Humanin and diabetes mellitus:A review of in vitro and in vivo studies
2022-03-23ChrysoulaBoutariPanagiotisPappasTheodorosTheodoridisDimitriosVavilis
lNTRODUCTlON
Twenty years ago,three independent laboratories discovered humanin(HN)(MTRNR2),the first mitochondrial small open reading frame(sORF)-encoded microprotein found to have biological activity.The Hashimoto laboratory discovered HN while searching for survival factors in the unaffected brain section of an Alzheimer’s patient[1].The investigators revealed a cDNA fragment that mapped back to the mitochondrial 16S rRNA.This microprotein was named humanin because it displayed protection against Alzheimer’s disease(AD)-related neurotoxicity,an action that the original authors though potentially could retrieve the “humanity” of patients suffering from dementia.Second,Ikonen
[2]found that HN bound insulin like growth factor binding protein 3(IGFBP3)using a yeast two-hybrid screening system and intensified the protective effects of IGFBP3 against amyloid-β(Aβ)toxicity.Also,Guo
[3]showed that HN can bind and suppress the apoptotic protein BAX and,subsequently,alleviate cell apoptosis.
The flax seemed to spread and increase; and when she wove a carpet or a piece of linen7, or made a shirt, she was sure to find a customer who paid her well, so that not only did she feel no want herself, but she was able to help those who did
Physiologically,HN is produced by tissues in several organs,including kidney,skeletal muscles,brain,heart,and liver[4-6].Subsequently,it is secreted into the blood circulation and transported to various target cells,protecting in parallel cells against several diseases strongly associated with oxidative stress,mitochondrial dysfunction,and cytotoxicity[7].Beyond the cytoprotection HN possesses a key role in cell metabolism and mediates the production and secretion of endocrine/paracrine/autocrine protective stress response factors[8].Additionally,it plays a role in age-related diseases and several metabolic disorders(
cardiovascular diseases[CVD],memory loss,stroke,diabetes type 2[T2DM]).
Diabetes is a chronic disease that occurs either due to autoimmune destruction of the pancreatic beta cells,leading to absolute insulin deficiency(T1DM)or due to progressive attenuation of insulin secretion on a background of insulin resistance resulting in relative insulin deficiency(T2DM).The number of people with diabetes rose from 108 million in 1980 to 422 million in 2014.Prevalence has been increasing faster in low- and middle-income countries than in high-income countries.The rising burden of T2DM is a major concern in health care worldwide.In 2017 6.28% of the worldwide population was affected by T2DM.It is disconcerting that the burden of the disease is rising globally,and at a more rapid rate in developed regions such as western Europe[9].As for the T1DM,its incidence is estimated 15 per 100000 people and the global prevalence 9.5%[10].Since diabetes and its complications affect individuals’ functional capacities and quality of life leading to significant morbidity and premature mortality,effective agents are required for its treatment.
STRUCTURE OF HUMANlN PEPTlDE
HN is encoded by a sORF within the gene for the 16S ribosomal subunit in the mitochondrial genome[11].HN has a positively charged N-terminal(Met-Ala-Pro-Arg),central hydrophobic region(Gly-Phe-Ser-Cys-Leu-Leu-Leu-Leu-Thr-Ser-Glu-Ile-Asp-Leu),and negatively charged C-terminal(Pro-Val-Lys-Arg-Arg-Ala)[1].Last three amino acid residues in the C-terminal are considered as dispensable because both 21 and 24-amino acid long peptides have indistinguishable intracellular and extracellular effects[12].Thirteen nuclear-encoded HN isoforms have been identified.HN-like ORF has been named MTRNR2L1 to MTRNR2L13 after the original humanin MTRNR2 gene in the mitochondrial genome.MTRNR2L1—MTRNR2L10 are expressed in most human tissues,with MTRNR2 being expressed in higher proportion in comparison to the other isoforms.Molecular manipulations of HN at key amino acids lead to changes in chemical characteristics.Additionally,single amino acid substitutions can lead to significant modifications to its biological functions and potency[13].
MECHANlSMS OF ACTlON
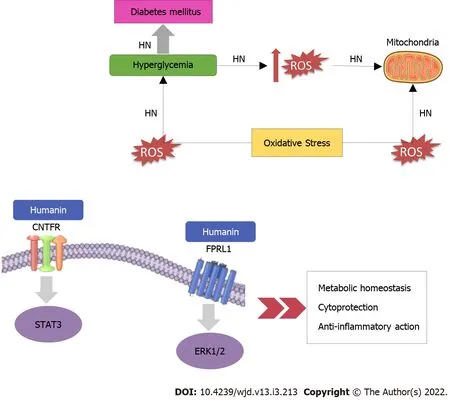
HN exerts its functions after connecting to either intracellular molecules or cell membrane receptors(Figure 1).Immediately after HN’s receptor binding,extracellular signal-regulated kinase 1/2(ERK1/2)phosphorylation increases[14].Once ERK1/2 is phosphorylated,it separates from its anchoring proteins,and transfers to other subcellular compartments.ERK1/2,a member of the mitogen-activated protein kinase pathway,participates in several essential cellular processes such as cell proliferation,survival,differentiation,mobility,and apoptosis[15,16].HN behaves as a link to two different types of receptors:the seven-transmembrane G protein-coupled receptor formyl peptide receptor-like 1(FPRL1)which plays a role in the cytoprotective properties of HN and a trimeric receptor,consisting of ciliary neurotrophic factor receptor(CNTFR),the cytokine receptor WSX-1 and the transmembrane glycoprotein 130(GP130)(CNTFR/WSX-1/GP130)which is essential for HN activity and its neuroprotective effects[17].As regards GP130,it is a transmembrane protein that acts as the signal transduction unit of the IL-6 receptor family[18].Dimerization of GP130 receptors provokes the stimulation of janus kinases(JAK1 and JAK2),then subsequently provokes signal transducer and activator of transcription 3(STAT3)and STAT1[19].The dimerized STATs move to the nucleus and control transcription.The second signaling pathway directed by GP130 recruits SHP-2.SHP-2 is phosphorylated by JAK and interacts with growth-factor receptor bound protein 2(Grb2),which allows the activation of mitogenactivated protein kinase(MAPK)[19].
As for the changes in HN levels with ageing,Voigt
[67]showed that HN decreased with age among individuals attending a diabetes complications screening clinic suggesting a protective function of HN and this observation was consistent with a previous study among human and mice[23].On the contrary,circulating levels of HN increase in age-associated diseases such as T2DM.With disease progression and additional oxidative stress,mitochondria may increase HN levels.
Especially concerning diabetes,HN provides protection against apoptosis by binding pro-apoptotic Bax,inhibiting its mitochondrial localization,and lessening Bax-mediated apoptosis activation[3],acting either directly on Bax or through the FPRL-1 receptor[17].As for its neuroprotective action,which has also a place in the neuroendocrine beta cells protection,it involves HN binding to a complex involving CNTFR/WSX-1/GP130[17]and activation of tyrosine kinases and STAT-3 phosphorylation[41].Moreover,an important mechanism of cell protection may be
interfering with Jun N-terminal kinase(JNK)activity[42].Important is also the interaction between HN and insulin-like growth factor binding protein-3(IGFBP-3)which prevents the activation of caspases[2].Furthermore,an alteration at position[Gly14]-HN(S14G,HNG)seems to induce neurosurvival activity and a substitution of phenylalanine in the 6
position with alanine(F6A,F6AHN)changes the binding of HN to IGFBP-3 and enhances its main effect on glucose metabolism and insulin sensitivity[5].
ROLE OF HUMANlN lN THE PATHOGENESlS OF TYPE 1 DlABETES
The role of HN in T1DM has been scarcely investigated.T1DM is characterized by the loss of pancreatic beta cells which results in insulin deficiency.The beta cells destruction,the dominant event in the pathogenesis of T1DM,occurs as a result of the IL-1,TNF-a,and IFN- γ actions which are originated from T cells and macrophages.Since HN is identified as a survival factor[43],it seems to serve also as a survival factor for neuroendocrine beta cells by decreasing cytokine-induced apoptosis and subsequently,improves glucose tolerance and onset of diabetes as it has been demonstrated in NOD mice
[44].Yet,no studies juxtaposing the HN levels in T1DM and T2DM have been published thus far.
Cola-Mattheo, who was, as I have said before, a great simpleton, made no reply; but before sunrise next morning he went to the wood and gathered a bunch of St
ROLE OF HUMANlN lN THE PATHOGENESlS OF TYPE 2 DlABETES
It was eight o clock when he took up his post, and for the first hour he was quite proud of his courage; during the second hour he was well pleased with the large reward that he would get, but in the third hour, when it was getting near eleven, the effects of the wine passed off, and he began to get uncomfortable, for he had heard about this post; that no one had ever escapeed alive from it, so far as was known
Mitochondrial dysfunction and oxidative stress are involved in the pathogenesis of diabetes.Mitochondria are principal elements for the maintenance of metabolic health and cellular energy homeostasis.Mitochondrial dysfunction causes glycaemic dysregulation and metabolic derangement[49].It causes inefficiency in the electron transport chain and beta-oxidation,thus trigging insulin resistance[50].Furthermore,hyperglycemia provokes ROS generation which,in turn,causes oxidative stress in several tissues,cellular lipids,proteins,and DNA,and subsequently,provokes chronic inflammation[51].The accumulation of oxidative damage leads to a decrement of mitochondrial function which can result in increased ROS production[29].It has been suggested that mitochondrial dysfunction is implicated in diabetes-related complications impairing the kidneys,nervous system,heart and retina,and that mitochondrial dysfunction-related oxidative stress contributes to these complications[52].Subsequently,an increase in ROS concentrations may provoke HN mobilization from various tissues to the impaired areas,where HN acts against oxidative stress,decreases ROS production,and promotes cell survival[51].Mitochondrial derived peptides(MDPs),such as HN,have been suggested to play a critical role in reducing oxidative stress[53-55]and improving T2DM[56].It has also been demonstrated that HN promotes mitochondrial biogenesis in pancreatic β-cells[57].
IN VITRO AND ANlMAL STUDlES
In vitro and animal studies
At the end of 2018 Ma
[78]evaluated HN concentrations in pregnant women with and without gestational diabetes mellitus(GDM)aiming to define the role of HN in the development of GDM.157 women were enrolled in the study.Serum HN levels were significantly lower in women with GDM compared to controls.Like Lee
[21],who found that HN was regulated by IGF-1 in mice and humans,they suggested that the IGF axis influenced the HN levels and affected its normal function in GDM.By performing logistic regression analysis,they also showed that low HN levels were the independent risk factor of GDM and,therefore,might be a predictor for the GDM diagnosis.Additionally,HN levels were significantly negatively correlated with the body weight,body mass index(BMI)and homeostatic model assessment for insulin resistance(HOMA-IR).
A year later,a group from California[44]investigated whether HN could improve the survival of beta cells and delay or even treat diabetes in NOD mice.HN prevented apoptosis induced by serum starvation in NIT-1 cells and decreased cytokine exposure-related apoptosis(caused by interleukin[IL]-1β,tumor necrosis factor[TNF]α,and interferon[IFN]γ).STAT3 is considered as a principal survivalsignaling protein in beta cells,regulating the pro-survival effects of various growth factors and cytokines.HN activated STAT3 and ERK over a 24-hour time course.Interestingly,HN improved glucose tolerance in NOD mice and after 6 wk of treatment decreased lymphocyte infiltration was observed in their pancreata.When the treatment was extended up to 20 wk the investigators noted that HN delayed or prevented the onset of diabetes in NOD mice.
From this time on no one asked the Cat to stand godmother; but when the winter came and there was nothing to be got outside, the Mouse remembered their provision and said, Come, Cat, we will go to our pot of fat which we have stored away; it will taste very good
A few years later,the group we mentioned first[23]hypothesized that HNGF6A,a potent non-IGFBP-3 binding HN analog,may affect acutely and independently insulin secretion,since insulin concentrations were not reduced along with hypoglycemia caused by HNGF6A in Sprague Dawley rats[22].Sprague Dawley rats that received HNGF6A presented higher insulin levels during hyperglycemic clamps compared to controls.Similarly,
,HNGF6A enhanced glucose-stimulated insulin secretion in isolated islets and cultured murine β cell line.This effect was dose dependent,combined with ATP production in the β cell,related to the KATP-channel-independent augmentation phase of insulin release[62],and associated with amplified glucose metabolism.These potent effects on insulin secretion in combination with the effects on insulin action suggested a role of HN in the treatment of T2DM.
The protective effects of[Gly14]-Humanin(HNG)against high glucose-induced apoptosis were investigated in human umbilical vein endothelial cells(HUVECs).Pretreatment of HUVECs with HNG inhibited cell death,nucleus pyknosis and deformation[63].Also,HNG diminished the expression of cleaved poly ADP-ribose polymerase(PARP)which reflects the level of apoptosis as well as reactive oxygen species(ROS).Regarding the level of bax,which is a pro-apoptotic protein,it decreased after pretreatment with HNG,while bcl-2,which exerts anti-apoptotic effects,it increased.
Another group identified a different sORF within the mitochondrial 12S rRNA encoding a 16-aminoacid peptide named MOTS-c(mitochondrial open reading frame of the 12S rRNA type-c)which also regulates insulin sensitivity and metabolic homeostasis[56].Particularly,MOTS-c treatment in mice protected against age-dependent and high-fat-diet-induced insulin resistance and diet-induced obesity as well.Finally,they suggested that MDPs,like MOTS-c and HN,with such systemic effects may be useful in ameliorating the abnormal metabolism associated with aging in humans and regulating biological processes like weight and metabolic homeostasis.
Kim and his colleagues from California tried to elucidate the signaling pathways underlying HN’S cytoprotective roles
and
[14].Utilizing multiple models,they showed that HN is a major GP130 agonist which acts through the GP130/IL6ST receptor complex and activates AKT,ERK1/2,and STAT3.PI3K,MEK,and JAK were suggested to be involved in the activation of those three signaling pathways,respectively.
Concerning the effects of HN on mitochondrial biogenesis in pancreatic β-cells,HN treatment in MIN6 β-cells increased the expression of peroxisome proliferator-activated receptor(PPAR)γ coactivator-1α(PGC-1α)[57]which promotes mitochondrial biogenesis by activating the expression of nuclear respiratory factor 1(NRF-1)and mtDNA transcription factor A(TFAM)[64].Also,HN treatment promoted mitochondrial biogenesis by increasing mitochondrial mass,elevating mitochondrial DNA(mtDNA)/nDNA ratio(reduced mtDNA copy number plays a key role in insulin resistance[65]),and increasing cytochrome B expression.Finally,HN treatment resulted in the phosphorylation of AMPK,which was involved in the induction of PGC-1α,NRF-1,and TFAM and improved mitochondrial respiration and stimulated ATP generation leading to a possible functional gain of the mitochondria.
In HUVECs also,HN displayed protective action against high-glucose-induced endothelial dysfunction and macrovascular complications[66].HN treatment promoted the expression of Krüppellike factor 2(KLF2),a principal transcriptional regulator of endothelial function,by activating ERK5.In addition,HN significantly reduced the expression of vascular cell adhesion molecule 1(VCAM-1)and E-selectin,which regulate the adhesion of circulating leukocytes to the endothelium,a principal procedure in the initiation of atherosclerosis.Furthermore,HN impeded the secretion of pro-inflammatory cytokines,such as TNF-α and IL-1β.
HUMAN SUBJECTS RESEARCH AND CLlNlCAL TRlALS
Human subjects research and clinical trials
The most recent study which attempted to evaluate MDP levels in normal,prediabetes and diabetes subjects enrolled 225 participants[49].The investigators found that serum HN concentrations are lower in T2DM(
0.0001)and correlate with HbA1c.Interestingly,HN levels decreased by 62% in the prediabetes group,66% in diabetes subjects with good control and 77% in uncontrolled diabetes patients compared to participants without diabetes.Also,this study confirmed that there are no significant differences in HN levels between healthy men and women and the levels of HN were not affected by the different anti-diabetic treatment(insulin,metformin,other hypoglycemic regimens)or the duration of therapy.Furthermore,since HN was associated with adiponectin,which has been suggested to be reduced in prediabetes and T2DM[79],it can be concluded that mitochondrial dysfunction contributes to glycemic dysregulation and metabolic effects in T2DM.Adiponectin levels were positively correlated with HN.Adiponectin concentrations decrease in pre-diabetes and DM[79].It has also been demonstrated that adiponectin knockout mice have reduced mitochondrial content combined with insulin resistance[80].In addition adiponectin may impair mitochondrial biogenesis[81].Therefore,the affected mitochondrial function may arise from the low adiponectin levels.
I d agreed to help my brother, Mac, move from the East Coast to California. He would drive a rental2 truck loaded with his belongings3 and I would follow him in his sedan, then fly back. We figured it would be a simple trip, with four or five motel stops along the way.。,,。,。
These conditions are related to extensive oxidative stress which is also a key feature of DM.Particularly,hyperglycemia causes extended free radical activity and mitochondrial dysfunction which induce oxidative stress and release more ROS[76].The advanced diseases MELAS and CPEO are associated with increased plasma HN levels.HN has a protective role and is upregulated with disease progression.On the contrary,the minor elevations of blood glucose levels are combined with a decrease in HN concentrations which supports the protective role of HN when levels are expected to decrease as a result of stimulation of oxidative stress-associated agents that are inhibited by HN.However,with disease progression to T2DM and further oxidative stress,mitochondria increase HN levels,as reported in MELAS and CPEO.
As he fell the lion whistled loudly three times with such force that the forest rang again, and the sound must have been heard for more than two leagues round, after which having apparently93 nothing more to do in the world he rolled over on his side and died
There were four nails on the right wall that were used to fix their wedding photo frame. But now there were only four nails without the frame. She still remembered he got hurt on his fingers when trying to drive the nails home. It was her who pushed him to receive an injection against tetanus() in the hospital.
T2DM is one of the most common metabolic diseases.This metabolic disorder and its comorbidities and complications,such as CVD,stroke,chronic kidney disease(CKD),and cancer,are global health problems which,noticeably,diminish quality of life and life expectancy[45-48].
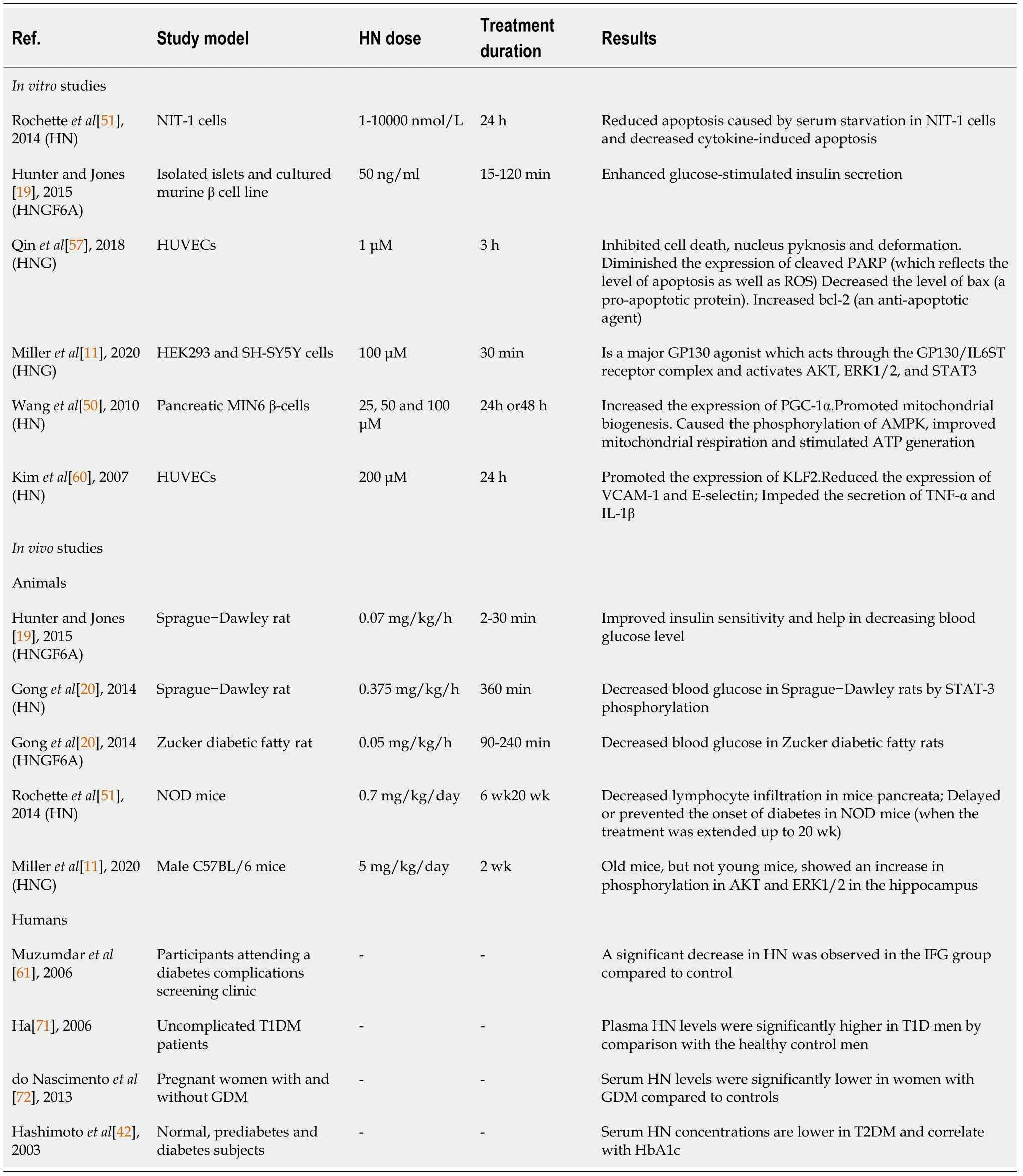
Considering that diseases related with ageing,named T2DM and neurodegeneration,have been suggested to be associated with mitochondrial dysfunction[58,59],it follows that the mitochondrialderived peptide HN regulates them(Table 1).Based upon the molecular interaction between HN and IGFBP-3,that prevents the activation of caspases,and since IGFBP-3,independent of IGF-1,provokes IR both at the liver and periphery[60,61],Muzumdar
[23]hypothesized that HN,besides its neuroprotective action,may regulate glucose homeostasis.Utilizing state of the art clamp technology,they investigated the role and the mechanism of action of central and peripheral HN in glucose metabolism.They finally demonstrated that infusion of HN improves both hepatic and peripheral insulin sensitivity and that hypothalamic STAT-3 activation is essential for the insulin-sensitizing action of HN.Moreover,treatment with a highly potent HN analog significantly lowered blood glucose in Zucker diabetic fatty rats.As for the levels of HN in tissues like hypothalamus,skeletal muscle,and cortex,they reduced with age in rodents,and its’ circulating levels were also diminished with age in humans and mice.
The first attempt to measure HN levels in a clinical population with impaired fasting glucose(IFG)was made in participants attending a diabetes complications screening clinic(DiabHealth)[67,68].Previous clinical studies reported noticeably increased HN levels in patients with mitochondrial encephalomyopathy,lactic acidosis,and stroke-like episodes(MELAS)and chronic progressive external ophthalmoplegia(CPEO),which are associated with excess oxidative stress[69,70].However,a significant reduction(
= 0.0001)in HN was reported in the IFG group(
= 23;204.84 ± 92.87 pg mL
)compared to control(
= 58;124.3 ± 83.91 pg mL
)in accord with an adaptive cellular response by HN to a slight raise in fasting blood glucose level(BGL).As we described above,HN protects neuroendocrine β-cells[44]and increases glucose tolerance and insulin sensitivity[20,44].Moreover,it is considered to interact with hydrogen peroxide and α-actinin-4 which rise during oxidative stress and IFG[71-73]and binds extracellularly with the CNTFR/WSX-1/GP130 receptor[69,74,75].Interestingly,mild to moderate levels of ROS result in positive adaptive mechanisms of the mitochondria[76].All these mechanisms,which benefit cell function and survival,lead to a reduction in HN levels,indicating a protective role of HN.However,with disease progression to T2DM and further oxidative stress,mitochondria may upregulate HN levels as observed in studies of Alzheimer's disease and in those of MELAS and CPEO.
The beggar there, said her husband, has joys of his own whichseem to him great, and cause him as much pleasure as a king would find in the magnificence of his palace. And then do you not think thatthe beast of burden, which suffers blows and hunger, and worksitself to death, suffers just as much from its miserable fate? Thedumb creature might demand a future life also, and declare the lawunjust that excludes it from the advantages of the higher creation. Christ said: In my father s house are many mansions, sheanswered. Heaven is as boundless as the love of our Creator; the dumb animal is also His creature, and I firmly believe that no life will be lost, but each will receive as much happiness as he can enjoy, which will be sufficient for him.
2. Little Red Riding Hood: The red riding hood is a popular and familiar symbol to much of Europe and North America. In the height of portraiture11 in the nineteenth century, many young daughters of wealthy families were painted wearing red capes12 or hoods13. Today, some little girls still want to wear red capes for Halloween or other imaginative play.
HN is regulated by insulin-growth factor 1(IGF-1)and growth hormone(GH).HN and IGF-1 Levels decrease with age[20].It has also been suggested that GH inhibits HN levels
IGF-1.Treatment with GH or IGF-1 reduces circulating HN levels in both mice and human subjects[21].To date,HN has been suggested to play a role in various diseases like T2DM[22,23],CVD[4,5],memory loss[24],amyotrophic lateral sclerosis(ALS)[25],stroke[26],and inflammation[12,27].The main mechanisms that dominate and link these age-related diseases are oxidative stress[28]and mitochondrial dysfunction[29].Mitochondria are principal sources of reactive oxygen species(ROS)which can cause oxidative stress and injure of the lipids,proteins,and DNA of the cells.This can afflict mitochondrial function,and,subsequently,enhanced ROS production occurs[29].These circumstances contribute to cellular damage,apoptosis,and cellular ageing,causing ageing and age-related diseases such as Parkinson’s disease[30],Alzheimer’s disease[31],atherosclerosis[32],heart failure[33],myocardial infarction[34],chronic inflammation[35],kidney disease[36],stroke[37],cancers[38],and many kinds of metabolic disorders[39,40].
Besides the initial and principal lifestyle interventions for glycemic control in DM,currently,we have various oral and injectable pharmacologic agents at our disposal including metformin,thiazolidinediones,sulfonylureas,glucagon-like peptide 1(GLP-1)receptor agonists,dipeptyl-peptidase 4(DPP-4)inhibitors,sodium-glucose co-transporter 2(SGLT-2)inhibitors,and insulin[82].These medicines can be administered in various dosages and in many combinations in each patient diagnosed with DM.However,there is still room for additional new factors that could efficiently contribute to the management of the disease.Given HN’s protective properties,it may represent a novel treatment option to decrease the cellular damage caused by diabetes.Altered HN levels in diabetes could serve as a potential biomarker.Nevertheless,no clinical trials investigating the effects of HN or its analogues(e.g.HNGF6a)administration have thus far been published,albeit it would be an innovative and promising breakthrough in diabetes prevention and treatment.
A few years earlier,another group from Toronto suggested that plasma HN levels were significantly higher in T1D men by comparison with the healthy control men(
0.0001)[77].
CONCLUSlON
In summary,HN shows cytoprotective effects in many biological processes,including oxidative stress and apoptosis.Altered HN levels could serve as a potential biomarker in prediabetes and T2DM,since they seem to be an effect or a response to the increased ROS production,oxidative stress,and reduced mtDNA copy number which all contribute to IR[83].However,further study is needed to define the role of age and other modifiable confounding factors,like fitness level,adiposity,other metabolic comorbidities,such as CVD,stroke,inflammation.Undoubtedly,the major and important question is whether HN could be used as a potential therapeutic option for diabetes,that could even replace the current diabetes mellitus treatment strategies soon.Towards this direction,further studies are needed to identify the contribution of HN in the metabolic dysregulation of T2DM.
FOOTNOTES
All authors contributed equally to the writing of the manuscript and have read and approve the final manuscript.
There are no conflicts of interest to declare.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:http://creativecommons.org/Licenses/by-nc/4.0/
United States
Chrysoula Boutari 0000-0002-0053-2440;Panagiotis D Pappas 0000-0002-9549-2807;Theodoros D Theodoridis 0000-0001-8723-2215;Dimitrios Vavilis 0000-0001-7768-1818.
Wang LL
A
Wang LL
1 Hashimoto Y,Niikura T,Tajima H,Yasukawa T,Sudo H,Ito Y,Kita Y,Kawasumi M,Kouyama K,Doyu M,Sobue G,Koide T,Tsuji S,Lang J,Kurokawa K,Nishimoto I.A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta.
2001;98:6336-6341[PMID:11371646 DOI:10.1073/PNAS.101133498]
2 Ikonen M,Liu B,Hashimoto Y,Ma L,Lee KW,Niikura T,Nishimoto I,Cohen P.Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis.
2003;100:13042-13047[PMID:14561895 DOI:10.1073/PNAS.2135111100]
3 Guo B,Zhai D,Cabezas E,Welsh K,Nouraini S,Satterthwait AC,Reed JC.Humanin peptide suppresses apoptosis by interfering with Bax activation.
2003;423:456-461[PMID:12732850 DOI:10.1038/nature01627]
4 Zhang X,Urbieta-Caceres VH,Eirin A,Bell CC,Crane JA,Tang H,Jordan KL,Oh YK,Zhu XY,Korsmo MJ,Bachar AR,Cohen P,Lerman A,Lerman LO.Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice.
2012;91:199-206[PMID:22820173 DOI:10.1016/J.LFS.2012.07.010]
5 Muzumdar RH,Huffman DM,Calvert JW,Jha S,Weinberg Y,Cui L,Nemkal A,Atzmon G,Klein L,Gundewar S,Ji SY,Lavu M,Predmore BL,Lefer DJ.Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice.
2010;30:1940-1948[PMID:20651283 DOI:10.1161/ATVBAHA.110.205997]
6 Brookmeyer R,Gray S,Kawas C.Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset.
1998;88:1337-1342[PMID:9736873 DOI:10.2105/AJPH.88.9.1337]
7 Widmer RJ,Flammer AJ,Herrmann J,Rodriguez-Porcel M,Wan J,Cohen P,Lerman LO,Lerman A.Circulating humanin levels are associated with preserved coronary endothelial function.
2013;304:H393-H397[PMID:23220334 DOI:10.1152/AJPHEART.00765.2012]
8 Zuccato CF,Asad AS,Nicola Candia AJ,Gottardo MF,Moreno Ayala MA,Theas MS,Seilicovich A,Candolfi M.Mitochondrial-derived peptide humanin as therapeutic target in cancer and degenerative diseases.
2019;23:117-126[PMID:30582721 DOI:10.1080/14728222.2019.1559300]
9 Khan MAB,Hashim MJ,King JK,Govender RD,Mustafa H,Al Kaabi J.Epidemiology of Type 2 Diabetes-Global Burden of Disease and Forecasted Trends.
2020;10:107-111[PMID:32175717 DOI:10.2991/JEGH.K.191028.001]
10 Mobasseri M,Shirmohammadi M,Amiri T,Vahed N,Hosseini Fard H,Ghojazadeh M.Prevalence and incidence of type 1 diabetes in the world:a systematic review and meta-analysis.
2020;10:98-115[PMID:32296622 DOI:10.34172/HPP.2020.18]
11 Miller B,Kim SJ,Kumagai H,Mehta HH,Xiang W,Liu J,Yen K,Cohen P.Peptides derived from small mitochondrial open reading frames:Genomic,biological,and therapeutic implications.
2020;393:112056[PMID:32387288 DOI:10.1016/J.YEXCR.2020.112056]
12 Zapała B,Kaczyński Ł,Kieć-Wilk B,Staszel T,Knapp A,Thoresen GH,Wybrańska I,Dembińska-Kieć A.Humanins,the neuroprotective and cytoprotective peptides with antiapoptotic and anti-inflammatory properties.
2010;62:767-777[PMID:21098860 DOI:10.1016/S1734-1140(10)70337-6]
13 Bodzioch M,Lapicka-Bodzioch K,Zapala B,Kamysz W,Kiec-Wilk B,Dembinska-Kiec A.Evidence for potential functionality of nuclearly-encoded humanin isoforms.
2009;94:247-256[PMID:19477263 DOI:10.1016/J.YGENO.2009.05.006]
14 Kim SJ,Guerrero N,Wassef G,Xiao J,Mehta HH,Cohen P,Yen K.The mitochondrial-derived peptide humanin activates the ERK1/2,AKT,and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus.
2016;7:46899-46912[PMID:27384491 DOI:10.18632/ONCOTARGET.10380]
15 Steelman LS,Chappell WH,Abrams SL,Kempf RC,Long J,Laidler P,Mijatovic S,Maksimovic-Ivanic D,Stivala F,Mazzarino MC,Donia M,Fagone P,Malaponte G,Nicoletti F,Libra M,Milella M,Tafuri A,Bonati A,Bäsecke J,Cocco L,Evangelisti C,Martelli AM,Montalto G,Cervello M,McCubrey JA.Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging.
2011;3:192-222[PMID:21422497 DOI:10.18632/AGING.100296]
16 Chang F,Steelman LS,Lee JT,Shelton JG,Navolanic PM,Blalock WL,Franklin RA,McCubrey JA.Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors:potential targeting for therapeutic intervention.
2003;17:1263-1293[PMID:12835716 DOI:10.1038/SJ.LEU.2402945]
17 Hashimoto Y,Kurita M,Aiso S,Nishimoto I,Matsuoka M.Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130.
2009;20:2864-2873[PMID:19386761 DOI:10.1091/MBC.E09-02-0168]
18 Bauer S,Kerr BJ,Patterson PH.The neuropoietic cytokine family in development,plasticity,disease and injury.
2007;8:221-232[PMID:17311007 DOI:10.1038/nrn2054]
19 Hunter CA,Jones SA.IL-6 as a keystone cytokine in health and disease.
2015;16:448-457[PMID:25898198 DOI:10.1038/ni.3153]
20 Gong Z,Tas E,Muzumdar R.Humanin and age-related diseases:a new link?
2014;5:210[PMID:25538685 DOI:10.3389/FENDO.2014.00210]
21 Lee C,Wan J,Miyazaki B,Fang Y,Guevara-Aguirre J,Yen K,Longo V,Bartke A,Cohen P.IGF-I regulates the agedependent signaling peptide humanin.
2014;13:958-961[PMID:25040290 DOI:10.1111/ACEL.12243]
22 Kuliawat R,Klein L,Gong Z,Nicoletta-Gentile M,Nemkal A,Cui L,Bastie C,Su K,Huffman D,Surana M,Barzilai N,Fleischer N,Muzumdar R.Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell.
2013;27:4890-4898[PMID:23995290 DOI:10.1096/fj.13-231092]
23 Muzumdar RH,Huffman DM,Atzmon G,Buettner C,Cobb LJ,Fishman S,Budagov T,Cui L,Einstein FH,Poduval A,Hwang D,Barzilai N,Cohen P.Humanin:a novel central regulator of peripheral insulin action.
2009;4:e6334[PMID:19623253 DOI:10.1371/JOURNAL.PONE.0006334]
24 Mamiya T,Ukai M.[Gly(14)]-Humanin improved the learning and memory impairment induced by scopolamine in vivo.
2001;134:1597-1599[PMID:11739234 DOI:10.1038/SJ.BJP.0704429]
25 Matsuoka M,Hashimoto Y,Aiso S,Nishimoto I.Humanin and colivelin:neuronal-death-suppressing peptides for Alzheimer's disease and amyotrophic lateral sclerosis.
2006;12:113-122[PMID:16958985 DOI:10.1111/J.1527-3458.2006.00113.X]
26 Xu X,Chua CC,Gao J,Hamdy RC,Chua BH.Humanin is a novel neuroprotective agent against stroke.
2006;37:2613-2619[PMID:16960089 DOI:10.1161/01.STR.0000242772.94277.1F]
27 Zhao ST,Zhao L,Li JH.Neuroprotective Peptide humanin inhibits inflammatory response in astrocytes induced by lipopolysaccharide.
2013;38:581-588[PMID:23277413 DOI:10.1007/S11064-012-0951-6]
28 Dai DF,Chiao YA,Marcinek DJ,Szeto HH,Rabinovitch PS.Mitochondrial oxidative stress in aging and healthspan.
2014;3:6[PMID:24860647 DOI:10.1186/2046-2395-3-6]
29 Cui H,Kong Y,Zhang H.Oxidative stress,mitochondrial dysfunction,and aging.
2012;2012:646354[PMID:21977319 DOI:10.1155/2012/646354]
30 Jenner P.Oxidative stress in Parkinson's disease.
2003;53 Suppl 3:S26-36;discussion S36[PMID:12666096 DOI:10.1002/ANA.10483]
31 Markesbery WR.Oxidative stress hypothesis in Alzheimer's disease.
1997;23:134-147[PMID:9165306 DOI:10.1016/S0891-5849(96)00629-6]
32 Singh U,Jialal I.Oxidative stress and atherosclerosis.
2006;13:129-142[PMID:16757157 DOI:10.1016/J.PATHOPHYS.2006.05.002]
33 Tsutsui H,Kinugawa S,Matsushima S.Oxidative stress and heart failure.
2011;301:H2181-H2190[PMID:21949114 DOI:10.1152/AJPHEART.00554.2011]
34 Di Filippo C,Cuzzocrea S,Rossi F,Marfella R,D'Amico M.Oxidative stress as the leading cause of acute myocardial infarction in diabetics.
2006;24:77-87[PMID:16961722 DOI:10.1111/J.1527-3466.2006.00077.X]
35 Federico A,Morgillo F,Tuccillo C,Ciardiello F,Loguercio C.Chronic inflammation and oxidative stress in human carcinogenesis.
2007;121:2381-2386[PMID:17893868 DOI:10.1002/IJC.23192]
36 Ozbek E.Induction of oxidative stress in kidney.
2012;2012:465897[PMID:22577546 DOI:10.1155/2012/465897]
37 Allen CL,Bayraktutan U.Oxidative stress and its role in the pathogenesis of ischaemic stroke.
2009;4:461-470[PMID:19930058 DOI:10.1111/J.1747-4949.2009.00387.X]
38 Sosa V,Moliné T,Somoza R,Paciucci R,Kondoh H,LLeonart ME.Oxidative stress and cancer:an overview.
2013;12:376-390[PMID:23123177 DOI:10.1016/J.ARR.2012.10.004]
39 Roberts CK,Sindhu KK.Oxidative stress and metabolic syndrome.
2009;84:705-712[PMID:19281826 DOI:10.1016/J.LFS.2009.02.026]
40 Furukawa S,Fujita T,Shimabukuro M,Iwaki M,Yamada Y,Nakajima Y,Nakayama O,Makishima M,Matsuda M,Shimomura I.Increased oxidative stress in obesity and its impact on metabolic syndrome.
2004;114:1752-1761[PMID:15599400 DOI:10.1172/JCI21625]
41 Hashimoto Y,Suzuki H,Aiso S,Niikura T,Nishimoto I,Matsuoka M.Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection.
2005;77:3092-3104[PMID:16005025 DOI:10.1016/J.LFS.2005.03.031]
42 Hashimoto Y,Tsuji O,Niikura T,Yamagishi Y,Ishizaka M,Kawasumi M,Chiba T,Kanekura K,Yamada M,Tsukamoto E,Kouyama K,Terashita K,Aiso S,Lin A,Nishimoto I.Involvement of c-Jun N-terminal kinase in amyloid precursor protein-mediated neuronal cell death.
2003;84:864-877[PMID:12562529 DOI:10.1046/J.1471-4159.2003.01585.X]
43 Eizirik DL,Mandrup-Poulsen T.A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis.
2001;44:2115-2133[PMID:11793013 DOI:10.1007/S001250100021]
44 Hoang PT,Park P,Cobb LJ,Paharkova-Vatchkova V,Hakimi M,Cohen P,Lee KW.The neurosurvival factor Humanin inhibits beta-cell apoptosis
signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice.
2010;59:343-349[PMID:19800083 DOI:10.1016/J.METABOL.2009.08.001]
45 Grundy SM.Adipose tissue and metabolic syndrome:too much,too little or neither.
2015;45:1209-1217[PMID:26291691 DOI:10.1111/ECI.12519]
46 Fang X,Liu H,Zhang X,Zhang H,Qin X,Ji X.Metabolic Syndrome,Its Components,and Diabetes on 5-Year Risk of Recurrent Stroke among Mild-to-Moderate Ischemic Stroke Survivors:A Multiclinic Registry Study.
2016;25:626-634[PMID:26725129 DOI:10.1016/J.JSTROKECEREBROVASDIS.2015.11.017]
47 Esser N,Paquot N,Scheen AJ.Inflammatory markers and cardiometabolic diseases.
2015;70:193-199[PMID:26103538 DOI:10.1179/2295333715Y.0000000004]
48 Shimabukuro M,Kozuka C,Taira S-I,Yabiku K,Dagvasumberel M,Ishida M.Ectopic fat deposition and global cardiometabolic risk:New paradigm in cardiovascular medicine.
2013
49 Ramanjaneya M,Bettahi I,Jerobin J,Chandra P,Abi Khalil C,Skarulis M,Atkin SL,Abou-Samra AB.Mitochondrial-Derived Peptides Are Down Regulated in Diabetes Subjects.
2019;10:331[PMID:31214116 DOI:10.3389/FENDO.2019.00331]
50 Wang CH,Wang CC,Wei YH.Mitochondrial dysfunction in insulin insensitivity:implication of mitochondrial role in type 2 diabetes.
2010;1201:157-165[PMID:20649552 DOI:10.1111/J.1749-6632.2010.05625.X]
51 Rochette L,Zeller M,Cottin Y,Vergely C.Diabetes,oxidative stress and therapeutic strategies.
2014;1840:2709-2729[PMID:24905298 DOI:10.1016/J.BBAGEN.2014.05.017]
52 Blake R,Trounce IA.Mitochondrial dysfunction and complications associated with diabetes.
2014;1840:1404-1412[PMID:24246956 DOI:10.1016/J.BBAGEN.2013.11.007]
53 Klein LE,Cui L,Gong Z,Su K,Muzumdar R.A humanin analog decreases oxidative stress and preserves mitochondrial integrity in cardiac myoblasts.
2013;440:197-203[PMID:23985350 DOI:10.1016/J.BBRC.2013.08.055]
54 Bachar AR,Scheffer L,Schroeder AS,Nakamura HK,Cobb LJ,Oh YK,Lerman LO,Pagano RE,Cohen P,Lerman A.Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress.
2010;88:360-366[PMID:20562421 DOI:10.1093/CVR/CVQ191]
55 Oh YK,Bachar AR,Zacharias DG,Kim SG,Wan J,Cobb LJ,Lerman LO,Cohen P,Lerman A.Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice.
2011;219:65-73[PMID:21763658 DOI:10.1016/J.ATHEROSCLEROSIS.2011.06.038]
56 Lee C,Zeng J,Drew BG,Sallam T,Martin-Montalvo A,Wan J,Kim SJ,Mehta H,Hevener AL,de Cabo R,Cohen P.The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance.
2015;21:443-454[PMID:25738459 DOI:10.1016/J.CMET.2015.02.009]
57 Qin Q,Jin J,He F,Zheng Y,Li T,Zhang Y,He J.Humanin promotes mitochondrial biogenesis in pancreatic MIN6 βcells.
2018;497:292-297[PMID:29432738 DOI:10.1016/J.BBRC.2018.02.071]
58 Frederiksen AL,Jeppesen TD,Vissing J,Schwartz M,Kyvik KO,Schmitz O,Poulsen PL,Andersen PH.High prevalence of impaired glucose homeostasis and myopathy in asymptomatic and oligosymptomatic 3243A>G mitochondrial DNA mutation-positive subjects.
2009;94:2872-2879[PMID:19470628 DOI:10.1210/jc.2009-0235]
59 Loeb LA,Wallace DC,Martin GM.The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations.
2005;102:18769-18770[PMID:16365283 DOI:10.1073/PNAS.0509776102]
60 Kim HS,Ali O,Shim M,Lee KW,Vuguin P,Muzumdar R,Barzilai N,Cohen P.Insulin-like growth factor binding protein-3 induces insulin resistance in adipocytes
and in rats in vivo.
2007;61:159-164[PMID:17237715 DOI:10.1203/pdr.0b013e31802d8a30]
61 Muzumdar RH,Ma X,Fishman S,Yang X,Atzmon G,Vuguin P,Einstein FH,Hwang D,Cohen P,Barzilai N.Central and opposing effects of IGF-I and IGF-binding protein-3 on systemic insulin action.
2006;55:2788-2796[PMID:17003344 DOI:10.2337/DB06-0318]
62 Straub SG,Sharp GW.Glucose-stimulated signaling pathways in biphasic insulin secretion.
2002;18:451-463[PMID:12469359 DOI:10.1002/DMRR.329]
63 Xie Y,Liu ZH,Li XY,Zhou YD,Xu X,Hu LF,Zhang YL,Liu CF.Protection effect of[Gly14]-Humanin from apoptosis induced by high glucose in human umbilical vein endothelial cells.
2014;106:560-566[PMID:25451915 DOI:10.1016/J.DIABRES.2014.09.020]
64 Scarpulla RC.Nuclear activators and coactivators in mammalian mitochondrial biogenesis.
2002;1576:1-14[PMID:12031478 DOI:10.1016/S0167-4781(02)00343-3]
65 Cheng Z,Almeida FA.Mitochondrial alteration in type 2 diabetes and obesity:an epigenetic link.
2014;13:890-897[PMID:24552811 DOI:10.4161/CC.28189]
66 Wang X,Wu Z,He Y,Zhang H,Tian L,Zheng C,Shang T,Zhu Q,Li D.Humanin prevents high glucose-induced monocyte adhesion to endothelial cells by targeting KLF2.
2018;101:245-250[PMID:30029058 DOI:10.1016/J.MOLIMM.2018.07.008]
67 Voigt A,Jelinek HF.Humanin:a mitochondrial signaling peptide as a biomarker for impaired fasting glucose-related oxidative stress.
2016;4[PMID:27173674 DOI:10.14814/PHY2.12796]
68 Jelinek HF,Wilding C,Tinely P.An innovative Multi-disciplinary Diabetes Complications Screening Program in a Rural Community:A Description and Preliminary Results of the Screening.
2006;12:14-20[DOI:10.1071/PY06003]
69 Kariya S,Hirano M,Furiya Y,Sugie K,Ueno S.Humanin detected in skeletal muscles of MELAS patients:a possible new therapeutic agent.
2005;109:367-372[PMID:15759134 DOI:10.1007/S00401-004-0965-5]
70 Kin T,Sugie K,Hirano M,Goto YI,Nishino I,Ueno S.Humanin expression in skeletal muscles of patients with chronic progressive external ophthalmoplegia.
2006;51:555-558[PMID:16639504 DOI:10.1007/s10038-006-0397-2]
71 Ha TS.High glucose and advanced glycosylated end-products affect the expression of alpha-actinin-4 in glomerular epithelial cells.
2006;11:435-441[PMID:17014558 DOI:10.1111/J.1440-1797.2006.00668.X]
72 do Nascimento JF,Canani LH,Gerchman F,Rodrigues PG,Joelsons G,dos Santos M,Pereira S,Veronese FV.Messenger RNA levels of podocyte-associated proteins in subjects with different degrees of glucose tolerance with or without nephropathy.
2013;14:214[PMID:24103534 DOI:10.1186/1471-2369-14-214]
73 Francés DE,Ronco MT,Monti JA,Ingaramo PI,Pisani GB,Parody JP,Pellegrino JM,Sanz PM,Carrillo MC,Carnovale CE.Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical:new insights into the insulin effect.
2010;205:187-200[PMID:20164374 DOI:10.1677/JOE-09-0462]
74 Pagano G,Talamanca AA,Castello G,Cordero MD,d'Ischia M,Gadaleta MN,Pallardó FV,Petrović S,Tiano L,Zatterale A.Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies:toward mitochondria-targeted clinical strategies.
2014;2014:541230[PMID:24876913 DOI:10.1155/2014/541230]
75 Katayama Y,Maeda K,Iizuka T,Hayashi M,Hashizume Y,Sanada M,Kawai H,Kashiwagi A.Accumulation of oxidative stress around the stroke-like lesions of MELAS patients.
2009;9:306-313[PMID:19393775 DOI:10.1016/J.MITO.2009.04.002]
76 Long YC,Tan TM,Takao I,Tang BL.The biochemistry and cell biology of aging:metabolic regulation through mitochondrial signaling.
2014;306:E581-E591[PMID:24452454 DOI:10.1152/ajpendo.00665.2013.-Cellular]
77 Lytvyn Y,Wan J,Lai V,Cohen P,Cherney DZ.The effect of sex on humanin levels in healthy adults and patients with uncomplicated type 1 diabetes mellitus.
2015;93:239-243[PMID:25615723 DOI:10.1139/CJPP-2014-0401]
78 Ma Y,Li S,Wei X,Huang J,Lai M,Wang N,Huang Q,Zhao L,Peng Y,Wang Y.Comparison of serum concentrations of humanin in women with and without gestational diabetes mellitus.
2018;34:1064-1067[PMID:29909696 DOI:10.1080/09513590.2018.1482869]
79 Kim JY,Bacha F,Tfayli H,Michaliszyn SF,Yousuf S,Arslanian S.Adipose Tissue Insulin Resistance in Youth on the Spectrum From Normal Weight to Obese and From Normal Glucose Tolerance to Impaired Glucose Tolerance to Type 2 Diabetes.
2019;42:265-272[PMID:30455334 DOI:10.2337/DC18-1178]
80 Civitarese AE,Ukropcova B,Carling S,Hulver M,DeFronzo RA,Mandarino L,Ravussin E,Smith SR.Role of adiponectin in human skeletal muscle bioenergetics.
2006;4:75-87[PMID:16814734 DOI:10.1016/J.CMET.2006.05.002]
81 O'Brien LC,Graham ZA,Chen Q,Lesnefsky EJ,Cardozo C,Gorgey AS.Plasma adiponectin levels are correlated with body composition,metabolic profiles,and mitochondrial markers in individuals with chronic spinal cord injury.
2018;56:863-872[PMID:29559683 DOI:10.1038/s41393-018-0089-8]
82 American Diabetes Association.9.Pharmacologic Approaches to Glycemic Treatment:
.
2020;43:S98-S110[PMID:31862752 DOI:10.2337/DC20-S009]
83 Tan BL,Norhaizan ME,Liew WP.Nutrients and Oxidative Stress:Friend or Foe?
2018;2018:9719584[PMID:29643982 DOI:10.1155/2018/9719584]
杂志排行
World Journal of Diabetes的其它文章
- Beyond diabetes remission a step further:Post bariatric surgery hypoglycemia
- Free fatty acids,glucose,and insulin in type 2 diabetes mellitus
- Sodium-glucose co-transporter 2 inhibitors induced euglycemic diabetic ketoacidosis within four days of initiation
- Age at diagnosis of type 2 diabetes and cardiovascular risk factor profile:A pooled analysis
- Hemoglobin within normal range is negatively related to hemoglobin A1c in a nondiabetic American population aged 16 years and older
- Functional annotation and enrichment analysis of differentially expressed serum proteins in patients with type 2 diabetes after dapagliflozin
