a 9min run with good reproducibility as well as sensitivity.Since the main derivatives of nucleotides with MTBSTFA could only occupy one hydroxyl group of phosphonates,the polarity of nucleoside triphosphates was higher than that of diphosphates,then monophosphates.The MRM chromatograms of derivatives of all the analytes are presented in Fig.3.As shown in this figure,the triphosphate nucleotides mainly appeared with the retention time in the range of 3.9-4.4 min and the diphosphate nucleotides were eluted during 5.3-6.1 min.Finally,the monophosphate nucleotides were eluted in less than 9min.The order of these characteristic peaks may attribute to their polar difference.The completely chromatographic separation among the mon-,di-and triphosphate nucleotides not only indicated their detectable polarity differences,but also enhanced the specificity via removing mis-annotation of in-source fragmentation[36].Both positive and negative ionization modes were compared for these analytes,and the negative mode was more selective and sensitive than that of the positive mode due to the high polarity and the acidic nature of the target analytes.To optimize the MS responses,some instrument parameters including tube lens and collision energy were optimized and the settings of the mass spectrometric parameters are listed in Table 1.
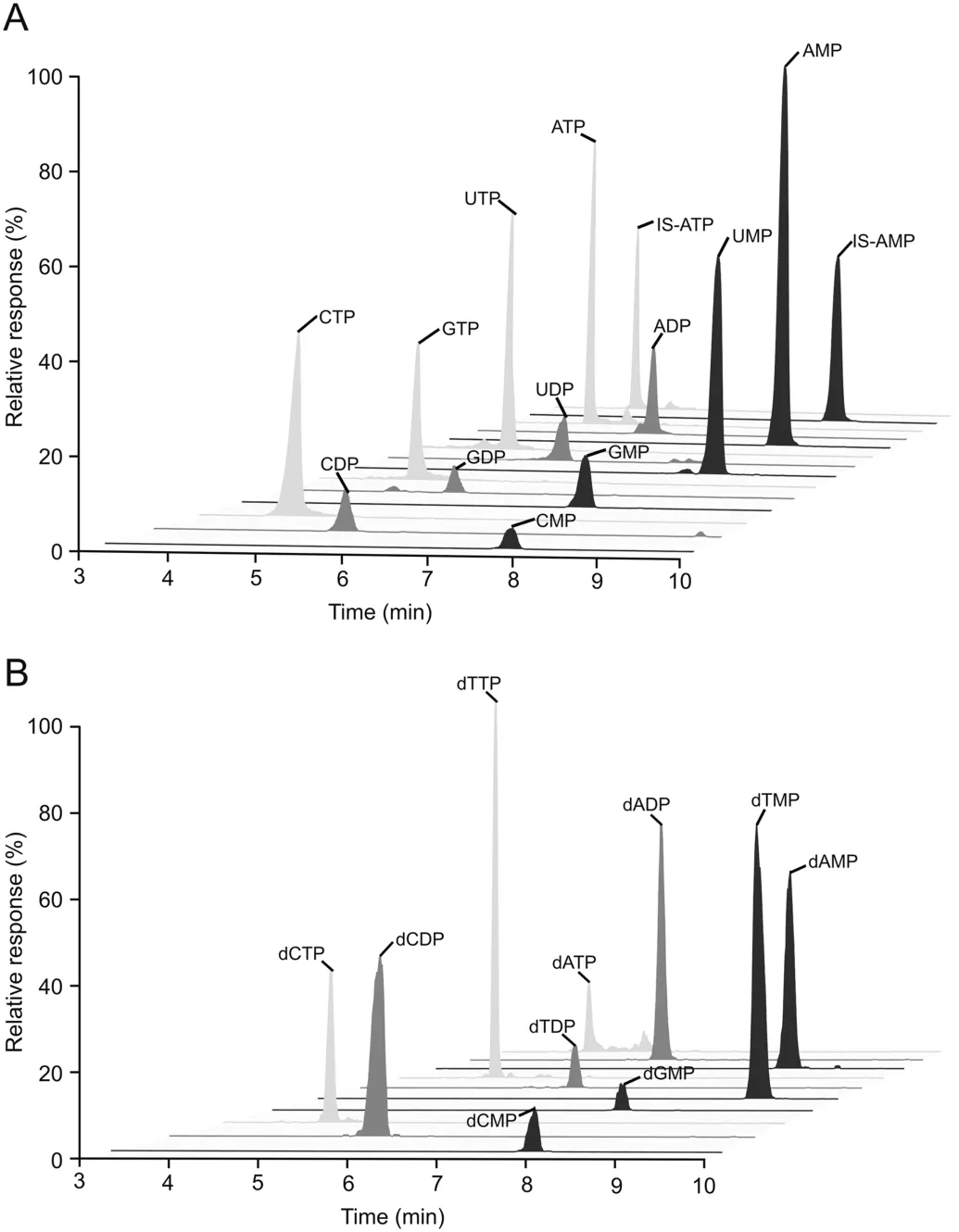
Fig.3.Selected reconstructed MRM chromatograms of derivatives of(A)RNs and(B)dRNs using the established LC-MS/MS method.
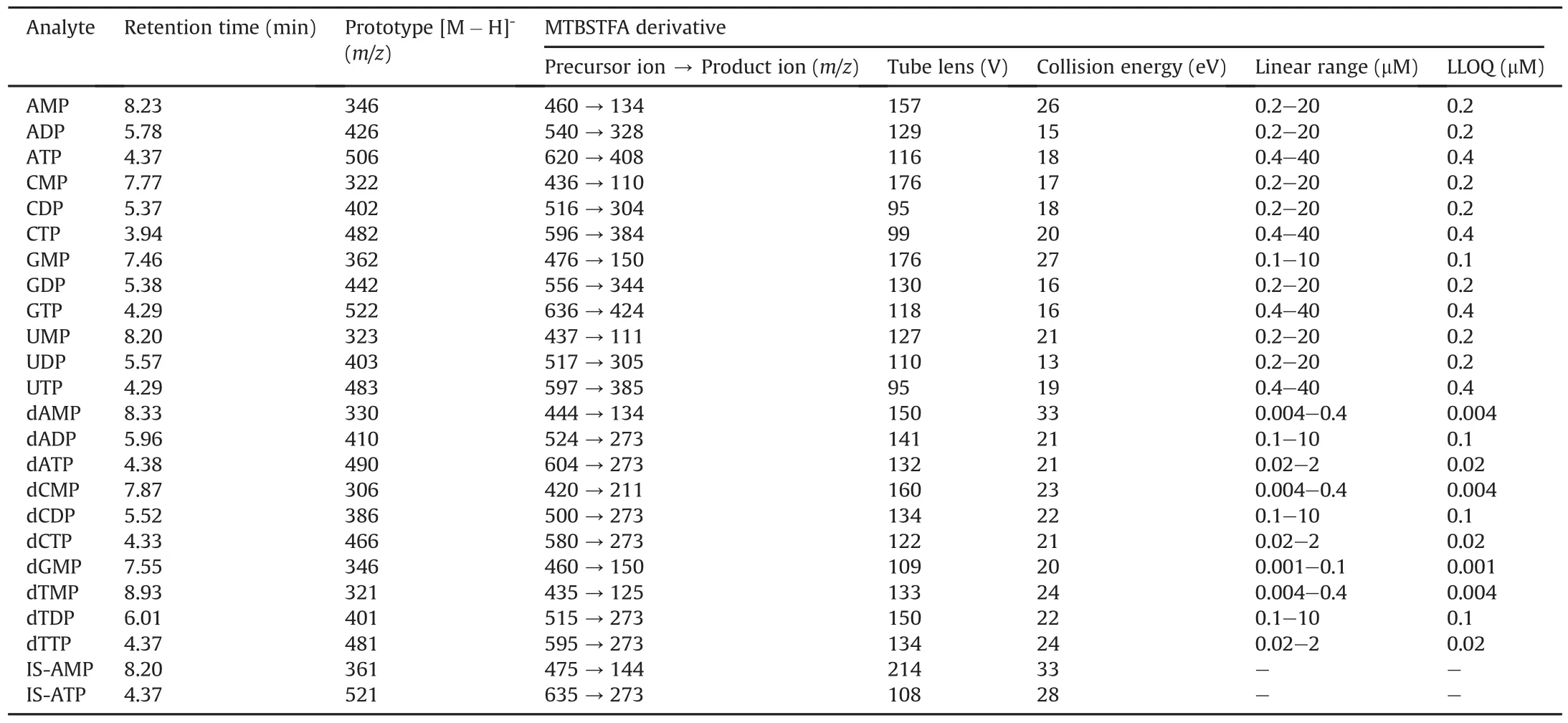
Table 1Retention time,mass spectrometric parameters,and linear range or the quantification of each nucleotide derivative and internal standard in negative ion mode in the LC-MS/MS method.
3.4.Method validation
In this work,activated charcoal was used to prepare the analytefree surrogate matrix for method validation according to previous study[27].Briefly,activated charcoal was added to the broken cells with the ratio of 60 mg/mL.The cell suspension containing activated charcoal was shaken for 4 h.Analyte-free matrices were collected after centrifugation(12,700r/min)for 40 min at 4°C,and stored at-80°C until utilized.The analyte-free matrix showed no obvious interfering peaks for any of the analytes when determined by the established LC-MS/MS method,indicating that activated charcoal could efficiently remove nucleotides RNs and dRNs from cell lysis.The validation data are summarized in Tables 1 and 2.All the calibration curves exhibited good linearities with correlation coefficients(r2)>0.99.The LLOQ for the tested RNs and dRNs were in the range of 0.1-0.4 and 0.001-0.1μM,respectively.The intraday precision(CV)of the low,medium and high QC levels was respectively in the range of 3.2%-12.9%,1.3%-14.4%,and 3.1%-12.4%.The inter-day precision(CV)of the three spiked concentrations was 6.3% to 13.3%,3.4%-13.3%,and 4.7%-14.8%,separately.The intra-day and inter-day accuracy(RE)was respectively in the range of-10.8% to 9.4% and-10.4% to 11.2%.No significant matrix effect was observed within the range from 91.1% to 113.9%.The tested RNs and dRNs derivatives were confirmed to be stable in the autosampler at 4°C for 24h with RE in the range of 86.4%-112.2% and RSD less than 10.4%.Therefore,it is suggested that this method can be used to quantify endogenous RNs and dRNs in a sensitive and reproducible manner.
As reported by Kuskovsky et al.[36],it is critical to choose suitable ISs for the quantification of nucleotides;thus the stable isotope labeled ISs are optimal for their identity with the analytes in terms of structure,chemical property,and the obstacles during detection,such as in-source fragmentation and matrix effect.Practically,the application of isotope labeled ISs for every analytes was limited because of the requirements of multiple isotope labeled compounds,their commercial unavailability,the potential safety hazards and high cost.Thus,we just chose two readilyaccessible ATP-13C10,15N5and AMP-13C10,15N5as ISs for nucleoside triphosphates,and for nucleoside mono- and diphosphates,respectively.
3.5.Determination of RNs and dRNs of the selected HCT 116cells
10-hydroxycamptothecin is a DNA-topoisomerase I inhibitor and it could restrain the proliferation of many tumor cells on the basis of inhibiting the synthesis of DNA.The investigation of cellular RNs and dRNs could facilitate the understanding of underlying mechanism of this compound.The described novel method was applied for the determination of RNs and dRNs concentrations in HCT 116 cells treated with 10-hydroxycamptothecin.The amounts of intracellular RNs and dRNs pools as expressed in pmol/106cell are shown in Table 3.These intracellular metabolic levels obviously varied in different groups.Briefly,the contents of all the RNs were much higher than those of the dRNs.In addition,the adenosine ribonucleotides and thymidine deoxyribonucleotides contained the highest portion of RNs and dRNs,respectively. After the incubation of 0.1μM 10-hydroxycamptothecin,all the RNs and dRNs pools in HCT 116 cells increased extremely compared with those of the control group(P<0.01).By contrast,after the incubation of 1.0 μM 10-hydroxycamptothecine,the mono-and diphosphates mainly presented a significant decreasing tendency(P<0.01)except dCMP.Interestingly,the contents of all the triphosphates were still higher than those of the control group(P<0.01).The levels of RNs and dRNs in eukaryotic cells vary on the basis of the cell cycle.In this study,cell cycle profiles were performed by using flow cytometry analysis at the same time.From the results in Fig.4,the reduction of cell population in G0/G1 phase was accompanied by a concomitant increase in S phase when cells exposed to 0.1μM 10-hydroxycamptothecine which was consistent with the previous study reported by Shao et al.[37].Cellular RNs and dRNs are dynamic during the progression of cell cycle and this process is related to the enzyme involved in their synthesis,especially ribonucleotide reductase(RR)[38,39].Mammalian RR is composed of three subunits,namely,RRM1,RRM2 and p53R2,which are expressed in a cell cycle-dependent manner[40].In cycling cells,the RRM1 protein is metabolically stable throughout the cell cycle whereas the expression and degradation of RRM2 protein limit the S-phasedependent activity of RR complex,leading to the high cellular dNTPs pools at S phase and low dNTPs pools outside S phase[41].It could be supposed that 10-hydroxycamptothecin may have the effect of these enzymes and such hypothesis needs to be further studied.
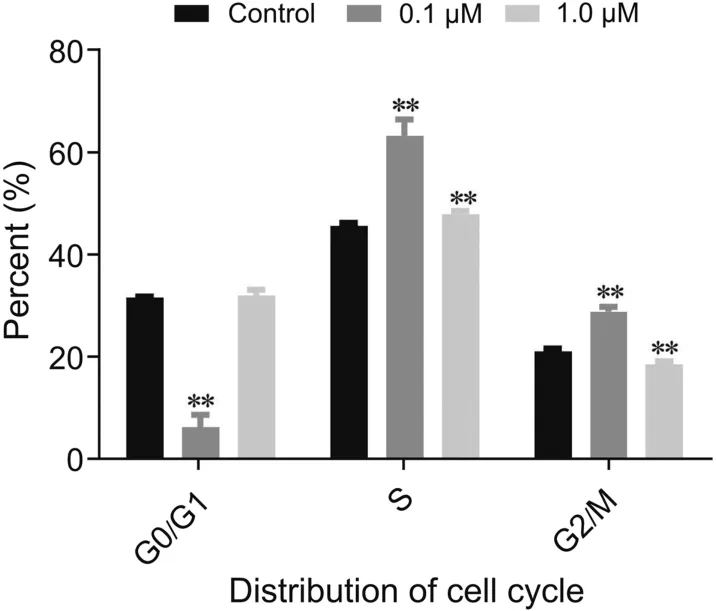
Fig.4.The statistical analysis of cell cycle distribution in HCT 116 cells upon the exposure of 10-hydroxycamptothecin.**P<0.01,compared with control group.
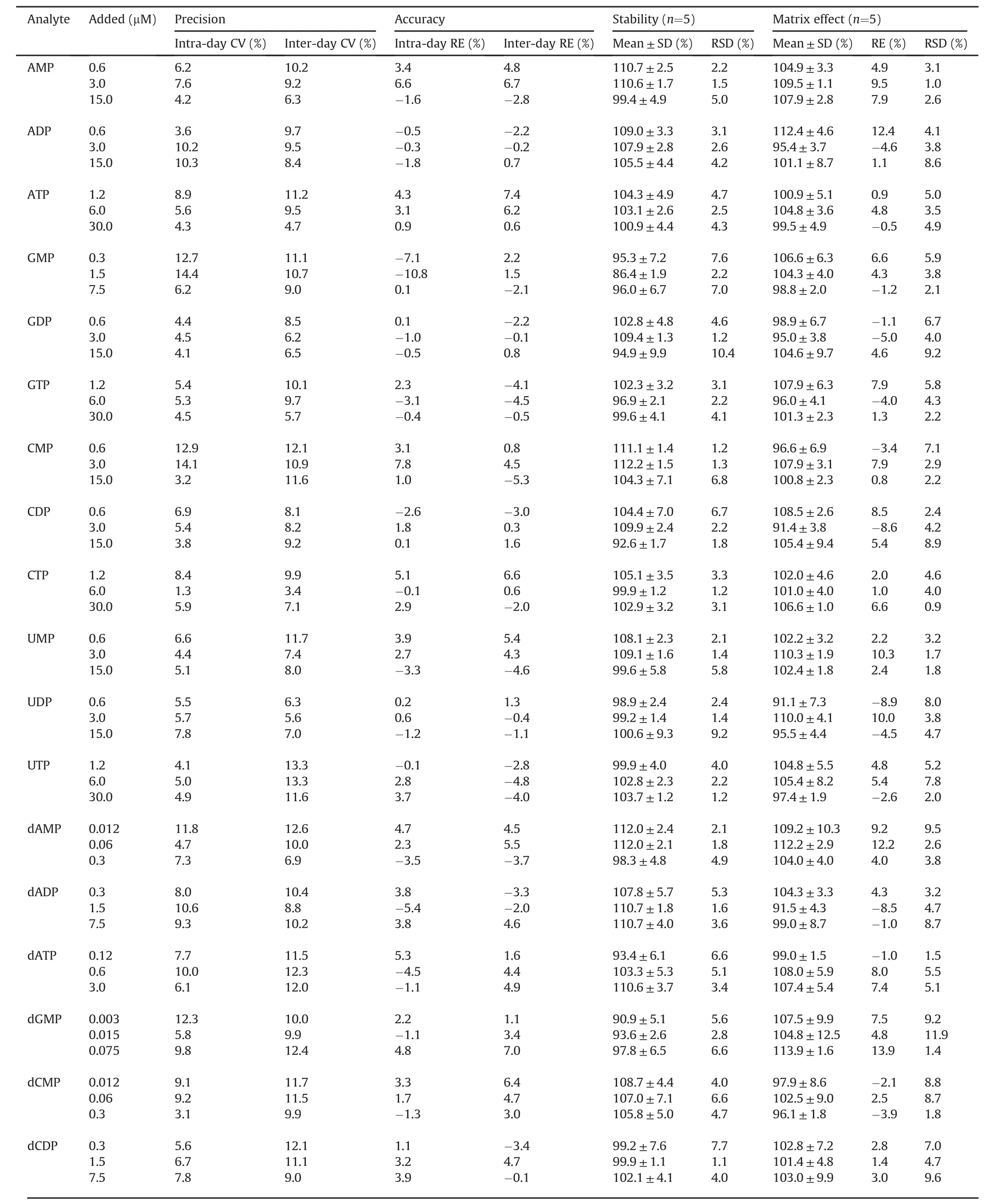
Table 2Intra-and inter-day precision,accuracy,stability and matrix effect of the developed method.
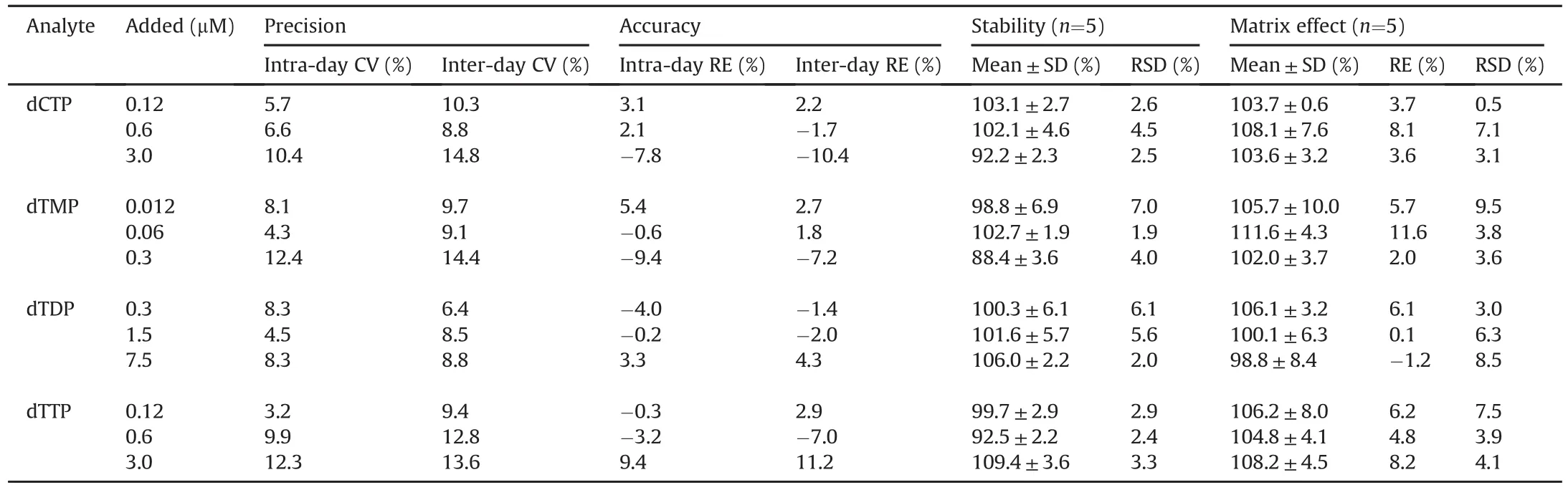
Table 2(continued)
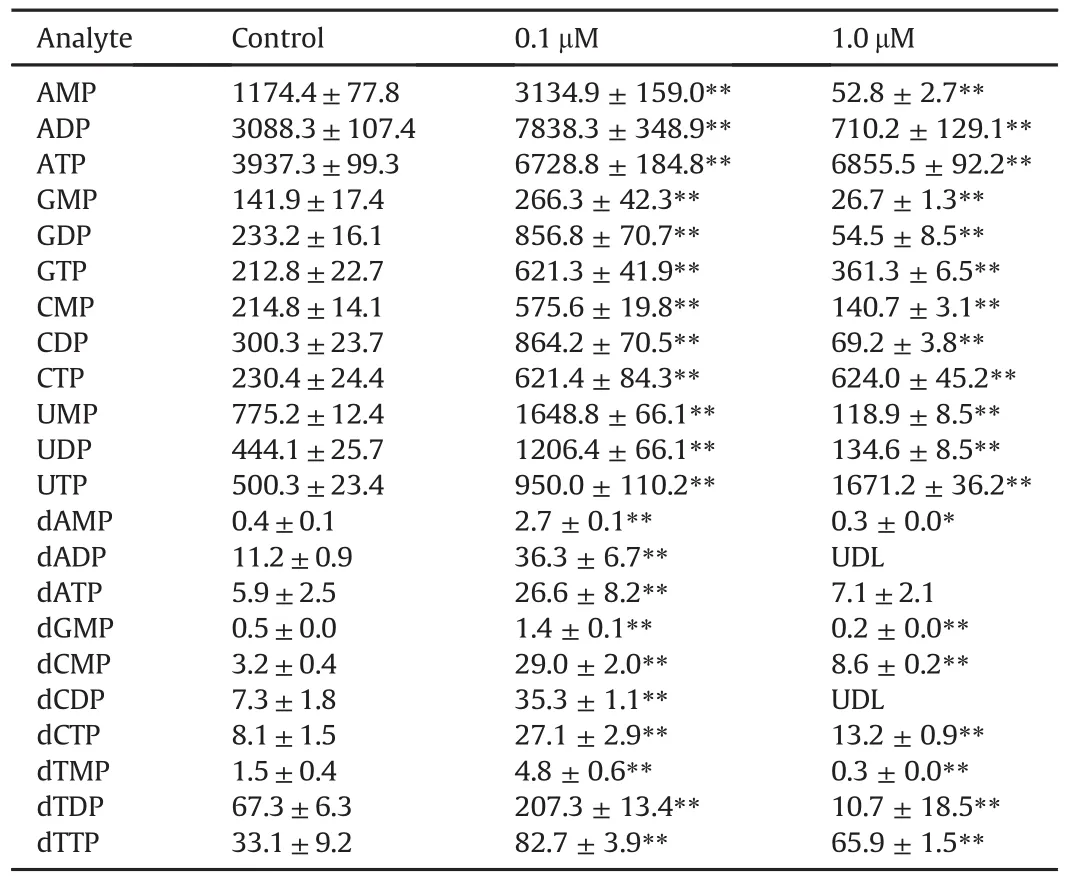
Table 3Amounts of RNs and dRN pools in HCT 116 cells treated with different dosages of 10-hydroxycamptothecin(pmol/106cells,n=3).
4.Conclusions
An optimized LC-MS/MS method for the simultaneous quantitative of 22 endogenous RNs and dRNs in cancer cells has been established after pre-column MTBSTFA derivatization.A one-step extraction of cells with 85% methanol followed by a simple derivatization reaction within 5min at room temperature contributed to shortened analysis time.The derivatives were retained on the typical C18column and eluted by ammonium acetate and acetonitrile in 9min.This approach has been validated and successfully applied to the analysis of nucleotides in HCT 116 cells exposed to 10-hydroxycamptothecin.It can be concluded that this newly described LC-MS/MS method for nucleotides detection and quantification is simple,rapid,specific and efficient.Further,it provides a new insight into the quantification of the metabolites of nucleoside analogs and other phosphonate-containing drugs like bisphosphonates.
CRediT author statement
Huixia Zhang:Conceptualization,Methodology,Formal analysis,Investigation,Writing-Original draft preparation;Yan Li:Methodology,Validation,Investigation,Writing-Original draft preparation;Zheng Li:Conceptualization,Formal analysis;Christopher Wai-Kei Lam:Writing-Reviewing and Editing;Peng Zhu:Resources;Caiyun Wang:Visualization;Hua Zhou:Writing-Reviewing and Editing;Wei Zhang:Writing-Reviewing and Editing,Supervision,Funding acquisition.
Declaration of competing interest
Authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by Science and Technology Development Fund,Macau SAR(File Nos.:0052/2018/A2 and 0077/2019/A2)and the National Natural Science Foundation of China(Grant No.:61827819).
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Time-course monitoring of in vitro biotransformation reaction via solid-phase microextraction-ambient mass spectrometry approaches
- Erythrocyte sphingolipid species as biomarkers of Alzheimer's disease
- Biological analysis of an innovative biodegradable antibiotic eluting bioactive glass/gypsum composite bone cement for treating experimental chronic MRSA osteomyelitis
- Global characterization of modifications to the charge isomers of IgG antibody
- Pharmacokinetics,distribution,and excretion of sodium oligomannate,a recently approved anti-Alzheimer's disease drug in China
- An integrated strategy for comprehensive characterization of metabolites and metabolic profiles of bufadienolides from Venenum Bufonis in rats
