Molecular targeted therapy causes hepatic encephalopathy in patients after Transjugular intrahepatic portosystemic shunt (TIPS): A case report and literature review
2022-03-10ChenZhouYangChenJiachengLiuQinShiBinXiong
Chen Zhou, Yang Chen, Jiacheng Liu, Qin Shi, Bin Xiong,*
a Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, Hubei, China
b Hubei Province Key Laboratory of Molecular Imaging, Wuhan, 430022, Hubei, China
Keywords:Transjugular intrahepatic portosystemic shunt(TIPS)Hepatic encephalopathy Molecular targeted therapy Case report
ABSTRACT We report two cases of hepatic encephalopathy caused by molecular targeted drugs after the Transjugular intrahepatic portosystemic shunt (TIPS) procedure in our center. The liver toxicities and anti-angiogenic effects induced by targeted drugs may generate an imbalance in ammonia metabolism,elevating blood ammonia levels.TIPS diverts partial blood supply from the liver, aggravates liver impairment, and shunts ammonia-rich blood from the intestine into the systemic circulation.These may be the mechanisms leading to hepatic encephalopathy caused by molecular targeted drugs following TIPS. When clinicians choose molecular targeted therapy as the second or third targeted therapy for patients who have undergone TIPS,the consequence of drug-induced hepatic encephalopathy should also be considered.
1. Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) is an effective method for treating cirrhotic portal hypertension, especially upper gastrointestinal bleeding and refractory ascites caused by the rupture of esophageal and gastric varices. However, hepatic encephalopathy may occur after TIPS.1The incidence of hepatic encephalopathy or its aggravation following TIPS has been reported to be 5%-35%.2Hepatic encephalopathy following TIPS is caused by a variety of factors,including a high-protein diet and constipation.However,no studies have reported on hepatic encephalopathy caused by molecular targeted therapy following TIPS. This study focused on two cases of hepatic encephalopathy that were reported in our center in patients who underwent TIPS,caused by molecular targeted drugs.Additionally,by reviewing the literature, this study aimed to discuss the treatment and corresponding mechanisms of hepatic encephalopathy related to this report.The patient has consented to the publication of this manuscript and any identifying images or data.
2. Case presentation
Our first case is a 60-year-old male patient with liver cancer and portal hypertension. He had been treated with multiple transarterial chemoembolization since 2013, combined with targeted therapy using sorafenib at a dosage of 0.2g bid.The status of liver cancer was evaluated as partial remission.The patient underwent TIPS in December 2018 due to gastrointestinal bleeding (Fig. 1A and B). Owing to disease progression,sorafenib was switched to regorafenib at a dosage of 160 mg qd,and the patient was regularly followed-up every 3 months. The second case was a 73-year-old male patient with esophageal cancer and portal hypertension. He underwent TIPS for liver cirrhosis and gastrointestinal bleeding in 2019 and recovered well after the procedure. Because of esophageal cancer with dysphagia, he underwent stent placement in August 2020, followed by arterial chemoembolization combined with oral apatinib at a dosage of 500mg qd to treat esophageal cancer,and the patient was regularly followed-up every 3 months.(Fig.1C and D).
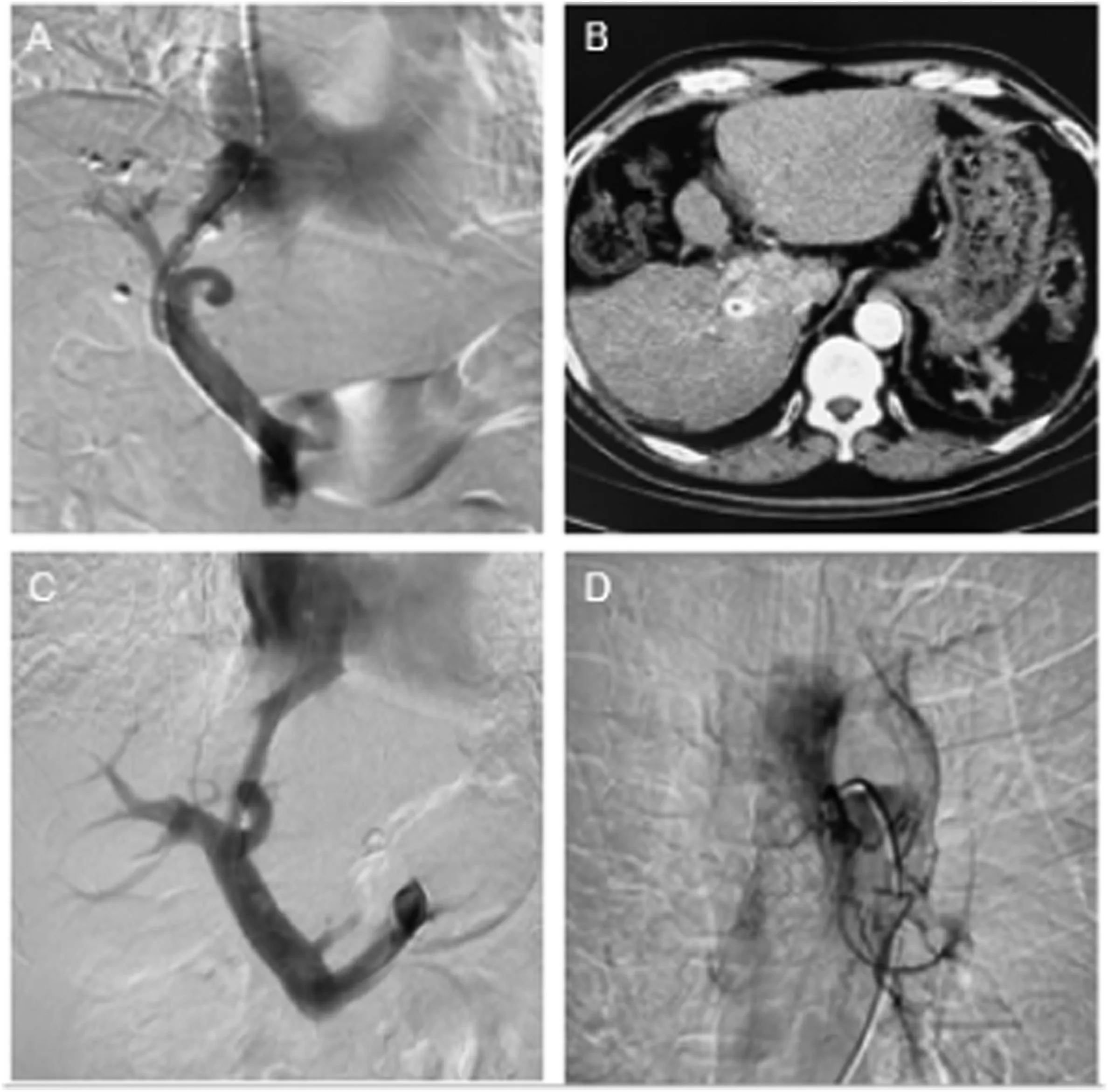
Fig.1. Picture A and B:Digital subtraction angiography(DSA)and postoperative computed tomography(CT)images of the patient with liver cancer combined with portal hypertension.Picture C and D:The TIPS and esophageal arterial chemoembolization DSA images of the patient with esophageal cancer and portal hypertension.

Table 1 Changes in blood ammonia and liver functions of two patients.
Both patients had no symptoms of drowsiness before taking the targeted drugs, and their plasma ammonia levels were within the normal range.Moreover,their liver function was classified as Child-Pugh class A(Table 1). Symptoms of hepatic encephalopathy, such as cognitive dysfunction,elevated blood ammonia level,and coma,occurred within a week of taking the targeted drug, and their liver function worsened(Table 1). Both patients were diagnosed with grade 4 hepatic encephalopathy after excluding other possible causes of brain dysfunction according to the West Haven criteria.1The targeted drugs were stopped immediately, and intravenous infusion of branched-chain amino acids,ornithine aspartate, was administered to lower blood ammonia levels,refreshing the brain, and lactulose was administered orally to induce catharsis. The patient's consciousness became clear, and their blood ammonia levels and liver function returned to normal (Table 1). These two patients were subsequently treated with interventional therapy and symptomatic treatment, and the targeted drugs were not rechallenged.Therefore, the patient no longer exhibited symptoms of hepatic encephalopathy.
3. Discussion
The increasing use of TIPS and the prolonged survival time of patients after surgery may lead to cancer following TIPS.3,4The two patients in our center had portal hypertension and tumors. Targeted antitumor treatments were administered following TIPS, and symptoms of hepatic encephalopathy appeared that improved after discontinuation of the drug. Therefore, it was evident that the symptoms of hepatic encephalopathy, such as lethargy and fatigue, were caused by the intake of targeted drugs. Molecular targeted therapy is a revolutionary treatment approach that halts cancer growth, progression, and metastasis by interfering with specific molecules.5Many molecular targeted therapies approved by the United States Food and Drug Administration have achieved significant clinical efficacy in treating liver, lung, esophageal,colorectal, and other cancers. Owing to their anti-angiogenic effect,molecular targeted drugs can also inhibit the growth of the cerebral vascular endothelium,causing increased permeability of the blood-brain barrier.6Consequently, high levels of ammonia cross the blood-brain barrier and disrupt the glutamate and glutamine pathways in the astrocytes of the brain,leading to neurological disorders.7This may be one of the mechanisms leading to hepatic encephalopathy.
The liver is the main site of ammonia metabolism.The liver toxicities induced by targeted drugs may generate an imbalance in ammonia metabolism, elevating the blood ammonia level.1TIPS diverts partial blood supply from the liver, aggravates liver impairment, and shunts ammonia-rich blood from the intestine into the systemic circulation.8The use of targeted drugs reduces the blood flow to the liver and worsens liver function.9Poor liver function and hepatotoxicity caused by these drugs have also been suggested as possible reasons for the occurrence of hepatic encephalopathy.Events that lead to increased levels of ammonia in the blood or brain have been implicated to worsen hepatic encephalopathy, whereas reducing blood ammonia levels improves hepatic encephalopathy.10,11Therefore, special attention should be paid to the development of hepatic encephalopathy when targeted drugs are used.
In conclusion, an increase in serum ammonia levels remains important to our understanding of hepatic encephalopathy, and therapies remain directed toward lowering ammonia levels in patients with signs of hepatic encephalopathy. When clinicians choose molecular targeted therapy as the second or third targeted therapy for patients who have undergone TIPS, we should have the diagnosis and treatment consciousness to detect mild hepatic encephalopathy, and drug-induced hepatic encephalopathy should be considered to avoid iatrogenic triggers.
Financial support
This work was funded by the National Natural Science Foundation of China(81873917).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all colleagues for helping us during the current study.
杂志排行
Journal of Interventional Medicine的其它文章
- Safety and efficacy of Endovascular Management of high-grade blunt renal injury
- A low-grade cerebral arteriovenous malformation suspected of being a metastatic tumor: A case report and literature review
- Angiography findings and endovascular management of acute nonvariceal gastrointestinal bleeding: A pictorial essay
- Comparison of tumor response following conventional versus drug-eluting bead transarterial chemoembolization in early- and very early-stage hepatocellular carcinoma
- Complications from port-a-cath system implantation in adults with malignant tumors: A 10-year single-center retrospective study
- Paclitaxel-coated balloons angioplasties for extra-long femoropopliteal artery atherosclerotic lesions(>30 cm):12 months outcomes from a single center
