Capillary electrophoresis methods for impurity profiling of drugs:A review of the past decade
2022-04-07MnsiShhNrupeshPtelNgjTripthiVivekVys
Mnsi Shh,Nrupesh Ptel,Ngj Tripthi,Vivek K.Vys
aDepartment of Pharmaceutical Analysis,Institute of Pharmacy,Nirma University,Ahmedabad,382 481,Gujarat,India
bDepartment of Pharmacognosy,Institute of Pharmacy,Nirma University,Ahmedabad,382 481,Gujarat,India
cDepartment of Pharmaceutical Chemistry,Institute of Pharmacy,Nirma University,Ahmedabad,382 481,Gujarat,India
Keywords:
Capillary electrophoresis
Impurities
Impurity profiling
Drugs
A B S T R A C T
Capillary electrophoresis(CE)is widely used for the impurity profiling of drugs that contain stereochemical centers in their structures, analysis of biomolecules,and characterization of biopharmaceuticals.Currently,CE is the method of choice for the analysis of foodstuffs and the determination of adulterants.This article discusses the general theory and instrumentation of CE as well as the classification of various CE techniques.It also presents an overview of research on the applications of different CE techniques in the impurity profiling of drugs in the past decade.The review briefly presents a comparison between CE and liquid chromatography methods and highlights the strengths of CE using drug compounds as examples.This review will help scientists,fellow researchers,and students to understand the applications of CE techniques in the impurity profiling of drugs.
1.Introduction
The presence of impurities in any of the pharmaceutical dosage forms can be extremely dangerous to patients.Currently,special attention is paid to the impurity profiling of pharmaceutical substances.Impurities are the major cause of this risk as they may affect the quality of drugs.There are many methods of impurity profiling,such as nuclear magnetic resonance(NMR),mass spectrometry(MS),high-performance thin-layer chromatography(HPTLC),high-performance liquid chromatography(HPLC),gas chromatography(GC),and other hyphenated techniques such as liquid chromatography-mass spectrometry(LCMS),gas chromatography-mass spectrometry(GC-MS)and capillary electrophoresis-mass spectrometry(CE-MS)[1].It is very important to assure the quality and purity of any pharmaceutical dosage form for the wellbeing of patients.The purity of any drug is based on the percentage of the amount of the labeled active pharmaceutical ingredient present in the pharmaceutical dosage form and approved by regulatory agencies,such as International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use(ICH)[2].According to the ICH guidelines,drug impurities can be classified into three categories:inorganic impurities,organic impurities,and residual solvents.Organic impurities may arise during the manufacturing process or storage of drug substances,including starting materials,by-products,intermediates,or degradation products.Inorganic impurities also arise during the manufacturing process,which consist of reagents,catalysts,heavy metals,or salts[3].Drug substances may contain both organic and inorganic impurities,which need to be identified and evaluated for their toxic effects,such as mutagenicity and DNA reactivity,which may lead to carcinogenic risks[4].The nature and quantity of impurities are identified using HPLC,GS,capillary electrophoresis(CE),supercritical fluid chromatography,thin layer chromatography(TLC),and high-performance thin layer chromatography(HPTLC),followed by further characterization using Fourier transform infrared spectroscopy(FT-IR),NMR,ultraviolet-visible spectroscopy(UV-vis),and MS.In the past few years,besides HPLC,other analytical methods such as CE have played a vital role in the identification of impurities present in the drugs[5,6],and CE has become a method of choice for the analysis of biologics,food materials,and supplements.CE is preferable over other methods as it provides higher resolution and faster results.For analytical purposes,CE is more sensitive and requires a small amount of analytes.It is a useful technique because a wide range of detection methods can be paired with it,and it can be used for drugs with stereochemical centers in their structures,biomolecules,and biologics.Compound separation is based on several factors,such as the applied voltage,pH,injection time,temperature of the capillary used,and presence of surfactants.The advantages of CE over the other sophisticated analytical methods are high efficiency,high resolution,fast sample analysis,and less reagent consumption in the analysis of drugs[4].
2.Theory and instrumentation of capillary electrophoresis
CE separates ions using an applied voltage based on the electrophoretic mobility,which depends on the atomic radius,charge,and viscosity of a particular molecule.The rate at which the particles move depends on the applied electric field.As the field strength increases,the mobility of particles increases and is affected by the charge of the particles;however,neutral particles remain unaffected[3].Here,charged ions migrate under the effect of an applied electric field.If two ions of similar sizes are present,the ion with the greater charge will move faster;in the case of ions of similar charges,small-sized ions have less friction and faster migration rates(Fig.1).Cations migrate toward the cathode and anions move toward the anode.The separation of these ions is based on the electric field and remains stagnant.Under normal conditions,the buffer solution moves toward the cathode and so do the molecules present in the solution.In CE,the sample is injected into a buffer solution present in a capillary tube.When an electricfield is applied to the capillary tube,the sample components migrate because of the two types of mobility:electro-osmotic flow(EOF)and electrophoretic mobility.
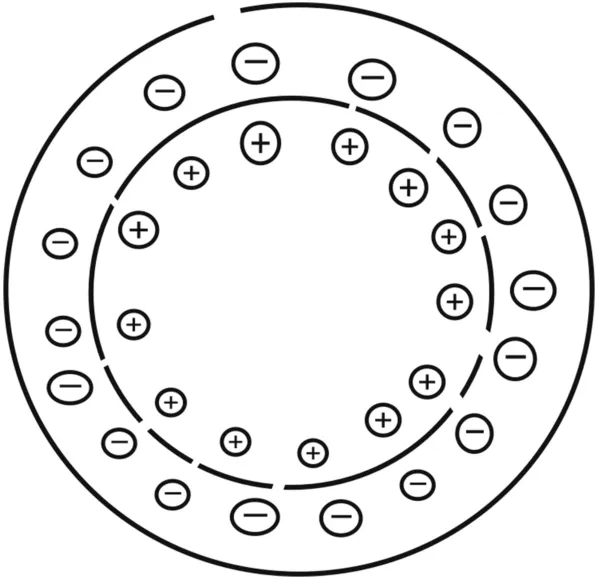
Fig.1.Cross section of capillary in capillary electrophoresis(CE).
2.1.Electro-osmotic flow(EOF)
EOF is based on the application of a high voltage to an electrolyte present in a capillary tube.EOF takes place as a buffer(pH>3)and causes a proton loss from the SiOH group from a silica capillary tube to form SiO-ions[7].The capillary wall imbibes a negative charge,which then develops a double layer of cations.The inner cation layer is stationary,whereas the outer layer is free to move along the capillary.The applied electric field causes free cations to move toward the cathode,creating a powerful bulk flow.The movement of the conductive medium through a capillary in response to an applied electric field is depicted in Fig.2.The magnitude of the EOF can be expressed in the terms of velocity or mobility.Because the charges are strongly pH-dependent,the magnitude of the EOF varies with pH.For an effective separation,a change in the pH of the buffer plays an important role.The change in pH from above the isoelectric point to below the isoelectric point changes the net charge of the solute from negative to positive.An increase in buffer pH generally increases the EOF.The EOF rate can be controlled by the field strength and the charge density of the capillary wall.
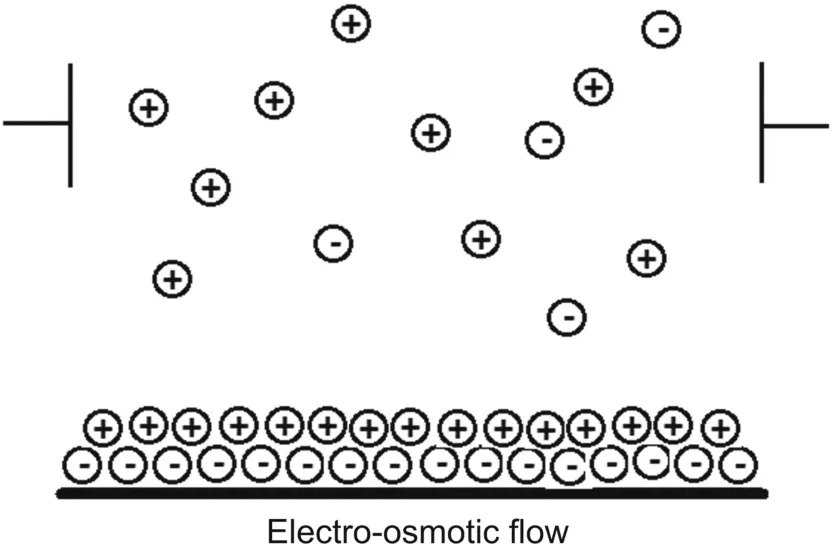
Fig.2.Movement of a conductive medium through a capillary.
2.2.Electrophoretic mobility
Electrophoresis is a method in which ions migrate owing to the applied voltage[8].The force applied to the ions is the cumulative net charge of the products and the electric field strength.This combined expression leads to electrophoretic mobility,which is a measure of solute mobility that helps a solute to move through a conductive medium in response to an applied electric field[9-11].CE is an electrophoretic method where a buffer is added into a narrow bore silica capillary/fused silica capillary with an internal diameter of approximately 25-100μm,and the capillary wall is covered with ionizable silanol groups(SiOH)(Fig.3).When the voltage is applied to the solution,molecules move through the solution toward the electrodes of opposite charges,and a detector helps to measure the absorbance of the molecules as they pass through the solution.The various types of detectors used in this system include UV/vis absorption detectors,fluorescence detectors,radiometry detectors for radioactive substances,laser-induced fluorescence detectors,conductivity detectors,electrochemical detectors,and mass spectrometers.The absorbance is analyzed using a computer system and presented graphically.
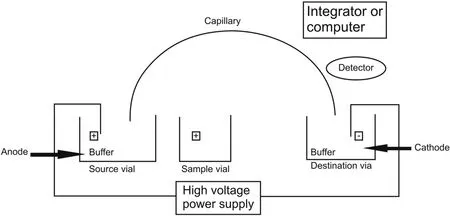
Fig.3.Instrumentation of CE.
3.Types of capillary electrophoresis
The different types of CE include capillary zone electrophoresis(CZE),capillary gel electrophoresis(CGE),micellar electrokinetic chromatography(MEKC),microemulsion electrokinetic chromatography(MEEKC),capillary electrochromatography(CEC),capillary isoelectric focusing(CIEF),and capillary isotachophoresis(CITP).Electrophoresis is divided into two major parts:a continuous system and a discontinuous system(Fig.4).The continuous system has a background electrolyte(BGE)acting throughout the capillary as a buffer and is further divided into two subtypes:the kinetic and the steady-state processes.The kinetic process has a persistent electrolyte content,whereas the steady-state process consists of varying electrolyte content.The sample component in a discontinuous system is divided into two different electrolytes,i.e.,one with higher mobility known as leading electrolyte and other one with buffer with lowest component,which is slower than molecule[12-14].

Fig.4.Classification of various CE techniques under the continuous and discontinuous systems.CITP:capillary isotachophoresis;CIEF:capillary isoelectric focusing;MEKC:micellar electrokinetic chromatography;CZE:capillary zone electrophoresis;CGE:capillary gel electrophoresis.
3.1.Capillary zone electrophoresis(CZE)
CZE is the most widely used CE method,and it is also referred to as the free solution CE technique.In CZE,the separation is quick and easy for a mixture in solution.The principle of this method is based on the change in electrophoretic mobility,which is directly proportional to the molecular charge and inversely proportional to the atomic radius and viscosity of a particular solvent.The fused silica capillary has silanol groups,and gets ionized in the buffer solution.Anions present in the solution are attracted toward the anode;however,a few of them migrate toward the cathode.Cations with the largest charge-to-mass ratio are separated first,followed by anions and finally neutral compounds.The electro-osmotic velocity can be changed by changing parameters,such as the viscosity,strength,pH,and dielectric constant(Fig.5).

Fig.5.Capillary zone electrophoresis(CZE).EOF:electro-osmotic flow.
3.2.Microemulsion electrokinetic chromatography(MEEKC)
MEEKC is a special method of electrokinetic chromatography that accomplishes electrokinetic separation using microemulsions as background electrolytes.MEEKC separates solutes based on the hydrophobicity and differences in electrophoretic mobility,and it offers highly efficient separation of charged and neutral solutes covering a wide range of water solubility.Analytes may be partitioned between the aqueous phase of the microemulsion and its oil droplets,which act as a pseudo-stationary phase.This technique is well suited for the separation of neutral species containing charged oil droplets(obtained by the addition of an anionic or cationic surfactant).The components that are immiscible organic solvents(n-heptane),co-solvents(n-butanol),and surfactants act as buffers.
3.3.Micellar electrokinetic electrophoresis(MEKC)
MEKC is a technique based on the transfer of solute partitioning between micelles and solvents.The aggregation of micelles occurs when a surfactant is mixed with a solution above the critical micelle concentration.The aggregated micelles comprise a polar negatively charged surface that is the cathode and are attracted toward a positively charged surface that is the anode.Because of the EOF toward the cathode,the micelles are attracted toward the cathode but at a slower speed.The hydrophilic molecules do not stay for longer periods and migrate through the solvent,whereas hydrophobic molecules require more time to migrate.In the absence of micelles,neutral species move with the help of EOF and separation does not occur(Fig.6)[10,11].
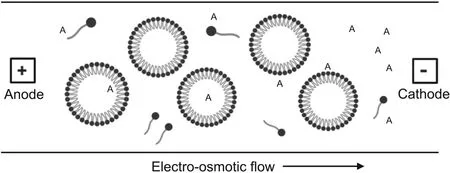
Fig.6.Micellar electrokinetic capillary electrophoresis(MEKC).
3.4.Non-aqueous capillary electrophoresis
In non-aqueous CE,separation is based on differences in the physicochemical properties of molecules.The migration of the solute depends on the nature of the solvent and its mixture.
4.Previous reviews on capillary electrophoresis
Many reviews have been published on the applications,current progress,and updates on recent advances in CE in terms of analysis,identification,compound characterization,and impurity profiling of drugs,biopharmaceuticals,and other biomolecules.Many authors have described the applications of different CE methods for the assay and identification of metabolites from different biological matrices,such as serum,plasma,urine,and blood;and biomolecules,such as carbohydrates,proteins,lipids,such as fatty acids,vitamins,and nucleic acids.In this section,we summarize the past reviews published on CE year-wise as shown in Table 1[15-29].
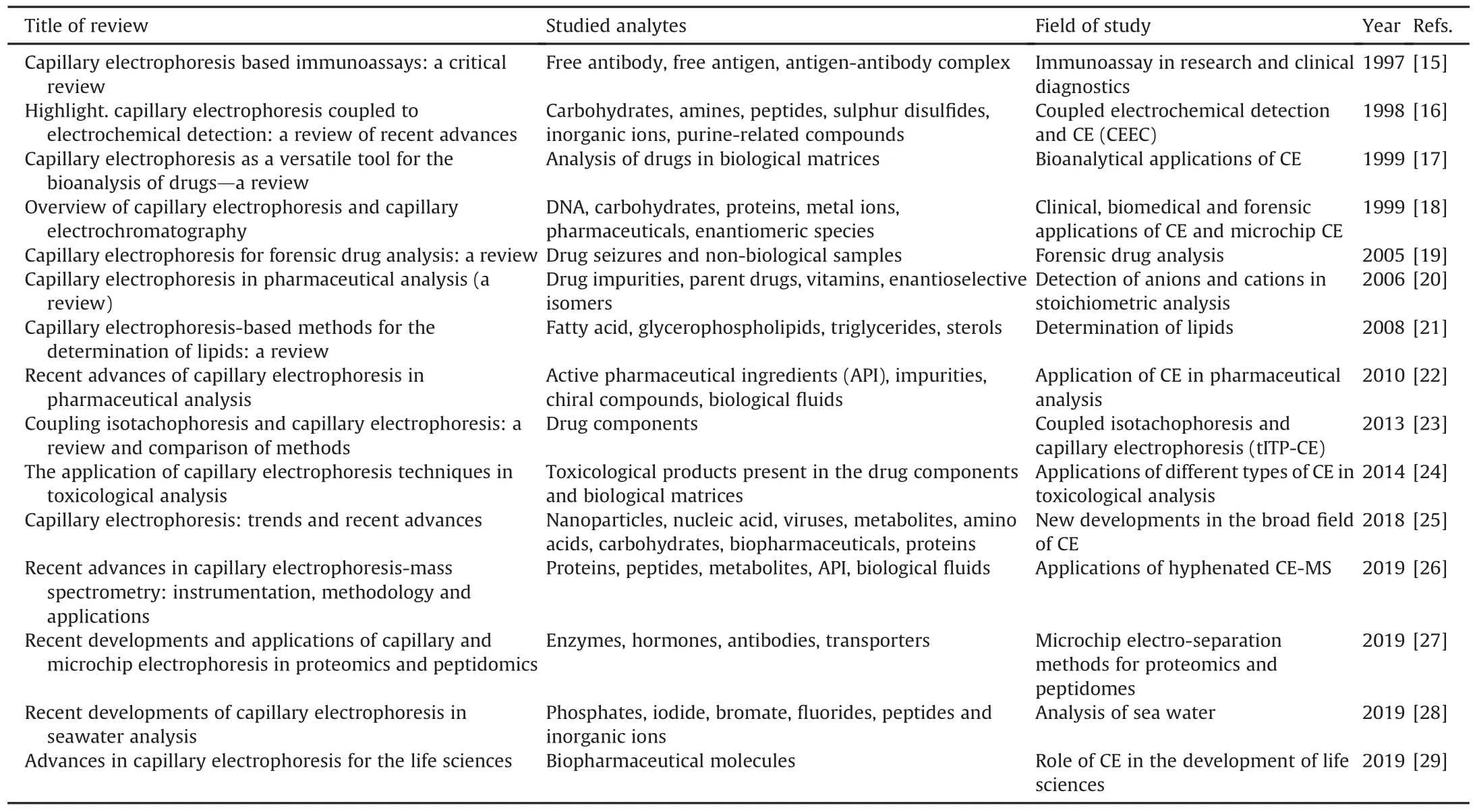
Table 1Year-wise details of past reviews on CE along with studied analytes and field of study.
5.Applications of different capillary electrophoresis methods for impurity profiling of drugs
5.1.Capillary zone electrophoresis(CZE)
Elbashir et al.[30]developed and validated a CZE method for the identification of quinocide impurities(Fig.7)in primaquine.For evaluation purposes,native cyclodextrin(CD)and crown ether were used for the capillary ion analysis.The notable finding by the authors was the interactions of quinocide and primaquine with buffer additives(β-CD),which led to the reverse order of elution of the analytes.The method could separate primaquine and quinocide in less than 5 min with a resolution of 3.5-3.8 between analytes.The authors also explained the mechanism of complex formation and separation of analytes using buffer additives(Table 2)[31-38].El-Attug et al.[31]developed a method to determine kanamycin sulfate and its related impurities using the CZE technique with the help of a P/ACE MDQ instrument(Beckman Coulter,USA)(Fig.7).The advantages of this method are 1)its ability to resolve kanamycin base,its sulfate salt,and related impurities in a single run,and 2)it was applied to drugs lacking chromophoric groups,and separation was achieved in less than 6 min(Table 2).The authors[32]drafted a method for the development and validation of amikacin,tobramycin,and its related impurities,nebramine and niamine,using CZE with the help of a P/ACE MDQ instrument(Beckman Coulter,USA).A similar approach was applied and used for kanamycin,and a method was developed for drugs lacking chromophores(Table 2).Orlandini et al.[33]applied the quality by design(QbD)approach for the development of a CE method for the simultaneous assay of metformin hydrochloride and its main impurities(Fig.7).In this study,the authors selected seven critical process parameters related to capillary,injection,BGE,and instrumental settings.This method allowed the separation of a significant amount of impurities in a very short time and obtained satisfactory results for the validation parameters and analysis of real samples.The method could separate metformin hydrochloride,internal standard imidazole hydrochloride,and metformin hydrochloride impurities in less than 9 min.This was the first report on method development using CD-CZE and QbD approaches.QbD can save experimental efforts for analytical method development(Table 2).Orlandini et al.[34]developed and validated a CZE method for the determination of levosulpiride(Fig.7)and its enantiomeric impurity using the QbD approach.The method could separate chiral S-and R-levosulpiride enantiomers in less than 12 min.This was the first reported method for the determination of the enantiomeric purities of a chiral substance using the QbD approach(Table 2).The CD-modified micellar electrokinetic chromatography was devised for enantioseparation and impurity determination of ambrisentan(Fig.7)using the CZE method[35].The method was developed using the QbD approach and the response surface methodology.The study showed an extensive effect of pH and γ-CD concentration on the enantiomeric separation of analytes(Table 2).The authors[36]developed a stability-indicating CZE method,a first report,for the assay of tipranavir(Fig.7,Table 2)and its two major organic impurities,which were recognized by high-resolution mass spectrometry.The results of this study indicated that tipranavir was more prone to oxidative degradation than other stress conditions following second-order degradation kinetics.The CZE method[37]was used for the identification of zofenopril calcium and hydrochlorothiazide in the presence of two main impurities of hydrochlorothiazide,chlorothiazide,and salamide(Fig.7)using an Agilent 7100 CE instrument equipped with a photodiode array(PDA)detector.The advantage of this method is that it can separate four analytes of interest in less than 5 min.The authors used the QbD approach for the development and application of this method and claimed it to be a green technology compared to the conventional analytical methods(Table 2).The CZE analytical method[38]was used for the enantioseparation and analysis of cinacalcet and its impurities(Fig.7)using the QbD approach and HP 3D CE(Agilent technology)equipped with UV-vis diodearray detector(DAD).The analytes were separated within 10 min(Table 2).
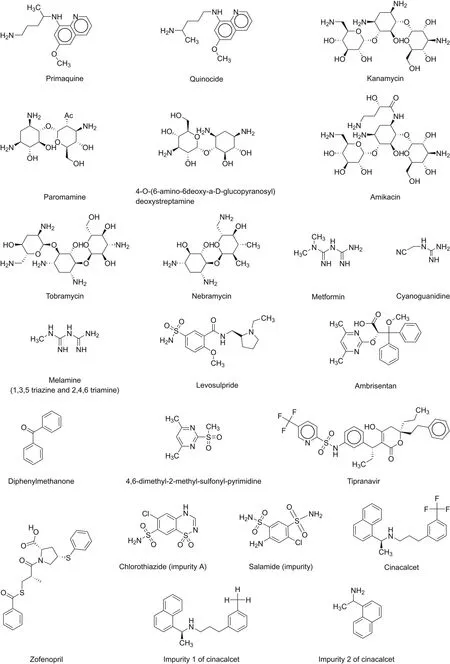
Fig.7.Chemical structures of drugs and their related substances/impurities identified/searched using CZE.
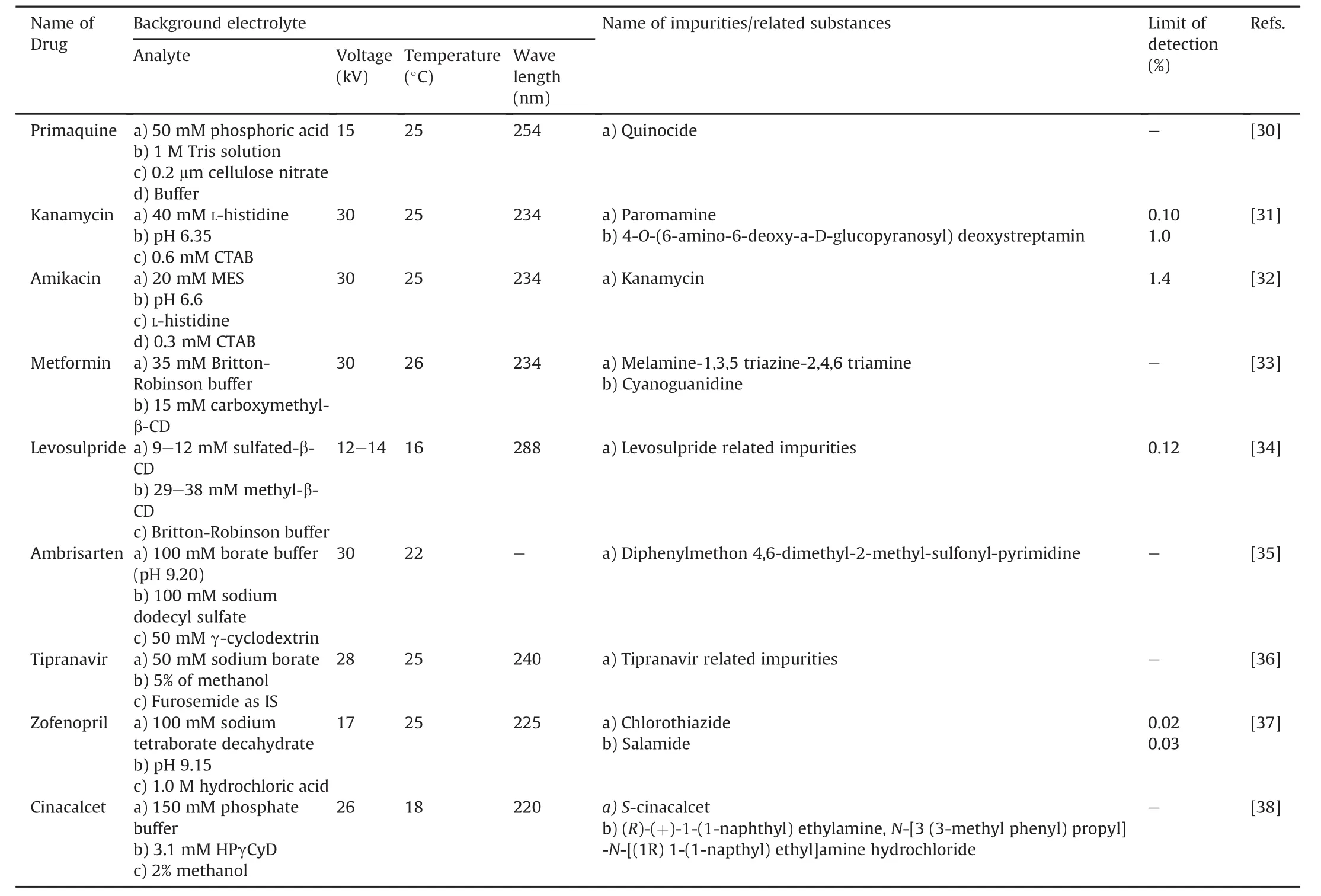
Table 2Review of capillary zone electrophoresis(CZE)method with the details of background electrolyte conditions for impurity profiling of drugs.
CZE is a powerful separation technique employed for the impurity profiling of various drugs.Various researchers have applied this technique for the impurity profiling of drugs,such as primaquine,kanamycin,amikacin,metformin,levosulpiride,ambrisentan,tipranavir,zofenopril,and cinacalcet.The technique could detect impurities below the reporting threshold according to the ICH Q3A and Q3B guidelines.
5.2.Microemulsion electrokinetic chromatography(MEEKC)
Orlandini et al.[39]used the MEEKC method with the QbD approach to determine clemastine and its three main impurities(Fig.8)using an Agilent Technologies 3DCE system equipped with a UV-vis detector and thermosetting system microemulsion.This dual CD system-modified MEEKC method was reported for the first time as an analytical method for the analysis of clemastine and its impurities(Table 3).Wongwan et al.[40]reported a modified CD MEEKC method for the estimation of charged and uncharged impurities of dexamphetamine,mainly levorotary enantiomer in dexamphetamine sulfate(Fig.8).Separation was performed using a Beckman Coulter P/ACE system.The percentage of levoamphetamine in dexamphetamine ranged from 3.2% to 3.8%.The method could quantify impurities at the 0.1% level,except for the Z-stereoisomer.The method was claimed to be the first reported CD-modified MEEKC method for the simultaneous estimation of charged and uncharged impurities,including stereoisomers(Table 3).Orlandini et al.[41]used the MEEKC method for the identification of ramipril and its eight main impurities(Fig.8)using an Agilent Technologies 3DCE system equipped with a UV-vis DAD.Separation was achieved within 10 min of the analysis.This method was able to resolve degradation products,which isomerize during acidic degradation under the selected experimental conditions(Table 3).The authors[42]developed an assay method using MEEKC for determination of almotriptan and its impurities(Fig.8)using the QbD approach and multiple process variables,which were further verified on an Agilent Technologies 3DCE system equipped with a UV-vis DAD(Table 3).Kaale et al.[43]developed and validated an analytical method for fiuoroquinolones and their impurities using MEEKC(Fig.8),where the separation was carried out using Beckman P/ACE MDQ CE.This method could separate five fluoroquinolone impurities in a run time of 20 min.The MEEKC method has more weightage as it can resolve impurities A-E of ciprofloxacin and impurities A and E of ofloxacin in a single run,which is not possible with the official European Pharmacopoeial method(Table 3).
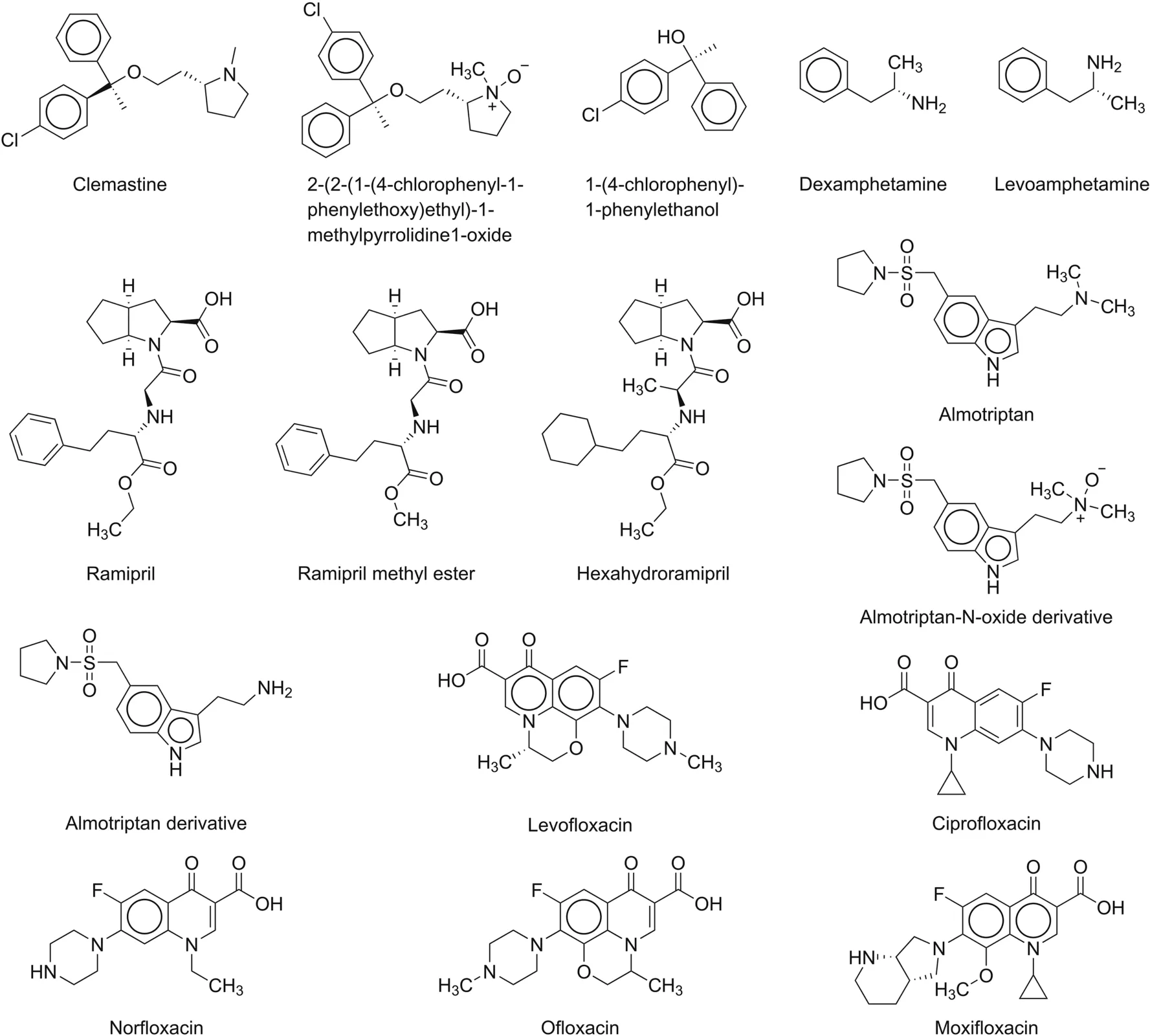
Fig.8.Chemical structures of drugs and their related substances/impurities identified/searched using microemulsion electrokinetic chromatography(MEEKC).
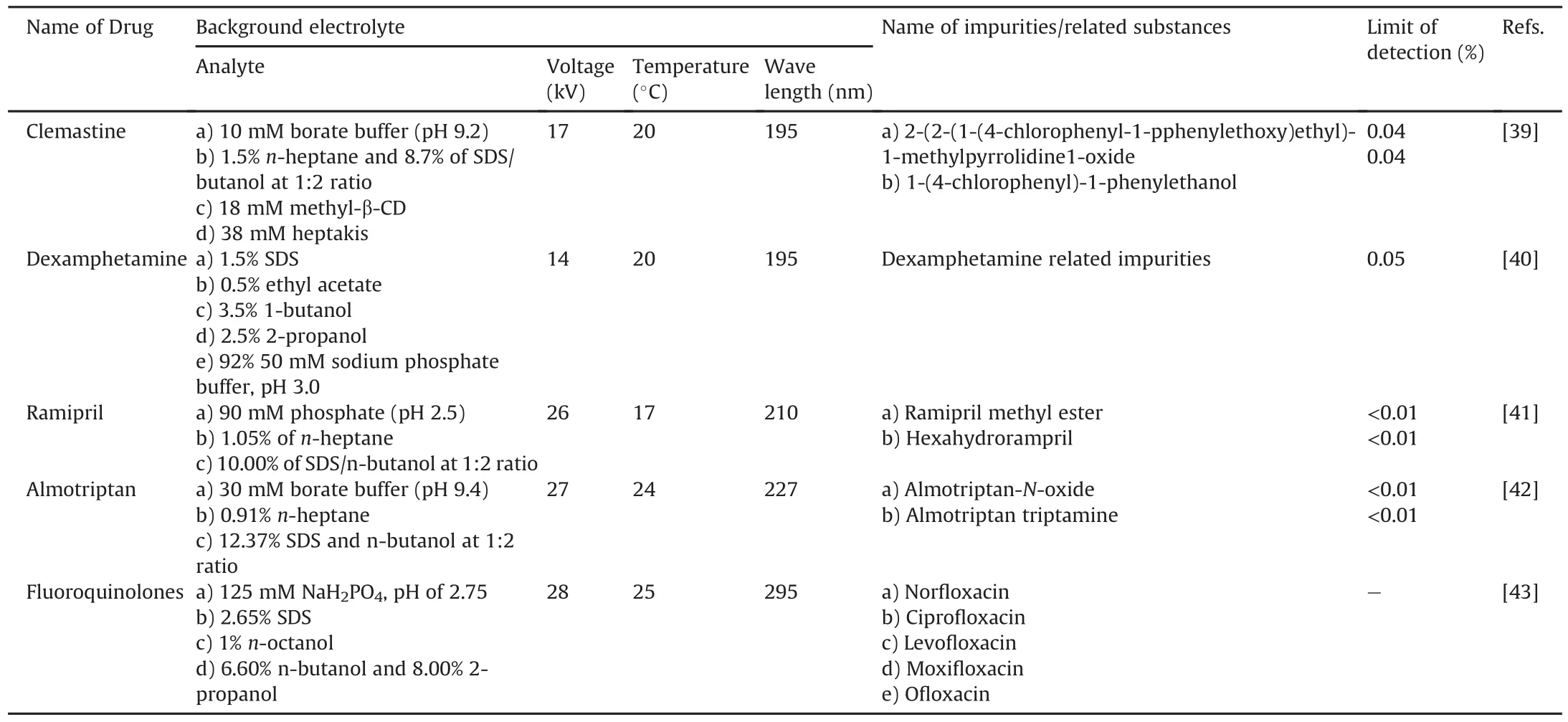
Table 3Review of microemulsion electrokinetic chromatography(MEEKC)method with the details of background electrolyte conditions for impurity profiling of drugs.
MEEKC was employed for the determination of impurities in various drugs mentioned in the API and formulations.Various researchers have used conventional and novel approaches such as the use of QbD for method development in their research work.The MEEKC method can resolve very low levels of impurities from the main analyte with a very low detection limit,complying with the requirements of the ICH Q3A and Q3B guidelines.
5.3.Micellar electrokinetic chromatography(MEKC)
Furlanetto et al.[44]developed an analytical MEKC method using the QbD approach and validated it for budesonide and its impurities(Fig.9,Table 4).Michalska et al.[45]devised a MEKC method for ertapenem and its impurities(Fig.9)where separation of the impurities was carried out on a Quanta 4000E CE system equipped with a UV spectrophotometric detector.The limit of detection was calculated at the level of 0.3μg/mL,whereas the limit of quantification was calculated at the level of 0.8μg/mL.Al Azzam et al.[46]developed an analytical MEKC method for valacyclovir and its impurities(Fig.9)where the separation of impurities was performed using the HP3DCE CZE system.The method was less sensitive than LC-MS;however,the separation time was less than 4 min,which is the most important feature of the developed method that can detect low concentrations(less than 0.7%)of guanine in accordance with the European Pharmacopeia(Table 4).Doomkaew et al.[47]developed a simple aqueous-based stability method,indicating the analytical MEKC method for gliclazide and its specified impurities,gliclazide impurities B and F(Fig.9)where separation was carried out using a 3DCE instrument model G1600A(Agilent Technologies,Waldron,Germany)equipped with a PDA detector.The method separate gliclazide and its impurities in less than 5 min.This method resolved impurity B and an unknown impurity generated during a stress degradation study.However,impurity F was not resolved using the developed method(Table 4).Hu et al.[48]developed an analytical method for the analysis of palonosetron hydrochloride(Fig.9)stereoisomers using sodium cholate as a chiral selector on a TH-3100 CE system equipped with a UV detector.The authors studied 10 different solvents for the separation of palonosetron stereoisomers.Methanol was found to be the best solvent for analyte resolution.The method could measure very low concentrations of palonosetron distomers in the presence of high concentrations of eutomers(Table 4).Orlandini et al.[49]devised a fast and selective CE method for the analysis of zolmitriptan and its five potential impurities(Fig.9)using the QbD approach(Table 4).Gonget al.[50]developed the MEKC method for the analysis of goserelin and its related impurities(Fig.9)using an Agilent 7100 CE instrument equipped with a DAD.This method was able to separate nine peptides with almost the same relative molecular mass.The separation of these nine peptides was achieved in less than 30 min.This proposed method resolved the impurities(DTyr5)-goserelin and(D-His 2)-goserelin,which cannot be resolved using the European Pharmacopoeial method(Table 4).

Fig.9.Chemical structures of drugs and their related substances/impurities identified/searched using MEKC.
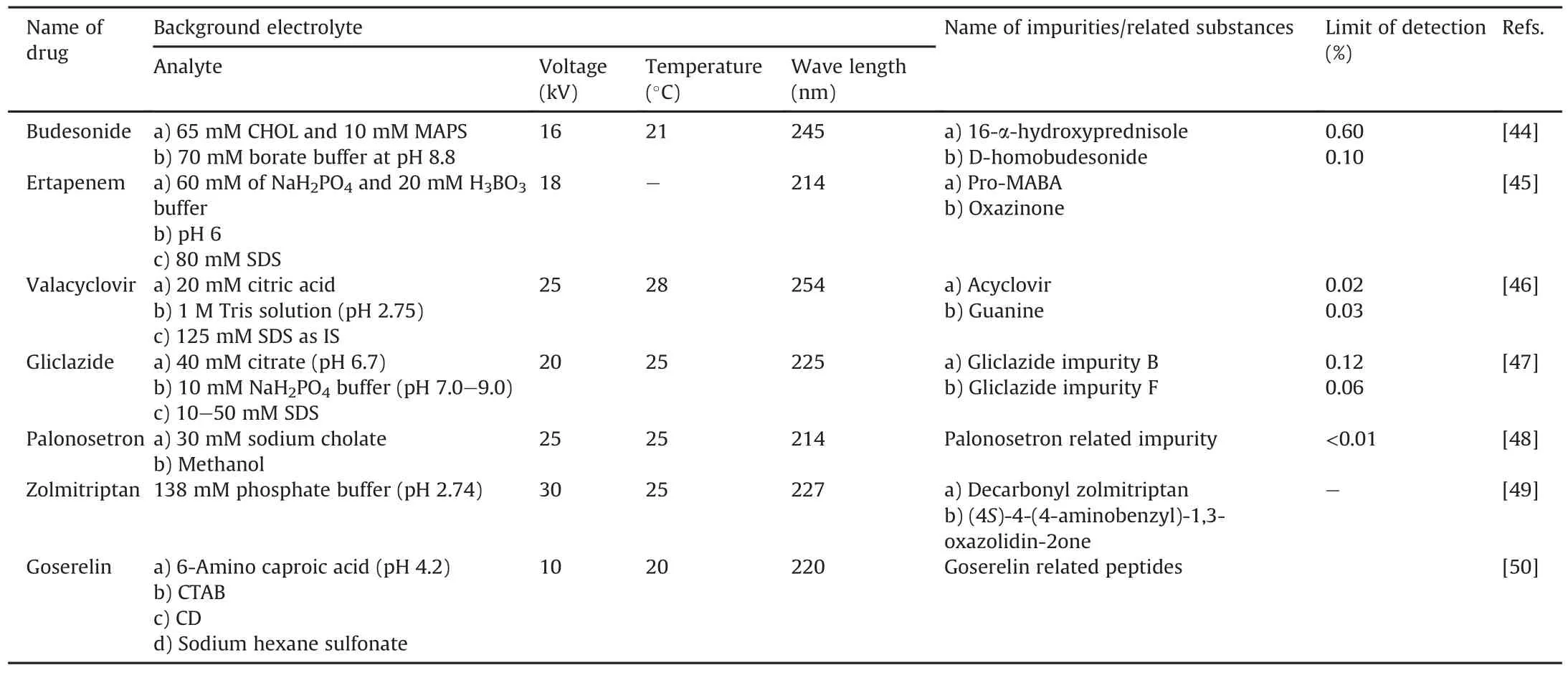
Table 4Review of micellar electrokinetic chromatography(MEKC)method with the details of background electrolyte conditions for impurity profiling of drugs.
MEKC is a widely adopted separation technique for the impurity profiling of drugs owing to its excellent resolution capabilities.In the present review, we summarize various MEKC methods employed for impurity profiling of drugs such as budesonide,valacyclovir,ertapenem,gliclazide,palonosetron,zolmitriptan,and goserelin.MEKC is sensitive enough to detect impurities below the reporting threshold level according to the ICH Q3A and Q3B guidelines.
5.4.Non-aqueous capillary electrophoresis
Rousseau et al.[51]established a non-aqueous CE method for the analysis of flurbiprofen and its impurities(Fig.10)using an HP 3D CE system equipped with a DAD.BGE is composed of phosphate buffer at pH 2.5,n-heptane,SDS,and n-butanol;the temperature was set at 17°C,voltage at 26 kV,and wavelength at 250 nm.In the developed method,IPA-β-CD was used as a chiral selector,and flufenamic acid was used as the internal standard.The method detected 0.1% S-flurbiprofen in R-flurbiprofen.
Tonon et al.[52]developed an electrokinetic enantioselective chromatography method for zopiclone and its impurities(Fig.11)using an Agilent CE system model G6100A equipped with a photodiode array detector.BGE comprised 80 mM sodium phosphate buffer(pH 2.5)and 5 mM carboxymethyl-β-CD as a buffer;the temperature was set at 25°C,voltage at 27 kV,and wavelength at 305 nm.The method was successfully applied for the determination of impurities in the zopiclone tablet formulation.
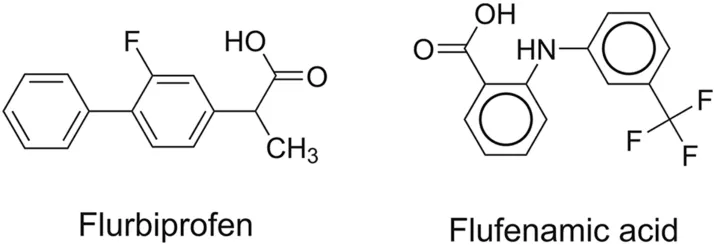
Fig.10.Chemical structures of flurbiprofen and flufenamic acid identified using nonaqueous capillary electrophoresis.
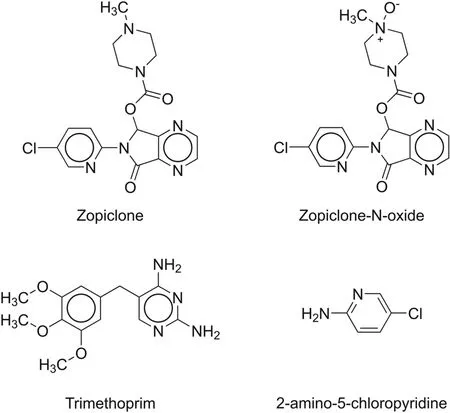
Fig.11.Chemical structures of zopiclone and its impurities identified using electrokinetic chromatography.
6.Analysis of pharmacopeial monographs using capillary electrophoresis methods
CE is a method of choice for the analysis of some published Pharmacopeial monographs,especially for analysis of glycan biomarkers,proteins,polar small molecules,hormones,and peptides.In the European Pharmacopeia(Ph.Eur.)monograph of aprotinin and its concentrated solution[53],CZE with a UV detector was used for the determination of related impurities.Similarly,CZE with a UV detector was used to determine erythropoietin isoforms in the erythropoietin concentrate as per the Ph.Eur and the United States Pharmacopeia-National Formulary(USP-NF)monographs[53,54].Aprotinin is a protein-based drug that is widely used for the analysis and characterization of biopharmaceuticals.Higher resolution,better separation efficiency,and shorter analysis time make CE a versatile technique for the analysis and identification of biologicals and biosimilars.CE,along with many other techniques,is currently used for the analysis of intact proteins and glycans.Almotriptan malate,a selective serotonin 5-HT1B/1D agonist,is a second-generation triptan.CE was found to be the method of choice for the determination of almotriptan impurities.As per the USP-NF[54]acceptance criteria,the resolution should be not less than 2.0 between almotriptanimpurity B and almotriptan peaks and between almotriptanimpurity D and almotriptan peaks.The relative standard deviation should not exceed 5.0% of the peak response ratio.CE proved to be one of the best methods for the detection of almotriptan impurities.In the case of alteplase for injection[55],complex peaks of glycopeptides were separated from the tryptic peptide using the CE method.In the recombinant DNA technology,for peptide mapping,separation is required between the test and reference proteins and CE plays a vital role in the analysis and characterization of dried factor VIII(rDNA)[55].As per the British Pharmacopeia(B.P.)monograph,the characterization of human α-1 proteinase was performed using CE techniques[55].
In this section,we have provided the details of the Pharmacopeial monographs referred from different Pharmacopeias,such as USP-NF,Ph Eur,and BP.The names,monograph numbers,related background electrolyte conditions,and names of the impurities/impurity-related substances are listed in Table 5.

Table 5Review of CE methods for Pharmacopeial monographs with the details of monograph number,background electrolyte conditions and name of impurities/related substances.
7.Comparison of capillary electrophoresis with liquid chromatography for the impurity profiling and analysis of drugs
CE is an analytical technique that has shown great potential in replacing many conventional analytical methods in the last few years.The main advantage of CE over LC is its high efficacy and low reagent consumption.It is a highly versatile and rapid method ofanalysis,which requires a very small amount of analytes and can analyze both neutral and charged species.Owing to its large peak capacity,this method is suitable for the separation of impurities from the main drug,which may resemble the chemical structure of the compounds.Moreover,its high efficacy and low reagent usage make it more visible against LC.A simple comparison of CE with LC is presented in Table 6.
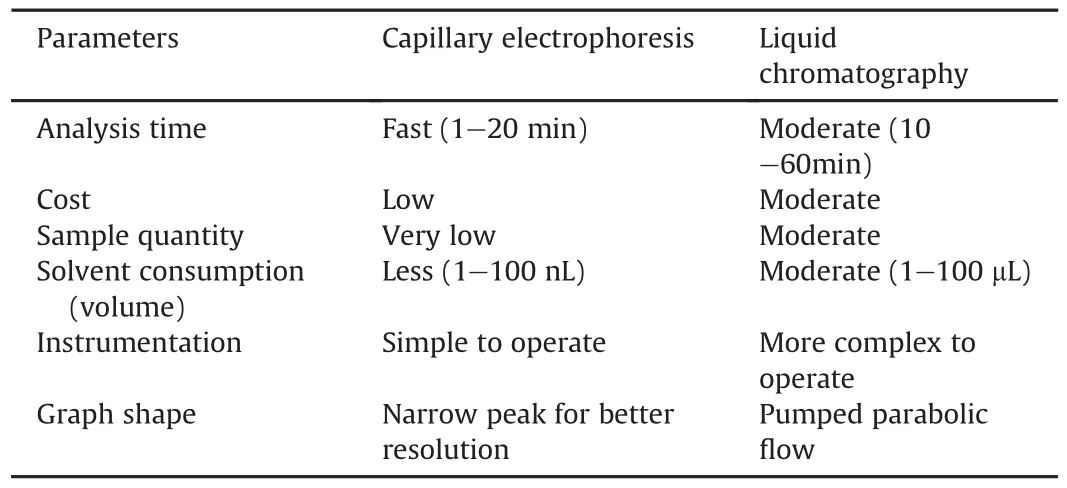
Table 6Comparison of CE with LC.
In this section,we compare CE and LC methods with examples of impurity profiling of drugs and CE has proven to be a better option in many cases than the LC methods;however,in some cases,LC is found to be better than CE.A comparison of CE and LC with examples of drugs along with more accurate information regarding the estimated cost of analysis is presented in Table 7[56-68].In these studies,the authors performed impurity profiling of drugs using CE and LC methods and compared both the methods.
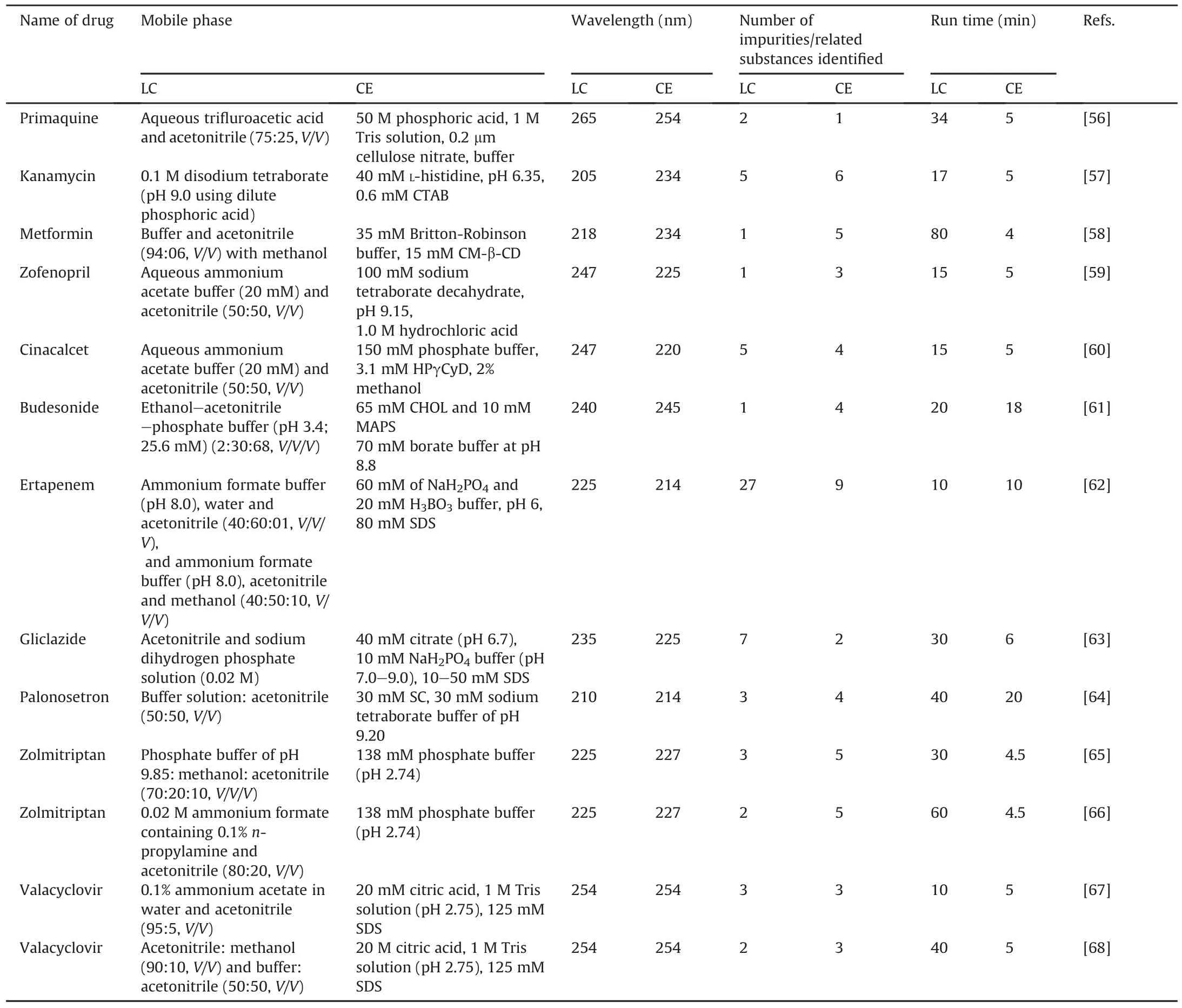
Table 7Comparison of LC and CE with examples of impurity profiling of drugs.
Based on the above comparison,it is clear that the LC method consumes more time and solvent than the CE method.In most of the examples,CE could scan more impurities than LC in the impurity profiling of the same drug;however,in some cases,the LC method separated and detected more impurities.In the maize protein characterization,both HPLC and CE methods were used,and CE was the preferred method of choice owing to its small scale,low sample and reagent consumption,and separation of large molecules in less time[69].CE proved to be a potential alternative method for the analysis of antifungal drugs such as nystatin,5-fluorocytosine,and miconazole in many ways[70]as it reduced the analysis time and solvent consumption,and the CE instrument was easier to handle.In the case of decaffeinated coffee,both separation techniques(CE and LC)were used and the CE method was found to be better because of faster analysis,low cost,and very low sample quantity.For the separation and quantification of D-tagatose[71]in the presence of its related impurities,CE showed better sensitivity and resolution than HPLC and was the preferred method for impurity profiling.CE and HPLC methods were developed and compared for the determination of carvedilol enantiomers in the serum[72].The limits of quantitation were lower in HPLC.The CE assay offered the advantage of a faster analysis time and lower consumption of solvents.Stereochemical analysis of duloxetine was performed using LC and CE methods[73].In an achiral and chiral analysis of duloxetine,CE was a viable alternative method because of its high efficiency,short analysis time,and low sample and reagent consumption.For the determination of diclofenac sodium in a tablet dosage form,both CE and LC analyses were performed and compared[74].Regarding some aspects of linearity,recovery,and specificity,CE provided faster analysis and better column efficiency,whereas LC provided superior repeatability and sensitivity.The determination of diazepam in the tablet dosage form was performed and compared using both CE and LC analysis methods[75].The authors found both methods to be suitable with similar performance in terms of the determination of the compounds;however,with regard to linearity,recovery,and specificity,CE provided faster analysis and column efficiency,whereas RP-HPLC presented superior repeatability and sensitivity.For quantitative determination of ketoconazole in drug formulations,both CZE and HPLC methods were performed and compared[76].The authors found that the CZE method was more selective,whereas the HPLC method was more sensitive(limit of detection was 2.5 times less)and provided better precision(relative standard deviation<2%).
With all the examples discussed above,it is suggested that CE is a versatile technique for not only the analysis,characterization,and impurity profiling of drugs but also for the research and development of new drugs and biopharmaceuticals as well as for pharmaceutical quality control.
8.Summary and conclusion
Recently,impurity profiling of pharmaceutical products has attracted significant attention because impurities can be detrimental to human health and can adversely affect the quality of pharmaceuticals.Currently,various methods are available for impurity profiling and other hyphenated techniques.The flexibility of CE has greatly increased with the possibility of attaching various types of detectors,and it can also be hyphenated with other sophisticated techniques to increase its sensitivity for the detection of various new chemical entities and different types of compounds[77].With the advent of highly automated multicapillary instruments and cost-effective kits,CE is being widely accepted in clinical laboratories.It can be used for the analysis of different APIs in various formulations.CE can also be efficiently used for the analysis of food products,food colors,vitamins,and natural products.CE has been effectively used for the determination of phenolic compounds,organic acids,toxins,and agricultural residues in food materials.Currently,CE is becoming a highly popular method for the analysis of biologicals and biopharmaceuticals and is also a preferred method for fast highresolution DNA sequencing.Advancements in CE,such as affinity CE,high-throughput capability techniques,multi-capillary arrays,and chip separations,hold a promising future and can be used extensively to study food-drug interactions.With this review,it is obvious that CE will continue to gain popularity and serve as a valuable technique in the pharmaceutical sector for drug development and quality control processes.
CRediT author statement
Mansi Shah:Literature reviewing,Writing-Original draft preparation;Nrupesh Patel:Visualization and Supervision;Nagja Tripathi:Data presentation;Vivek K.Vyas:Chemical structures preparation,Writing-Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interests.
Acknowledgments
The authors would like to thank Institute of Pharmacy,Nirma University,Ahmedabad,India for providing the necessary facilities.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Time-course monitoring of in vitro biotransformation reaction via solid-phase microextraction-ambient mass spectrometry approaches
- Erythrocyte sphingolipid species as biomarkers of Alzheimer's disease
- Biological analysis of an innovative biodegradable antibiotic eluting bioactive glass/gypsum composite bone cement for treating experimental chronic MRSA osteomyelitis
- Global characterization of modifications to the charge isomers of IgG antibody
- Pharmacokinetics,distribution,and excretion of sodium oligomannate,a recently approved anti-Alzheimer's disease drug in China
- An integrated strategy for comprehensive characterization of metabolites and metabolic profiles of bufadienolides from Venenum Bufonis in rats
