Mechanical circulatory support in cardiogenic shock and postmyocardial infarction mechanical complications
2022-03-24DanielRobJanlohlvek
Daniel Rob, Jan Bělohlávek✉
2nd Department of Medicine–Department of Cardiovascular Medicine, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic
ABSTRACT Despite advanced therapies, the mortality of patients with myocardial infarction (MI) complicated by cardiogenic shock (CS) remains around 50%. Mechanical complications of MI are rare nowadays but associated with high mortality in patients who present with CS. Different treatment strategies and mechanical circulatory support (MCS) devices have been increasingly used to improve the grim prognosis of refractory CS. This article discusses current evidence regarding the use of MCS in MI complicated by CS, ventricular septal rupture, free wall rupture and acute mitral regurgitation. Device selection should be tailored according to the cause and severity of CS. Early MCS initiation and multidisciplinary team cooperation is mandatory for good results. MCS associated bleeding remains a major complication and an obstacle to better outcomes. Ongoing prospective randomized trials will improve current knowledge regarding MCS indications, timing, and patient selection in the coming years.
Cardiogenic shock (CS) is a clinical syndrome of reduced cardiac output with critical end-organ hypoperfusion and encompasses wide spectrum of clinical scenarios from early shock states to refractory shock with multiorgan failure. Clinical presentation and symptoms onset of CS may significantly differ even in the most common ST-segment elevation myocardial infarction (MI) scenario. Patients may be in profound CS at admission, some patients can worsen during percutaneous coronary intervention (PCI), or may develop CS during the intensive care unit stay. Despite the importance of identification patients who will not respond to standard therapy and are candidates for mechanical circulatory support (MCS),generally accepted definition of refractory CS has not been established. The diagnosis of refractory CS should be based on easily accessible clinical criteria(signs of hypoperfusion, invasive blood pressure,serum lactate, SvO2levels, echocardiography, initial response to fluids and drug therapy) as the patient status may evolve rapidly. Persistent hypotension, high lactate (two consecutive values ≥ 3 mmol/L)and/or low central venous O2levels (SvO2two consecutive values < 50%), despite adequate pharmacological treatment (norepinephrine dose > 0.2 μg/kg per min + dobutamine dose > 5 μg/kg per min) proposed by Ostadal,et al.,[1]is closest to real-life scenario. In an effort to improve CS stratification, the Society of Cardiovascular Angiography and Interventions expert consensus statement on the classification of CS has been published and endorsed by several international societies.[2]Stages D and E of this classification implies refractory CS but definition of therapy where clinicians should consider MCS is too unclear compared to Ostadal,et al.[1]Whether these CS classifications will improve decision making (including MCS indication and timing) and patient outcomes must be clarified in future studies.Another clinically important question is whether patients with out-of-hospital cardiac arrest should be included in CS studies as shock after cardiac arrest is combined with distributive shock component and hypoxic brain injury plays a major role in survival of these patients. Inclusion of patients with out-of-hospital cardiac arrest makes the interpretation of CS studies and treatment effect difficult.Most patients with CS present with MI, of them approximately 10% have mechanical complications[acute mitral regurgitation, ventricular septal rupture (VSR), or rupture of the free wall].[3]Several studies showed that prevalence of CS complicating MI is decreasing in the last two decades, but mortality of these patients remains high, especially in patients who present with mechanical complications.[4,5]The optimal therapeutic strategy in refractory CS is a matter of ongoing debate and current clinical research focus on the use of MCS as the most promising methods to improve survival. This article summarizes current trends in the management of MI complicated with CS, including clinical scenarios of mechanical complications.
STANDARD CS THERAPY WITH RESPECT TO MCS USE
Standard therapy of CS consists of catecholamines, inotropes, fluids, oxygen therapy together with primary PCI in the acute MI setting.[6,7]
Despite the routine use of catecholamines and inotropes, there is only limited evidence from randomized trials comparing catecholamines and inotropes in CS.[7–9]The first-line vasopressor agent for CS is norepinephrine as it has been associated with a better outcome compared to dopamine and epinephrine.[8]The most frequently used inotropic agent for CS is dobutamine whereas levosimendan is preserved as a second-line agent or preferentially in patients previously treated with beta-blockers.[9]All vasopressors increase myocardial oxygen consumption, the risk of arrhythmias, may impair microcirculation and increase afterload.[6,7,9]Instead of detrimental vasopressors escalation to very high doses,early MCS initiation should be considered to avoid multiorgan dysfunction development.
Fluid therapy in CS should be individualized as there is a lack of evidence-based approach.[10]Fluid challenge in hypotensive patients is recommended but special consideration must be given to the risk of fluid overload and worsening of lung edema.[7,10]Fluid management in patients with MCS, especially during venoarterial extracorporeal membrane oxygenation (VA-ECMO) treatment is difficult. Positive fluid balance increases the risk of pulmonary edema and substantial amounts of fluids negatively impact survival.[11]On the other hand, VA-ECMO drainage is dependent on venous return and vascular volume, thus aggressive fluid therapy is sometimes inevitable for proper pump function and hemodynamic stabilization. The results of a retrospective study suggest that albumin fluid resuscitation may improve hospital survival.[12]
Early restoration of coronary blood flow is a wellknown major predictor of survival in CS and during the acute CS phase, culprit vessel only PCI is associated with better results compared to multivessel PCI.[6]Therefore, immediate PCI should never be delayed or postponed due to other therapeutic procedures (e.g., intubation). Close shock team cooperation, mainly between interventional cardiologists and intensivists, is of paramount importance. The critical question whether the patient is a suitable candidate for MCS should be made in all patients at risk of CS as soon as possible to avoid the risk of profound CS development and/or cardiac arrest.
CS is frequently complicated by multiorgan failure,the most common of which are respiratory and renal failure. In case of acute cardiogenic pulmonary edema, non-invasive ventilation may rapidly improve the respiratory distress and reduces the need for intubation.[13]Intubation of patient in CS is always a high-risk procedure and may lead to profound hypotension and peri-intubation cardiac arrest. If possible, we strongly prefer spontaneously breathing patients and consider MCS use before intubation as it may avoid both hemodynamic and infectious complications associated with mechanical ventilation. If mechanical ventilation is necessary, lung protective ventilation regimen (below 6 mL/kg predicted body weight tidal volume) should be respected to prevent pulmonary injury.[7]Acute renal failure is common among CS patients and associated with significant increase in mortality, renal replacement therapy is frequently required but its earlier initiation had no effect on outcome.[6]
MCS USE IN MI COMPLICATED BY CS
The use of MCS in MI complicated by CS has been increasing in the last two decades, despite limited high quality evidence.[14,15]Current European and American ST-elevation MI guidelines suggest the use of short-term MCS in CS patient as a rescue therapy on an individual basis (Class II, level of evidence C: expert consensus) without specification of the timing, patient selection or device type.[16,17]
Despite the persistent use of the intra-aortic balloon pump (IABP) in many cardiac centers, IABP did not improve outcomes in patients with ST-elevation MI and CS without mechanical complications in a large prospective trial and recently the six years’data of this trial confirmed no effect of IABP on allcause mortality and a poor prognosis of these patients as two thirds of them were dead after six years despite contemporary management.[18,19]IABP should not be routinely used in this indication anymore according to European Society of Cardiology guidelines(Class III, level of evidence B).[16]
Impella CP®(Abiomed Inc., Massachusetts, USA)has been increasingly used in the last decade but failed to prove effect in several retrospective as well as small prospective CS trials questioning the effect of Impella CP®as well as unrealistic and underpowered trials design.[7,15,20]The National Cardiogenic Shock Initiative published results of a singlearm, prospective, multicenter study with early Impella use in patients presenting with MI and CS treated by PCI and achieved high survival to discharge in 72% of patients.[21]However, the lack of randomization and comparative group does not allow any meaningful conclusion with respect to standard CS treatment or other MCS devices. Impella CP®can generate intermediate (2−3.5 L/min) blood flows which may not be sufficient for patients in refractory CS. Larger prospective trial with Impella CP®in CS patients (DanGER shock, clinicaltrials.gov: NCT01633502)is ongoing and will hopefully answer the question of Impella efficacy in CS patients.[22]
The use of VA-ECMO in CS has several advantages compared to other MCS currently used. It provides high flow support (≥ 5 L/min) sufficient even in cardiac arrest, biventricular and full oxygenation support in contrast to IABP and Impella CP®. Therefore, in CS patients with concomitant respiratory failure, right ventricular failure or cardiac arrest,VA-ECMO represents the MCS of choice.[15]Several retrospective studies reported improved outcomes with VA-ECMO compared to standard therapy in CS and a meta-analysis revealed a significant 33%higher thirty-day survival in patients with CS treated by VA-ECMO compared to IABP (P< 0.001, NNT 13).[23]To date, there is only one small prospective randomized study with poorly chosen primary endpoint evaluating the use of VA-ECMO in CS compared to standard treatment which was not able to show any differences between the treatment groups.[24]Ongoing prospective randomized trials focusing on the use of VA-ECMO in patients with CS will hopefully specify its clinical use in the near future.[1,25,26]One of the major drawbacks likely to occur in patients with severe left ventricle (LV) dysfunction after VA-ECMO implantation is the worsening or even loss of LV contractility.[27]The first strategy to lower the risk is to keep VA-ECMO flow as low as necessary for sufficient organ perfusion. Inotropes may sometimes help to sustain LV contractility and aortic valve opening. However, LV unloading is sometimes inevitable and has been associated with decreased mortality in adult patients with CS treated with VA-ECMO.[27]Concomitant implantation of Impella in addition to VA-ECMO (sometimes called ECPELLA or ECMELLA) successfully unloads the LV and eliminates the major disadvantage of VA-ECMO.[28,29]However, this approach should also be studied in rigorous prospective trials as the rates of complications with combined MCS is high and the reported rate of hemolysis are of particular interest.[29]
Until the new evidence is published, device selection in CS should first and foremost consider the type and severity of cardiac failure (left, right or biventricular). In addition, operator and medical staff experience, device availability, costs, patient characteristics are all important cofactors in clinical decision making.[7,15]Current management of patients with CS is showed in Figure 1.
One of the most important complication of MCS related to outcome in CS is bleeding.[5,15]Patients with CS and MI are treated with PCI and stent implantation requires dual antiplatelet therapy and MCS therapy needs effective anticoagulation. This“triple” therapy together with numbers of invasive procedures, and alterations in the coagulation pathway due to MCS and CS creates a high-risk bleeding situation. This risk can be mitigated by MCS cannulation under ultrasound and X-ray guidance by an experienced operator, strict coagulation and platelet levels control, minimizing blood loss in case of bleeding (discontinuation of heparin, plasma and coagulation factors infusions, surgery) and early MCS weaning when appropriate. Daily monitoring is required to provide early detection and treatment of MCS related complications. Prespecified institutional protocols should include careful monitoring of device components, hemodynamics, anticoagulation, blood gas analysis, the brain and cannulated limb tissue perfusion.[15]
MCS USE IN VENTRICULAR SEPTAL RUPTURE

Figure 1 Current approach to patient presenting with myocardial infarction complicated by cardiogenic shock.
VSR became a rare mechanical complication of MI in the era of PCI but is associated with extreme mortality in patients who present with CS. Substantial proportion of patients with VSR is already in profound CS at the time of diagnosis or following transport to a cardiac surgery center. These patients have an unacceptably high mortality with an urgent/emergent surgery approach.[30]In such situations,MCS may provide hemodynamic stabilization.[5]
The use of IABP in VSR remains debatable as it is a passive device providing only small and shortterm hemodynamic benefit. The use of IABP as a sole mechanical supportive measure was associated with continued clinical deterioration in retrospective studies.[5]Therefore, IABP should not be used in refractory CS and its benefit in mild CS is questionable and short-lasting.
VA-ECMO has been successfully used in patients with VSR as a bridge to scheduled surgical repair as well as the salvage method after VSR surgery.[5]Retrospective data suggest it can prevent irreversible multiorgan failure by improved end-organ perfusion.[5]However, increase in LV afterload and bleeding complications remain an important limitation of this approach.[5]
The successful early use of surgical Impella 5.0®as well as percutaneous Impella CP®as a bridge to surgical or percutaneous repair for posterior VSR has been reported.[31,32]Theoretically, Impella provides the most physiological hemodynamic support for VSR decreasing the pulmonary capillary wedge pressure and shunting compare to VA-ECMO.[33]On the other hand, the risks of Impella insertion into the anterior VSR and subsequent tissue damage and embolization is potentially high. Further,Impella CP®does not provide right ventricular support which must be considered before device selection.
MCS USE IN ACUTE MITRAL REGURGITATION AND PAPILLARY MUSCLE RUPTURE
Severe acute mitral regurgitation complicated by CS due to papillary muscle or chordae tendinae rupture is nowadays very rare complication. Afterload reduction with vasodilators in this setting is desirable, but is not possible in patients with CS.IABP is the most used MCS in this indication and may be effective in mild CS.[34]The use of VA-ECMO as a bridge to surgery in this setting is controversial due to the afterload increase consequently worsening mitral regurgitation and lung edema. Impella again represents the most physiological way to stabilize the patient and successful case reports have been published but in patients with refractory CS,the combination of Impella and VA-ECMO seems to be the ultimate way to stabilize the patient before surgery.[35,36]In the large retrospective analysis from 2002 to 2014, evaluating the use of MCS in patients with chordae tendinae and papillary muscle rupture complicating ST-elevation MI, the use of MCS remained almost exclusively limited to IABP (91%)with ECMO and Impella used in 5% and 4.1%.[34]A mortality rate was high (46%).[34]Recently, a retrospective study with combined strategy of Impella and MitraClip including CS patients has been published with 80% of survival rate.[37]This novel fully percutaneous management for patients presenting with acute, severe mitral regurgitation complicated by CS should be investigated in future studies.
MCS USE IN VENTRICULAR FREE-WALL RUPTURE
The use of VA-ECMO in ventricular free-wall rupture has been reported in case reports, case series and retrospective studies in diverse clinical scenarios.[38–41]Interestingly, few case reports of successful management solely with VA-ECMO with spontaneous ventricular rupture healing has been reported.[40,41]However, it is almost impossible to identify such patients, because a rupture can increase in size and cause tamponade with sudden cardiac arrest anytime. Therefore, patients who are hemodynamically stable with or without MCS and suitable for surgery should not be postponed as current surgical techniques, avoiding cardiopulmonary bypass,have improved survival of these patients. A successful case report with the postoperative use of Impella CP®has been also reported but the risk of subsequent tissue damage and rupture reoccurrence due to the presence of Impella makes this approach hazardous and more data are needed.[42]
Finally, it must be admitted that due to the rare occurrence of mechanical complications, it is hard to gather reliable evidence regarding optimal management and we must rely on retrospective data which are prone to selection bias. Current data suggest that VA-ECMO prevent irreversible multiorgan failure by improved end-organ perfusion in mechanical complications of MI at the high cost of bleeding. Impella is a promising MCS with few data. Based on our experience and currently published data, the use of MCS should be individually adapted according to the CS severity, patient, and mechanical complication characteristics and is depicted in Figure 2.
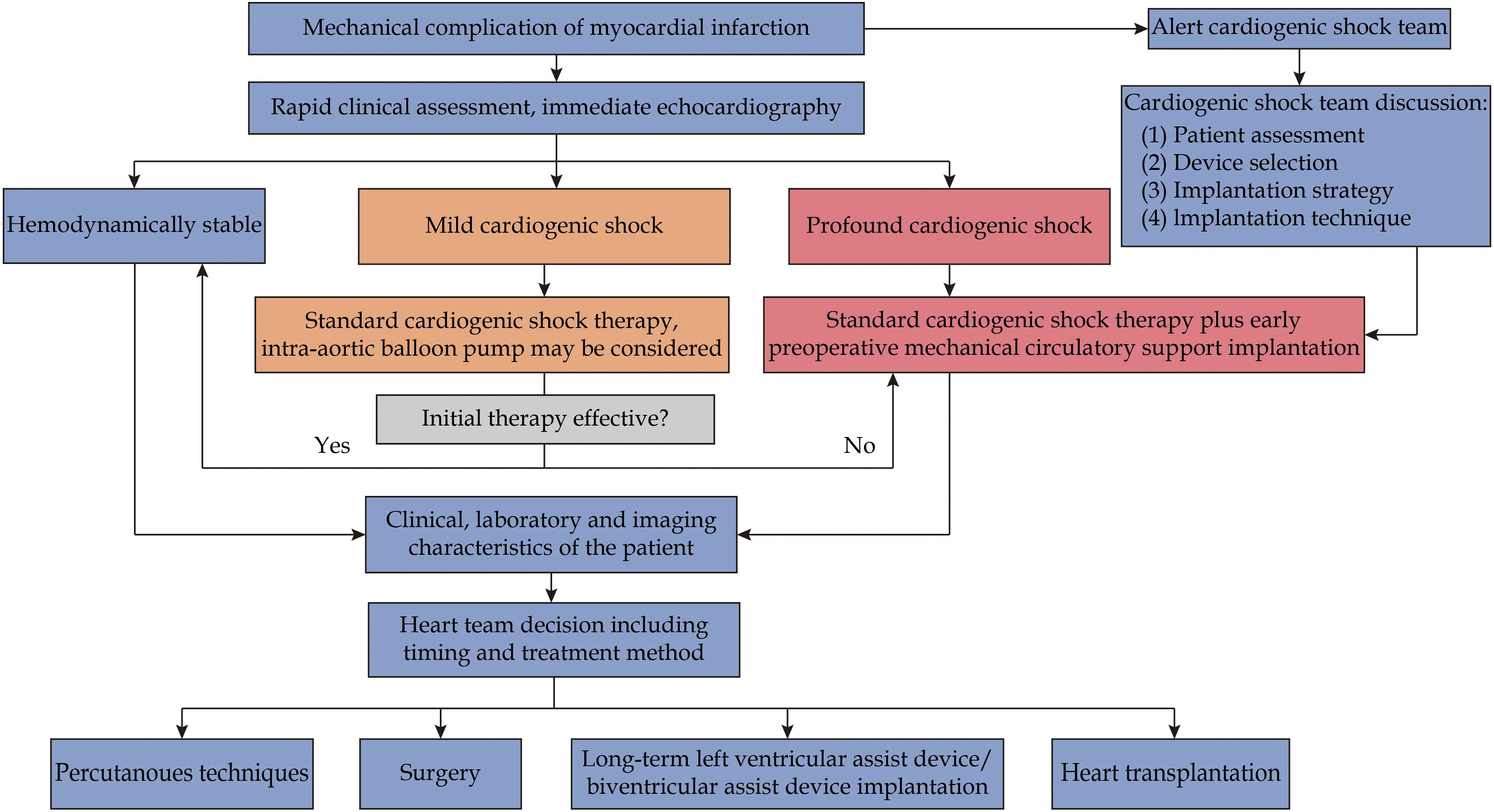
Figure 2 Current approach to patient presenting with mechanical complication of myocardial infarction.
CONCLUSIONS
Different treatment strategies and MCS have been increasingly used to improve the grim prognosis of refractory CS in the settings of MI and mechanical complications. The use of MCS is associated with significant complications, particularly bleeding complications remain a major barrier to better survival.Early MCS initiation and multidisciplinary team collaboration is necessary for good results. The benefits and risks of MCS must be carefully considered in each individual case. Due to the lack of data, device selection should be tailored according to the cause and severity of CS as well as the patient clinical status and cardiac center experience. Ongoing prospective randomized trials will improve current knowledge regarding the MCS use in the coming years.
ACKNOWLEDGMENTS
This study was supported by the Agency for the Czech Republic Health Research (No.15-27994A).All authors had no conflicts of interest to disclose.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Lactobacillus levels and prognosis of patients with acute myocardial infarction
- Usefulness of Impella support in different clinical settings in cardiogenic shock
- Translational proteomics in cardiogenic shock: from benchmark to bedside
- Challenges in the conduct of randomised controlled trials in cardiogenic shock complicating acute myocardial infarction
- Cardiogenic shock due to left main related myocardial infarction:is revascularization enough?
- The characteristic and dynamic electrocardiogram changes on hyperkalemia in a hemodialysis patient with heart failure:a case report
