DFT mechanistic insight into the modular strategy involved in the palladium-catalyzed synthesis of cyclopentenones from α,β-unsaturated acid chlorides and alkynes*
2022-03-19ZHONGLiangZHAORuihuaWANGZhixiang
ZHONG Liang,ZHAO Ruihua,WANG Zhixiang
(School of Chemical Sciences, University of Chinese Academy of Sciences,Beijing 100049, China)(Received 19 April 2021;Revised 19 May 2021)
Abstract Cyclopentenones are important synthetic building blocks and as motifs appear in bioactive molecules and natural products.We applied density functional theory(DFT)calculations to gain insight into the modular strategy involved in the palladium-catalyzed synthesis of cyclopentenone from α,β-unsaturated acid chlorides and alkynes in the presence of hydrosilane.The study unveils that the transformation proceeds via the sequence: the disassembly of α,β-unsaturated acid chloride into vinyl, carbonyl, and Cl fragments with the palladium catalyst; carbon monoxide release; coupling of alkyne with vinyl group; carbon monoxide re-coordination and migratory insertion to form another C—C bond with alkyne, ring-closure via CC bond insertion, transmetalation with hydrosilane, C, H-reductive elimination to release the product.Different from the mechanism proposed by the experimentalists, the CO group is involved in the reaction via separate liberation and re-coordination in the solvent cage, rather than persistent coordination with palladium.The transmetalation for H/Cl exchange takes place at the late stage and is a bottleneck of the transformation, instead of at early disassembly stage.
Keywords cyclopentenone synthesis; modular strategy; carbon monoxide surrogates; palladium catalysis; DFT calculation
Cyclopentenones are valuable building blocks and prevalent motifs in bioactive molecules and natural products[1-9].Development of effective and operationally simple methods to synthesize cyclopentenones is of significance in organic synthesis.The[2+2+1]cycloaddition of alkene, alkyne and carbon monoxide(CO), named as Pauson-Khand reaction(PKR), is a classical method to access cyclopentenones(Eq.(1)in Fig.1).Since the first discovery of PKR[10], considerable efforts have been devoted to improve PKR[11-21].A disadvantage of PKR is the use of toxic and flammable pressured CO gas, which is operationally inconvenient.To circumvent the problem, Chung et al.[22]in 2004 utilized α,β-unsaturated aldehydes to replace CO and alkene for the synthesis of cyclopentenones(Eq.(2)).In 2011, Montgomery group[23-24]and Ogoshi group[25]independently developed the nickel(0)-catalyzed synthesis of cyclopentenones from α,β-unsaturated esters and alkynes(Eq.(3)).Generally, these reactions undergo oxidative cyclization mechanism to form a five-membered metalacycle as a key intermediate(seeTS-OXandIM-OXin Eq.(3))[22-26].As such, these reactions maintain the integrity of the CO surrogates and add C1and C4to the ends of alkynes to afford cyclopentenones.Recently, Morandi and co-workers reported a mechanistically different method, called modular strategy, to synthesize cyclopentenones from α,β-unsaturated acid chlorides and alkynes, as exemplified by Eq.(5)[27].The same strategy was also applied to perform carboformylation of alkynes with acid chlorides(Eq.(4))[28].Interestingly, Eq.(5)affords no alkyne carboformylation product but cyclopentenones.Moreover, by fragmentation and reorganization, the strategy affords cyclopentenones with a quaternary carbon at C5, whereas the oxidative cyclization method furnishes cyclopentenones with C5limited to secondary or tertiary carbon.Continuing our interest in the cyclization involving alkyne[29-30], we here report a detailed DFT mechanistic study to understand how the modular strategy works.As compared in Fig.1, the study indicates that the reaction could proceed via the path-B mechanism, rather than path-A postulated by experimentalists[27].
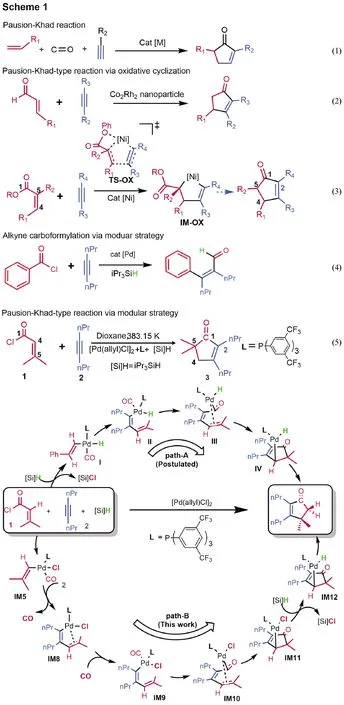
Fig.1 Researches on the synthesis of cyclopentenone and mechanisms of the modular strategy
1 Computational details
All structures were optimized at B3LYP[31]-D3[32]/BSI level in the gas phase.BSI represents a basis set with SDD[33]for Pd and 6-31G(d,p)for other atoms.Harmonic frequency analysis calculations were subsequently performed to verify the optimized geometries to be minima(no imaginary frequency)or transition states(TSs, having unique one imaginary frequency).The energies were then improved by M06-2X[34]/BSII single-point calculations with solvent effects accounted by the SMD solvent model[35], using the experimental solvent(dioxane).BSⅡ denotes a basis set with SDD for Pd and 6-311++G(d,p)for other atoms.All standard DFT calculations were carried out using Gaussian 09 program[36].
In Gaussian program, the Gibbs free energies were obtained by using the ideal gas model under the conditions of 383.15 K and 1 atm.Considering that the reaction was carried out in the solvent, we corrected the gas phase Gibbs free energies to the values under the conditions of 383.15 K and 1 mol/L.The correction factor(ΔGcorr= 2.6 kcal/mol)for a species A can be estimated from Eq.(6), where Csolis equal to 1 mol/L and p is the standard atmosphere pressure(101 325 Pa).
G[A(383.15 K,1 mol/L)]=G[A(383.15 K,1 atm)]+ΔGcorr.
(6)
2 Results and discussion

Examining the energy profile, the intermediates and transition states prior toIM8have high relative energies up to 32.6 kcal/mol and the transmetalation fromIM11toTS8has a high barrier of 31.5 kcal/mol.The somewhat unfavorable energetics explains why elevated temperature(383.15 K)was applied for the reaction.The energy profile suggests that the reaction could be improved from the following two aspects:(i)Because of the low barriers of the elementary reactions involved in the fragmentation of1, using a catalytic system which could be initiated to easily generate a palladium(0)active species irreversibly to facilitate the fragmentation of1.Note that Pd(PPh3)4was used to catalyze reaction, but gave low yield, which we attribute to that Pd(PPh3)4is not easy to give PdPPh3active species via dissociation.(ii)Using alternative hydrogen source to low the transmetalation barrier.
Morandi et al have considered four pathways for the reaction, among which path-A in Fig.1 was considered to be most likely.Comparing path-A and our predicted path-B, the two pathways are different with two key differences.First, as the transmetalation in path-B takes place at the very late stage, the process in path-A occurs at the early stage.Second, the CO group in path-A coordinates to palladium throughout the reaction after fragmentation, whereas the CO group in path-B is released fromIM5 and then re-coordinates afterIM5converts toIM8.The differences motived us to inspect our mechanism further.For transmetalation process, we considered various possible transmetalations, Fig.3(a).For the intermediates prior toTS5,IM1,IM4andIM5which possess no vacant sitecisto Cl are not viable for transmetalation.The transmetalation barriers of the coordinatively unsaturatedIM3/IM3aandIM6/IM6aare much higher thanTS5.The energetics indicates that fragmentation of the acid chlorides could not be interpreted by[Si]H and straightforwardly leads toIM5.Because the transmetalation barriers ofIM8/IM8aandIM10are much higher thanTS7, these transmetalations can be ruled out, affirming that the transmetalation can only take place after ring-closure to formIM11.
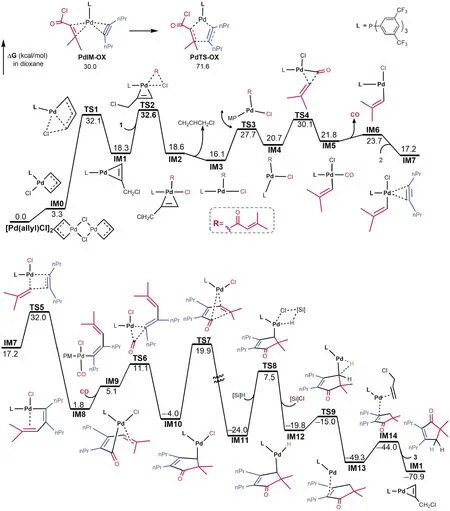
Fig.2 Energy profile for the reaction Eq.(5)
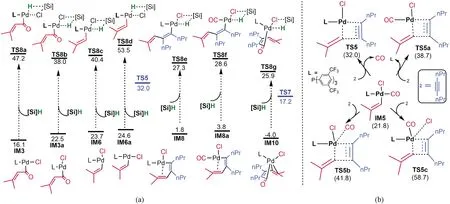
Fig.3 Alternative transmetalations(a)and comparing the alkyne-vinyl coupling with or without liberation of monoxide(b)
To address the second difference, we considered alternative mechanisms for the reaction ofIM5with2without releasing CO, as shown in Fig.3(b).The alkene migration described byTS5awith L released fromIM5is 6.7 kcal/mol less favorable than that(TS5)with CO released, due to the stronger coordination of phosphorus ligandLthan CO.Note that the CO release fromIM5only cost 1.9 kcal/mol(Fig.2).InTS5bandTS5c, the CO group or Cl atom maintain contact with Pd, however, these alkene migration transition states are significantly higher thanTS5.These energetic results indicate that the CO ligand inIM5must be liberated to drive the reaction forward.We considered two scenarios for CO liberation.The first scenario is that the CO release takes place in the solvent cage.When CO is released, it is confined by the solvent cage.AfterIM5reacts with2to generateIM8with accessible vacant site, the CO in the cage then coordinates toIM8to continue the reaction.Alternatively, the liberated CO escapes the solvent cage and enters the gas phase of the reaction system.Given that the process fromIM5toIM8is very facile and could be much faster than CO escape from the cage, it is more likely that the liberation and re-coordination of CO take place in the solvent cage.Experiments using isotopic acid chloride in the CO atmosphere or regular acid chloride in the isotopic CO atmosphere could verify our assumption.
As our path-B is supported by computed energetics, path-A was proposed on the basis of deuterium-labeling experimental results, raising a question if our path-B contradicts to the experimental results.As shown in Eq.(7)in Fig.4, H/D scrambling was observed at the hydrogen atoms bonded to both C4and C5.Experimentalists attributed their observations to that the palladium hydrideIVdin Eq.(7)could undergo competitive β-H elimination and reductive elimination.Because our path-B also involves a similar intermediate(i.e.IM12), our mechanism would not contradict to the experimental results if their elucidations were correct.However, according to our mechanism, we reasoned that the deuteration should not take place at the palladium hydride, because the barrier for subsequent reductive elimination is too low, being 4.8 kcal/mol fromIM12toTS9(Fig.2).Indeed as shown in Fig.4,TSd5for reductive elimination is 21.9 kcal/mol more favorable thanTSd6for β-H elimination.Thus, whenIMd6is formed, it would give3a-Dbdirectly, leaving no chance for β-H elimination to give3a-Da.According to our computed pathway in Fig.2, we reasoned that the structural isomerizations for deuteration should occur at the deeper valley corresponding toIM11.Unlike the achiralIM11, the counterparts of 1aare stereoisomers, namelyIMd1andIMd5in Fig.4.As such, we first examined which enantiomer is kinetically preferred.As compared,IMd1is more kinetically favorable thanIMd5by 9.7 kcal/mol, which can be attributed to the stronger coordination interaction inTSd0than inTSd0’.Supportively, the two marked Pd-C distances in the former are much shorter than those in the latter.AlthoughIMd1is not able to undergo β-H elimination, it is kinetically more favorable.As such, we should start withIMd1to study the deuteration process.ConsideringIMd1, it can be represented by two resonance structures, namelyIMd1-I andIMd1-Ⅱ.IMd1-I can lead to3a-Dcafter transmetalation and reductive elimination.IMd1-Ⅱ can convert toIMd5via β-H elimination with nPr group(TSd2),IMd4flip, and reverse β-H elimination(TSd3).Subsequently,IMd5proceeds via two pathways.On the one hand, it can afford3a-Dbafter transmetalation to giveIMd6, followed by reductive elimination visTSd5.Alternatively, it can convert toIMd8via β-H elimination(TSd7), structural adjustment, and reverse β-H elimination(TSd8).FinallyIMd8affords3a-Davia subsequent transmetalation and reductive elimination.The transmetalations ofIMd1-I,IMd6, andIMd8are the rate-determining step to give3a-Dc,3a-Db, and3a-Da, respectively.Relative toIMd1, the transmetalation barriers are 35.1(TSd9), 31.5(TSd4), and 34.5 kcal/mol(TSd1).The energetic results reasonably explain the experimental observations.
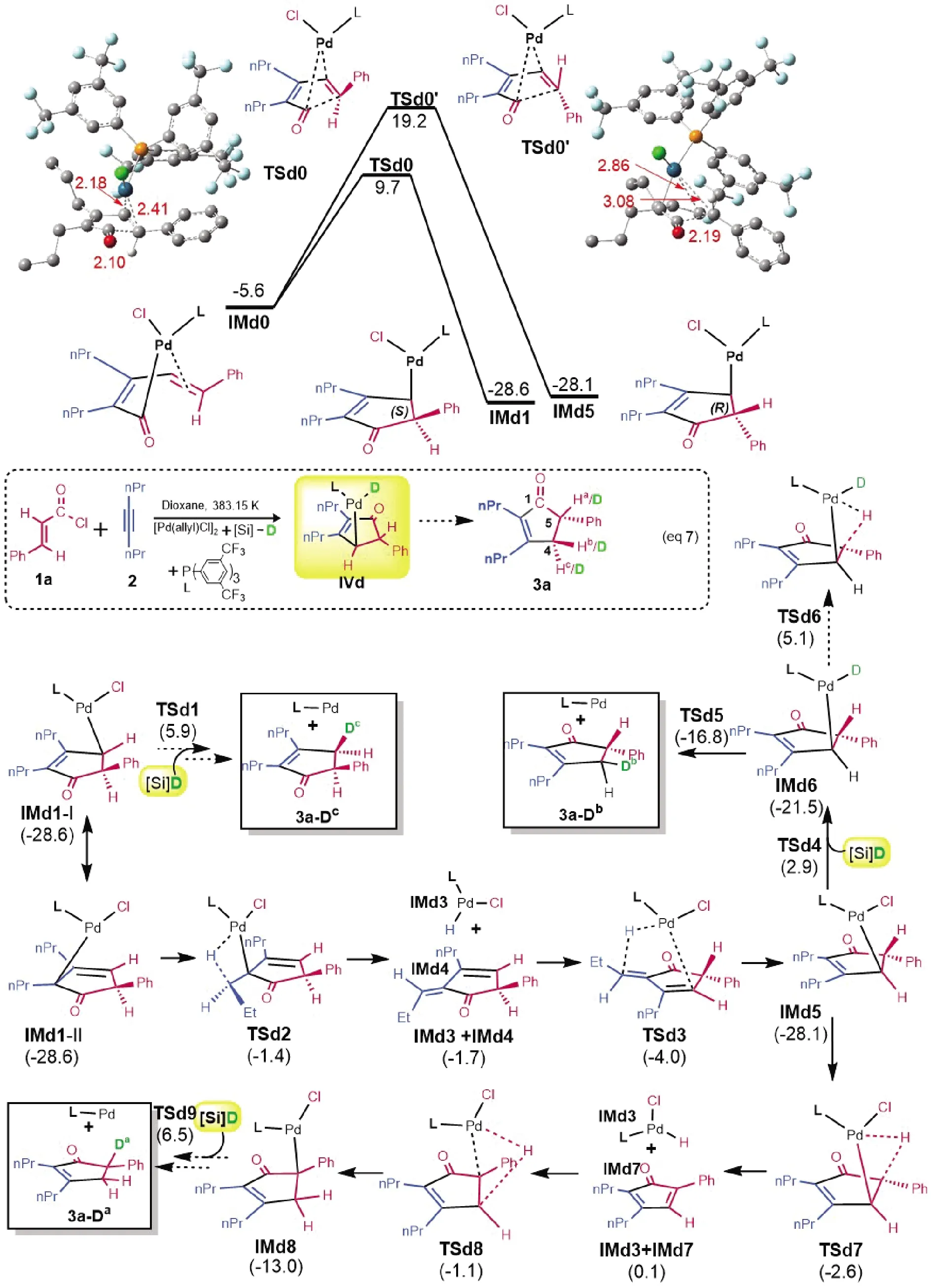
Fig.4 Rationalizing the deuterium-labeling experimental results
According to the experimental studies,[9-10]in addition to3, the reaction could also afford3a-3c(Fig.5).To further corroborate our mechanism and to understand the selectivity of the reaction, we examined if our mechanism can rationalize the selectivity of the reaction to give3.3ais the alkyne carboformylation product in the Pd-catalyzed reaction of benzoyl chloride with alkyne(Eq.(4)).According to the mechanism in Fig.2,3acould be produced via transmetalation ofIM10, followed by reductive elimination.The transition state(TS10)for transmetalation is 8.4 kcal/mol higher thanTS7for alkene insertion, excluding the production of3a.It should be noted that, since the transmetalation barrier(29.9 kcal/mol fromIM10toTS10)is accessible, if there is no vinyl group in acid chloride, the reaction could afford carboformylation product, as shown in Eq.(4).The formations of3band3cboth start fromIM3.First,cisIM3converts totransIM3a.Coordination of alkyne2toIM3aresults inIM15.Alkyne insertion to Pd-C(O)viaTS11givesIM17.On the one hand,IM17undergoes transmetalation, followed by reductive elimination, affording3b.On the other hand,IM17undergoes alkene insertion to giveIM18, which then undergoes transmetalation and reductive elimination, affording3c.Because the transition stateTS11(ΔG≠=37.5 kcal/mol)is much higher thanTS4(ΔG≠=30.1 kcal/mol)andTS5(ΔG≠=32.0 kcal/mol), the reaction channels should not open to the formations of3band3c.3ccould also be generated via oxidative cyclization, however, the highly unfavorable energies(i.e.see the process fromPdIM-OXtoPdTS-OXin Fig.1)for the process exclude the possibility.
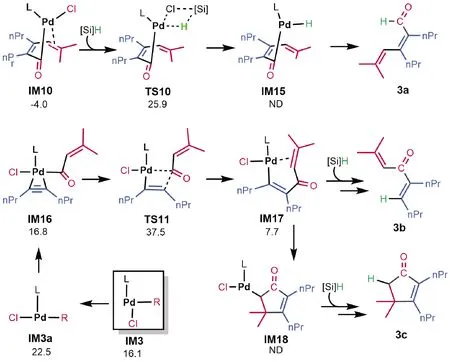
Fig.5 Explaining the selectivity of 3 over 3a-3c
3 Conclusion

