4-或6-芳基取代的二氢嘧啶-4-酮的合成
2022-03-10郭丽婷李轲谭重阳吴滨
郭丽婷,李轲,谭重阳,吴滨
(中南民族大学 药学院,武汉 430074)
二氢嘧啶酮(DHPM)是一种极具研究价值的分子骨架,存在于一系列具有多种药物活性的药物分子中[1]. 研究发现,许多二氢嘧啶-4-酮类化合物具有免疫缺陷病毒1 型(HIV-1)非核苷逆转录酶(RT)抑制剂(NNRTIs)的活性[2-3]. 一些二氢嘧啶-4-酮类化合物,如利培酮和帕利哌酮,在治疗精神分裂症方面有广泛应用[4-5]. 合成二氢嘧啶-4-酮骨架的传统方法包括β-氨基酰胺和醛之间的缩合反应[6-7],脒与丙烯酸甲酯的环化反应[6].
点击化学反应(CuAAC)是以芳基叠氮化合物和炔类化合物为底物,通过Cu(I)催化的环加成反应生成具有区域选择性的1,4-二取代-1,2,3-三氮唑[8]. 近年来,研究发现乙烯亚胺中间体可由铜催化叠氮化物与炔环加成反应形成[9]. 在早前报道中,化学家利用CuAAC 机制产生的乙烯亚胺中间体成功实现了许多高效的三组分反应研究[10-14].
本课题组拟以N-乙酰基-1-苯基炔丙基胺或N-苯甲酰基-炔丙基胺为底物,研究其在一价铜催化条件下与磺酰叠氮化合物反应制备二氢嘧啶-4-酮类化合物(图1),以期开发的合成方法为其在药物化学中的应用提供有力的帮助.
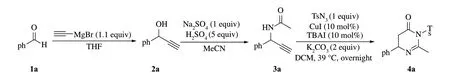
图1 二氢嘧啶-4-酮化合物4a的合成Fig.1 Synthesis of 4-phenyl substituted dihydropyrimidin-4-one 4a
1 实验部分
1.1 样品、试剂和仪器
苯甲醛、乙炔基溴化镁、四氢呋喃(99.5%)、硫酸钠、浓硫酸、乙腈、炔丙胺、苯甲酰氯、三乙胺、二氯甲烷(99.9%)、对甲基苯磺酰叠氮(97%)、碘化亚铜、四丁基碘化铵、碳酸钾、氘代试剂(Cambrige)等所有试剂均为市售分析纯.X-4A 显微熔点仪(上海仪电);傅立叶变换红外光谱仪(IRTracer-100,NULL);600 MHz 核磁共振波谱仪(德国布鲁克);液相色谱高分辨质谱联用仪(德国Thermo).
1.2 化合物2a,3a的合成
在惰性气体氛围下,苯甲醛(20 mmol,2.4 mL)加入到250 mL干燥圆底烧瓶中,0 ℃冰浴下搅拌,并将乙炔基溴化镁(22 mmol,44 mL)缓慢滴加进烧瓶中,待加完格氏试剂后,撤去冰浴,室温下搅拌,TLC监测反应完全后,分3次加入适量二氯甲烷萃取,合并有机相,饱和食盐水水洗有机相3次,加入适量无水硫酸钠干燥有机相,减压过滤浓缩得到黄色油状粗产物2a(图1),粗产物直接用于后续反应,无需进一步纯化.
在惰性气体氛围下,将溶解于10 mL 乙腈中的2a(10 mmol,2.11 g)加入装有无水硫酸钠(10 mmol,1.42 g)的250 mL干燥圆底烧瓶中,冰浴下搅拌,将浓硫酸(2.6 mL)溶解于40 mL 乙腈中的溶液缓慢滴加到烧瓶中(15 min),撤去冰浴,室温下搅拌2 h,直到反应完全.50 mL 水淬灭反应.乙酸乙酯萃取,无水硫酸钠干燥有机相,减压浓缩,最后通过柱层析分离纯化,展开剂比例为石油醚/乙酸乙酯=2∶1,得到淡黄色粉末3a 1.07 g,产率62%(图1).
1.3 化合物4a的合成
1.3.1 反应条件优化及4a合成
优化反应条件(表1).在筛选的9 种溶剂中,二氯甲烷表现的溶剂效应最好,确定二氯甲烷为溶剂. 又对铜催化剂进行筛选,发现大多数的铜催化剂都能促进反应进行,而其中一价铜盐的反应效果最好.对反应所需的碱进行优化,发现K2CO3与其他碱如Cs2CO3、DBU、Et3N、Na2CO3、NaHCO3等相比是最优的.最终确定在39 ℃下反应12 h是最优的反应条件.依次用硅藻土过滤反应液,乙酸乙酯冲洗,减压浓缩,再用薄层色谱板进行分离(石油醚/乙酸乙酯=2∶1),得到白色固体4a(25.7 mg,75%).
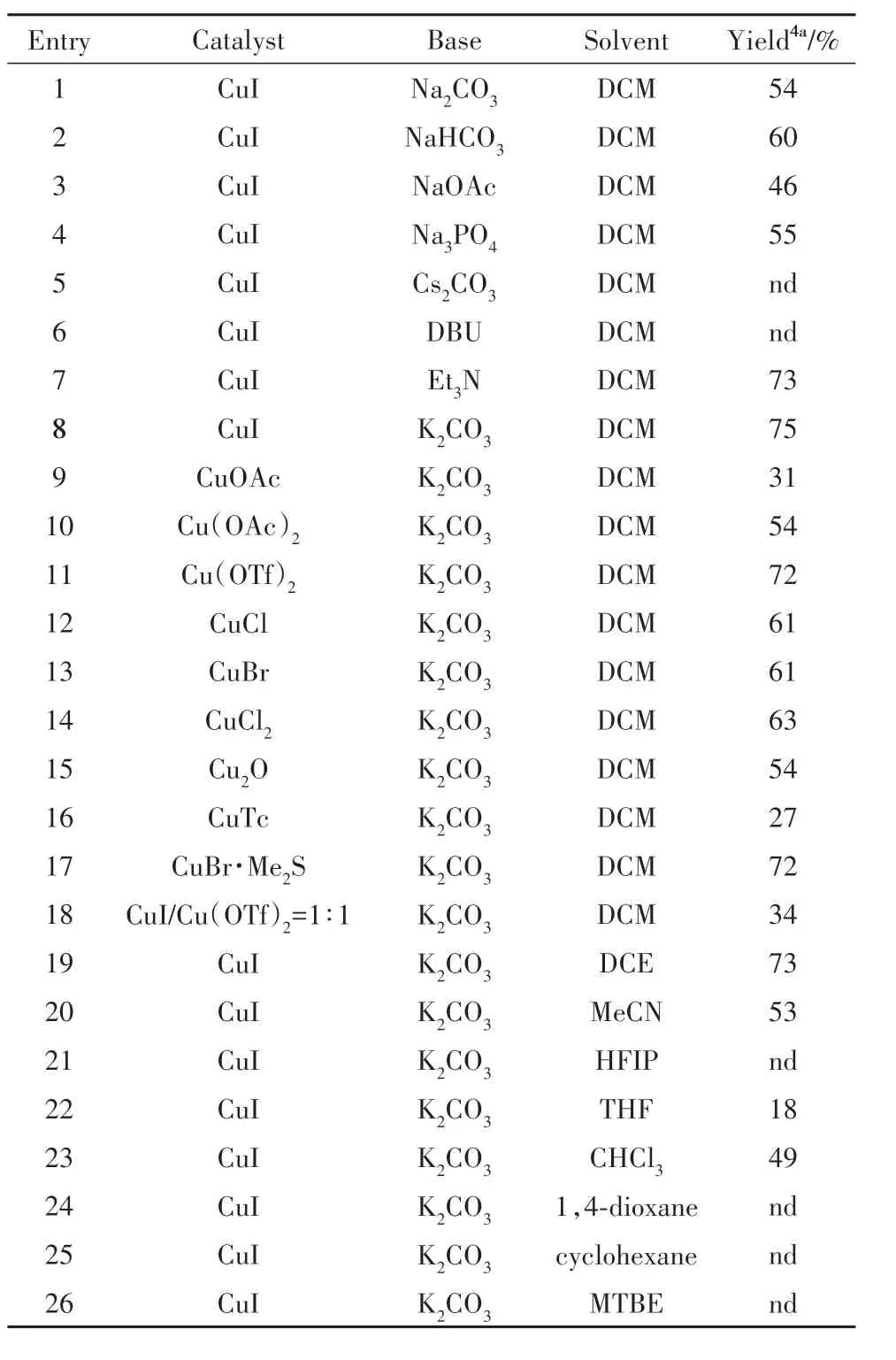
表1 反应条件的优化Tab.1 Optimization of reaction conditions
1.3.2 化合的4a晶体结构鉴定和描述
晶体保持在稳定的T= 150.0 K 下用Bruker APEX-II CCD 衍射仪收集,用BrukerShelxTL 软件包解析和优化该结构,使用多扫描方法(SADABS)对吸收效应进行数据校正,晶体结构用直接法求解,所有非氢原子使用全矩阵最小二乘法对F2 进行各项异性修正,用理论加氢法对氢原子进行加和至理论位置.
1.4 底物普适性考察
1.4.1 基于苯环的考察
以苯环上有不同吸电子和供电子基团的二级炔丙基胺类化合物3a~3l 为底物,在标准反应条件下,可以中等收率得到二氢嘧啶-4-酮类产物4a~4l.
1.4.2 取代基考察和化合物7a、8a的合成
在惰性气体氛围下,苯甲酰氯(17.23 mmol,2.2 mL),用25 mL 二氯甲烷溶解后加入到250 mL干燥圆底烧瓶中,冰浴下搅拌,将溶于二氯甲烷的炔丙胺(16.86 mmol,1.1 mL)缓慢滴加进烧瓶中,然后加入三乙胺,加完后撤去冰浴,室温下搅拌,TLC监测反应. 反应结束后饱和氯化铵水溶液和饱和NaCl水溶液分别萃取3次,合并有机相,加入适量无水硫酸钠干燥有机相,减压过滤浓缩得到白色粉末状产物7a 2.47 g,产率90%(图2).
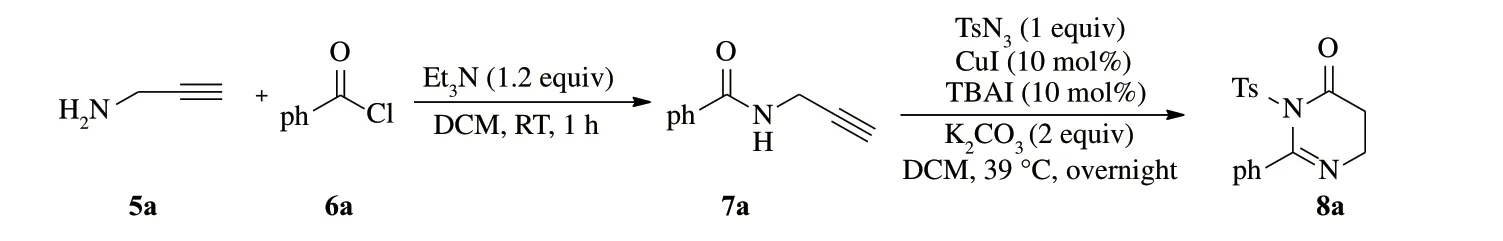
图2 化合物7a、8a的合成Fig.2 Synthesis of 7a,8a
化合物8a 的合成方法和操作步骤与4a 完全一样,反应在39 ℃下搅拌过夜,得到白色固体8a(19.6 mg,60%).
考察氮原上苯甲酰基的苯环上不同的取代基对反应的影响,得到化合物8a~8i.
2 结果与讨论
2.1 底物普适性考察
对底物的普适性进行考察(表2、3).合成苯环上有不同吸电子和供电子基团的二级炔丙基胺类化合物3a~3l.以这些化合物为底物,在标准反应条件下,可以中等收率得到二氢嘧啶-4-酮类产物4a~4l.研究发现,苯环上的取代基的性质和位置对该反应的影响不大.
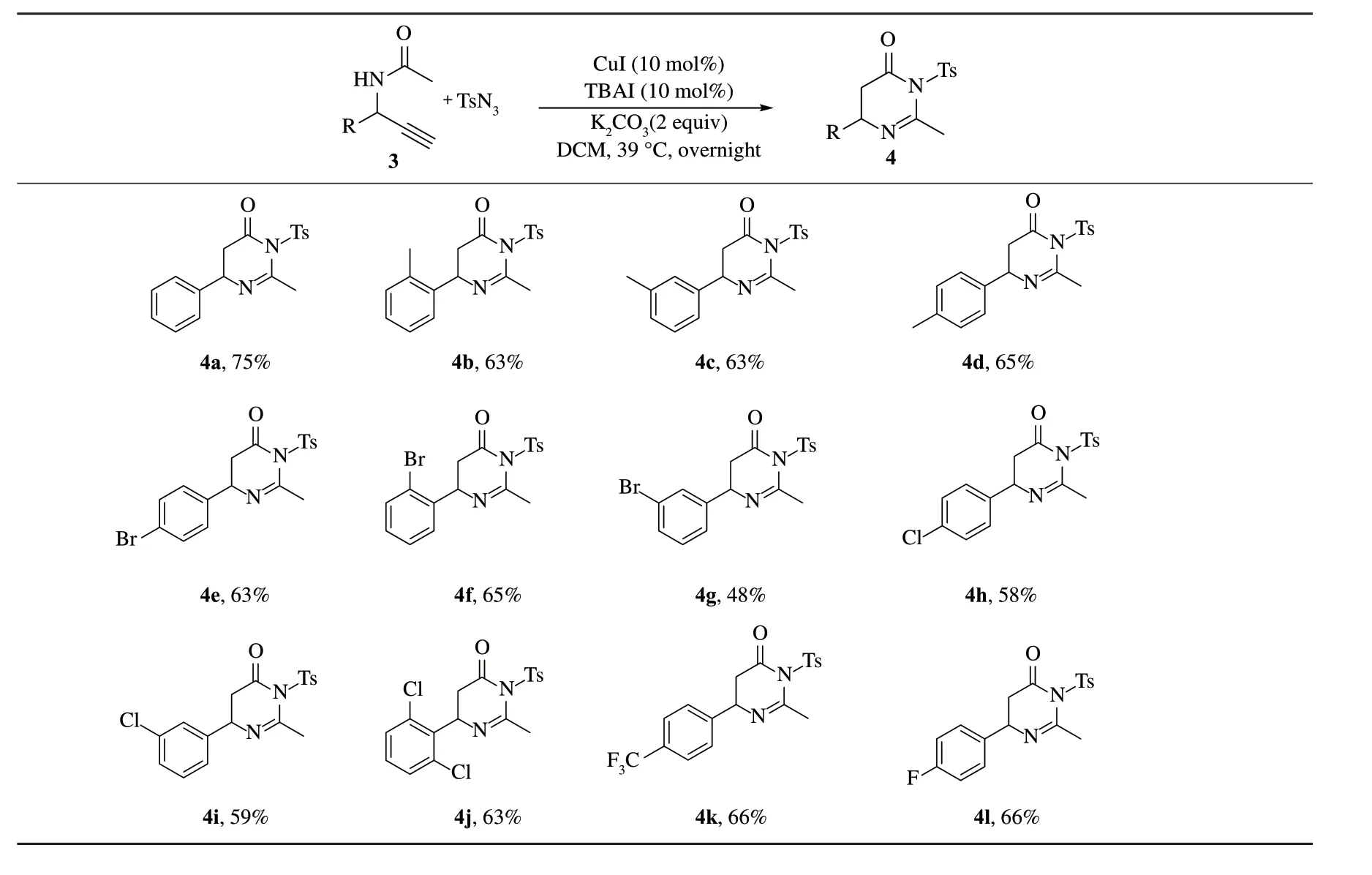
表2 酰胺的底物范围Tab.2 Amide substrate scope
表3 中,制备了氮原子不同酰基取代的一级炔丙基胺类化合物.以这些化合物为底物,考察了该反应的情况.发现氮原上苯甲酰基的苯环上不同的取代基对反应的影响很大.当取代基为供电子基团时,产物的收率明显优于吸电子基团的产物(如8a~8e对比8f~8i).
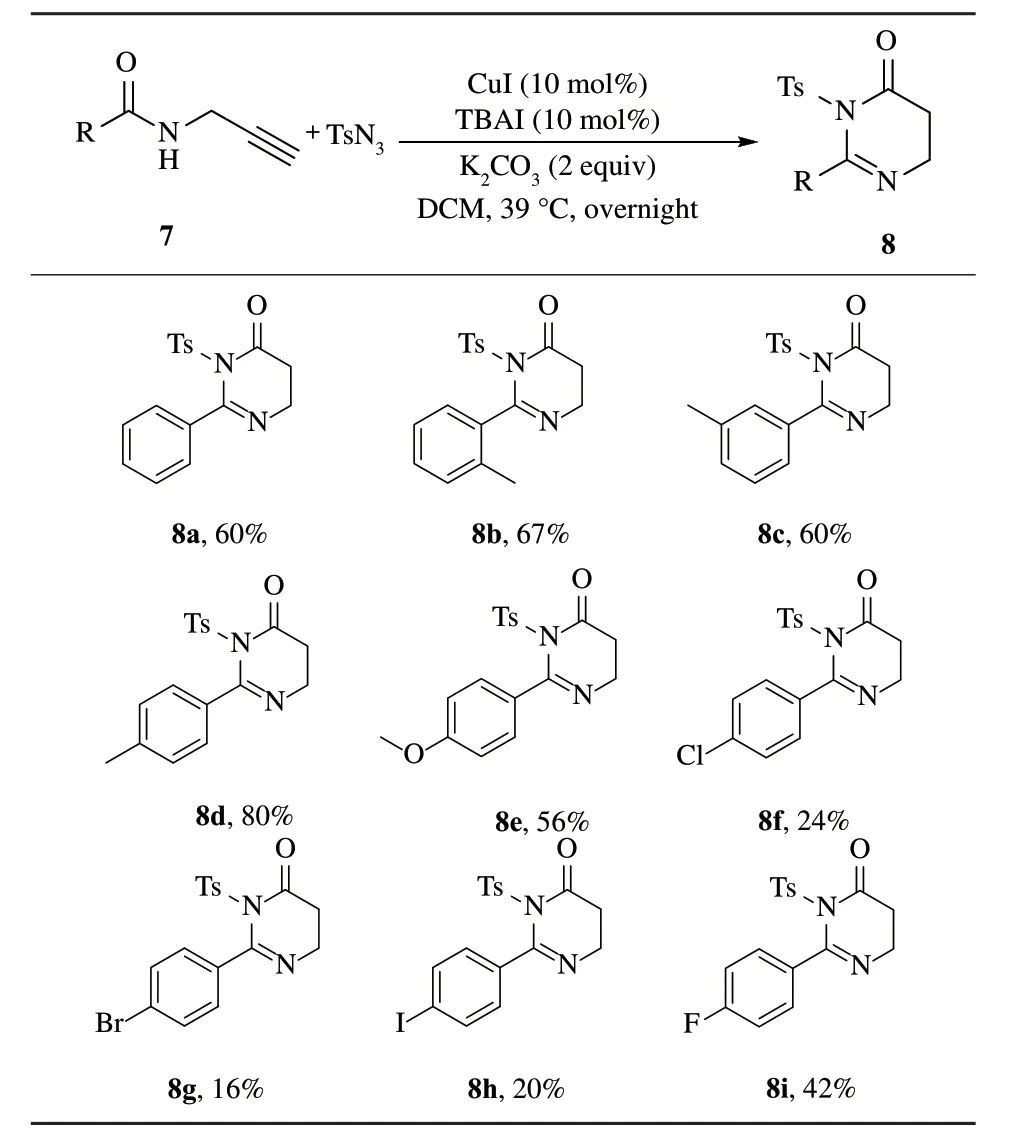
表3 酰胺的底物范围Tab.3 Amide substrate scope
2.2 反应机理研究
对比文献[2,15],对该反应可能的机理提出了设想.
炔丙基胺化合物3a 在碱作用下与一价铜盐反应生成末端炔铜化合物,与对甲苯磺酰叠氮发生环加成反应,脱去一分子氮气生成乙烯亚胺中间体A.中间体A 的官能团乙烯亚胺的中心碳原子与酰胺基团的氧原子发生分子内亲核加成反应得到B. B经过分子内开环/重新关环反应最终生成产物4a(图3).
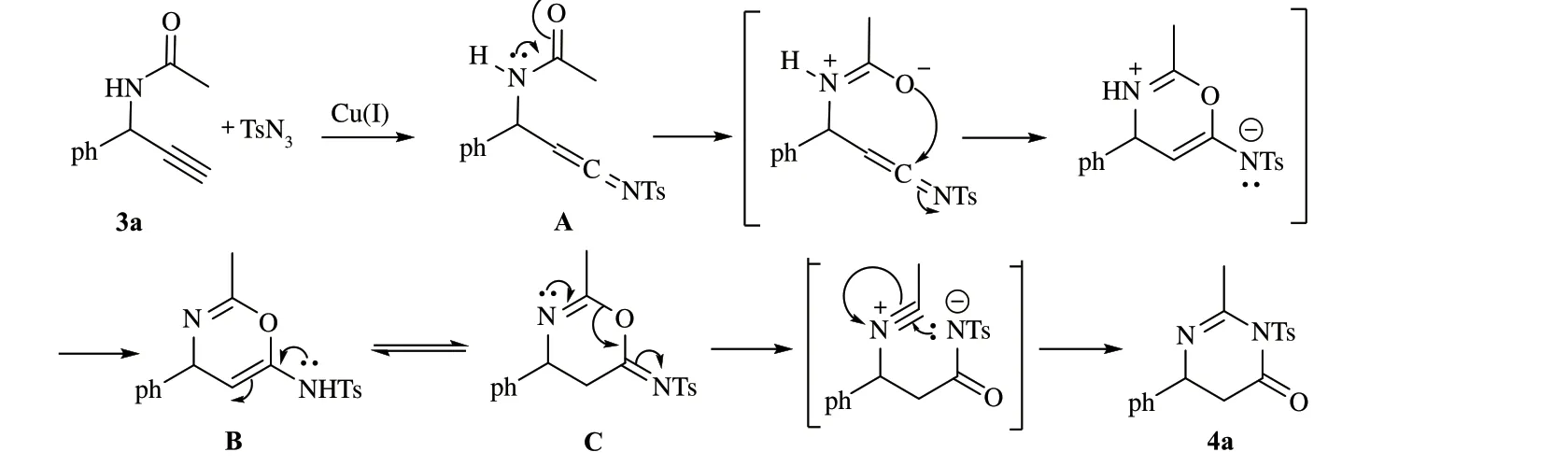
图3 可能的反应机理Fig.3 Plausible reaction mechanism
2.3 晶体结构鉴定和描述
化合物4a(C18H18N2O3S)的晶体数据和有关数据收集及结构精修数据列于表4.晶体编号:cu-1221-9.化合物4a 的晶体结构如图4 所示.化合物是一种无色块状晶体,属于三斜晶系,P-1 空间群,相对分子量342.40,近似尺寸为0.200 mm×0.150 mm×0.120 mm ,晶胞参数a= 8.3309(2)Å,b= 8.6621(2)Å,c= 13.1409(3)Å,α= 73.3410(10)°,β=83.8620(10)°,γ=64.9670(10)°,V=823.03(3)Å3.
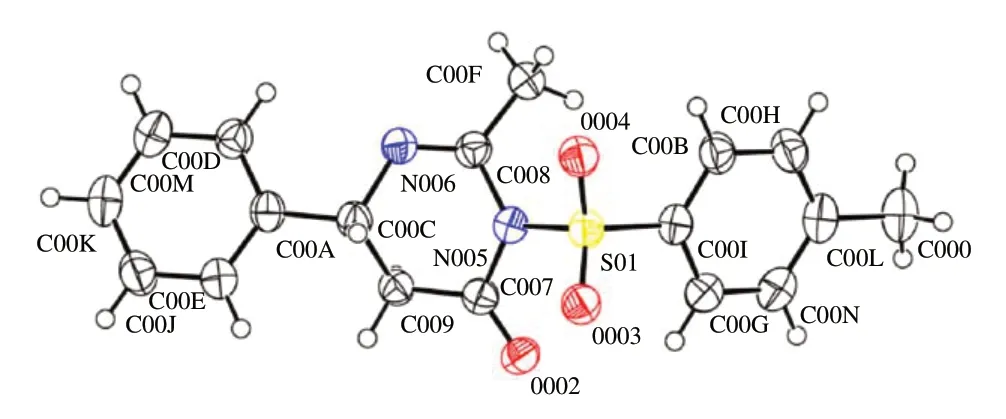
图4 化合物4a的晶体结构图Fig.4 Molecular structure of compound 4a
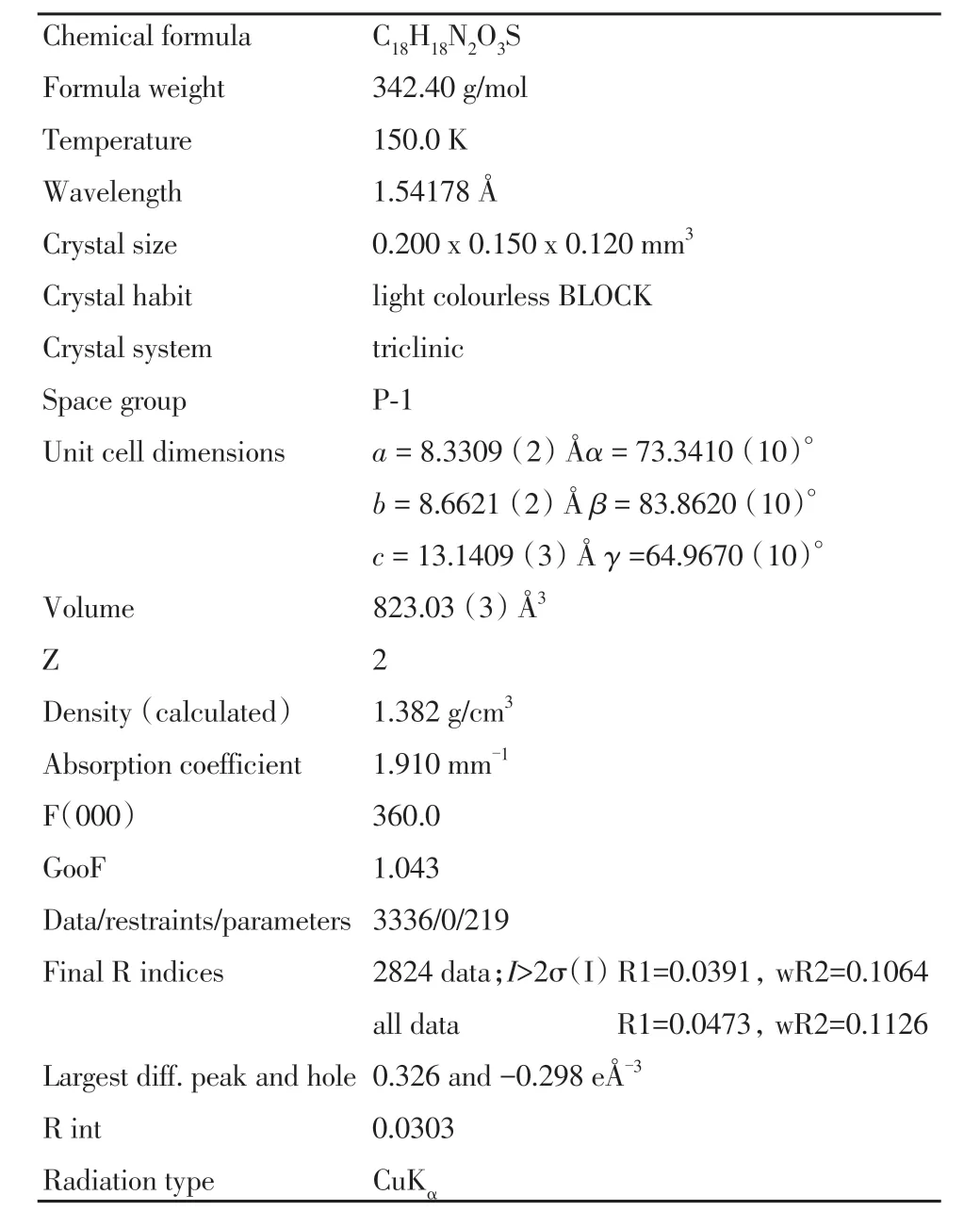
表4 化合物4a的晶体数据和结构精修数据Tab.4 Crystal data and structure refinement for compound 4a
2.4 化合物4a~4i的合成及表征
2-Methyl-6-phenyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4a):白色固体(25.7 mg,75%),已报道化合物[2].1H NMR(600 MHz,CDCl3)δ 8.00(d,J=8.4 Hz,2H),7.39-7.32(m,6H),7.32-7.28(m,1H),4.79-4.63(m,1H),2.77(dd,J= 17.4,3.9 Hz,1H),2.60(d,J= 1.9 Hz,3H),2.53-2.48(m,1H),2.46(s,3H);13C NMR(150 MHz,CDCl3)δ 169.2,150.8,145.7,140.1,135.8,129.6,128.9,128.8,127.7,126.4,56.2,40.6,24.3,21.7.
2-Methyl-6-(o-tolyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4b):白色固体(22.5 mg,63%).1H NMR(500 MHz,CDCl3)δ 8.07(d,J= 8.4 Hz,2H),7.42-7.35(m,3H),7.25-7.14(m,3H),4.87-4.79(m,1H),2.73(dd,J=17.4,3,8 Hz,1H),2.59(d,J=2.1 Hz,3H),2.47(s,3H),2.45-2.39(m,1H),2.33(s,3H);13C NMR(125 MHz,CDCl3)δ 169.4,150.6,145.7,138.2,135.9,134.9,130.7,129.6,128.9,127.6,126.6,126.3,53.3,39.7,24.2,21.7,19.2;HRMS(EI)Calcd for C19H21N2O3S[M+H]+:357.1273,Found 357.1266;IR (KBr) ν(cm-1):1732,1665,1352,1207,1161,675.
2-Methyl-6-(m-tolyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4c):白色固体(22.5 mg,63%).1H NMR(500 MHz,CDCl3)δ 7.99(d,J= 8.5 Hz,2H),7.35(d,J=8.2 Hz,2H),7.24(d,J=7.6 Hz,1H),7.16(s,1H),7.12(t,J= 7.6 Hz,2H),4.67-4.58(m,1H),2.76(dd,J=17.3,3.9 Hz,1H),2.60(d,J=1.9 Hz,3H),2.54-2.47(m,1H),2.46(s,3H),2.35(s,3H);13C NMR(125 MHz,CDCl3)δ 169.2,150.7,145.6,140.0,138.5,135.9,129.6,128.9,128.7,128.5,127.1,123.5,56.2,40.6,24.3,21.7,21.5;HRMS(EI)Calcd for C19H21N2O3S[M+H]+:357.1273,Found 357.1265;IR (KBr) ν(cm-1):1734,1668,1362,1209,1169,677,548.
2-Methyl-6-(p-tolyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4d):白色固体(23.2 mg,65%).1H NMR(500 MHz,CDCl3)δ 7.99(d,J= 8.1 Hz,2H),7.35(d,J= 8.1 Hz,2H),7.23(d,J= 8.0 Hz 2H),7.17(d,J=7.9 Hz 2H),4.63(d,J=11.9 Hz,1H),2.75(dd,J= 17.3,3.8 Hz,1H),2.60(d,J= 1.2 Hz,3H),2.52-2.47(m,1H),2.46(s,3H),2.34(s,3H);13C NMR(125 MHz,CDCl3)δ 169.3,150.6,145.6,137.4,137.1,135.9,129.5,129.4,128.9,126.3,55.9,40.5,24.3,21.7,21.1;HRMS(EI)Calcd for C19H21N2O3S[M+H]+:357.1273,Found 357.1264;IR(KBr)ν(cm-1):1732,1663,1362,1211,1169,855,660.
6-(4-Bromophenyl)-2-methyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4e):白色固体(26.6 mg,63%).1H NMR(500 MHz,CDCl3)δ 7.98(d,J= 8.4 Hz,2H),7.48(d,J= 8.5 Hz,2H),7.35(d,J= 8.2 Hz,2H),7.24(d,J= 8.5 Hz,2H),4.66-4.59(m,1H),2.75(dd,J= 17.3,3.9 Hz,1H),2.59(d,J= 2.0 Hz,3H),2.46(s,3H),2.45-2.39(m,1H);13C NMR(125 MHz,CDCl3)δ 168.8,151.3,145.8,139.2,135.7,131.9,129.6,128.9,128.2,121.6,55.6,40.4,24.3,21.7. HRMS(EI)Calcd for C18H18BrN2O3S[M+H]+:423.0201,Found 423.0193;IR(KBr)ν(cm-1):2918,2849,1740,1354,1188,1169,1148,1090,850,667.
6-(2-Bromophenyl)-2-methyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4f):白色固体(27.3 mg,65%).1H NMR(600 MHz,CDCl3)δ 8.06(d,J= 8.4 Hz,2H),7.64(dd,J=7.8,1.6 Hz,1H),7.54(dd,J= 8.0,1.0 Hz,1H),7.39(d,J= 8.2 Hz,2H),7.37-7.34(m,1H),7.16(td,J=7.8,1.6 Hz,1H),5.02-4.97(m,1H),2.93(dd,J= 17.5,3.8 Hz,1H),2.62(d,J= 2.0 Hz,3H),2.47(s,3H),2.26(dd,J= 17.4,13.4 Hz,1H);13C NMR(150 MHz,CDCl3)δ 154.0,139.2,134.7,129.4,126.4,123.8,121.2,120.8,120.5,120.3,119.8,115.1,59.5,45.6,37.3,32.9;HRMS(EI)Calcd for C18H18BrN2O3S[M+H]+:421.0222,Found 421.0217;IR(KBr)ν(cm-1):3536,3402,2918,1738,1368,1117,841,750,600,457.
6-(3-Bromophenyl)-2-methyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4g):白色固体(20.2 mg,48%).1H NMR(500 MHz,CDCl3)δ 8.00(d,J=8.3 Hz,2H),7.55(s,1H),7.43(d,J= 7.7 Hz,1H),7.36(d,J= 8.2 Hz,2H),7.29-7.20(m,2H),4.63(d,J= 12.5 Hz,1H),2.76(dd,J=17.3,3.7 Hz,1H),2.60(s,3H),2.46(s,3H),2.45-2.39(m,1H);13C NMR(125 MHz,CDCl3)δ 168.7,151.3,145.8,142.5,135.7,130.8,130.3,129.6,128.9,125.1,122.9,55.6,40.5,24.3,21.7;HRMS(EI)Calcd for C18H18BrN2O3S[M+H]+:423.0201,Found 423.0191;IR(KBr)ν(cm-1):1732,1368,1221,1171,997,867,754,665.
6-(4-Chlorophenyl)-2-methyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4h):白色固体(22.2 mg,58%).1H NMR(500 MHz,CDCl3)δ 7.99(d,J= 8.2 Hz,2H),7.36(d,J= 8.2 Hz,2H),7.31(dd,J =17.8,8.5 Hz,4H),4.64(d,J= 12.0 Hz,1H),2.75(dd,J= 17.3,3.7 Hz,1H),2.60(s,3H),2.46(s,3H),2.45-2.40(m,1H);13C NMR(125 MHz,CDCl3)δ 168.8,151.1,145.8,138.7,135.7,133.5,129.6,128.9,127.8,55.5,40.5,24.3,21.7;HRMS(EI)Calcd for C18H18ClN2O3S[M+H]+:377.0727,Found 377.0721;IR (KBr) ν(cm-1):2924,1736,1655,1460,1172,1144.
6-(3-Chlorophenyl)-2-methyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4i):白色固体(22.2 mg,59%).1H NMR(500 MHz,CDCl3)δ 8.01(d,J= 8.4 Hz,2H),7.40-7.38(m,1H),7.36(d,J= 8.1 Hz,2H),7.32-7.26(m,2H),7.22(dt,J= 6.7,2.1 Hz,1H),4.68-4.60(m,1H),2.77(dd,J=17.3,3.9 Hz,1H),2.60(d,J= 1.9 Hz,3H),2.47(s,3H),2.45-2.40(m,1H);13C NMR(125 MHz,CDCl3)δ 168.8,151.3,145.8,142.2,135.7,134.7,130.1,129.6,129.0,127.9,126.7,124.6,55.7,40.5,24.3,21.7;HRMS(EI)Calcd for C18H18ClN2O3S[M+H]+:377.0727,Found 377.0721;IR(KBr)ν(cm-1):2957,2924,2853,1736,1458,1261,870,764,750.
6-(2,6-Dichlorophenyl)-2-methyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4j):白色固体(26.1 mg,63%).1H NMR(500 MHz,CDCl3)δ 8.08(d,J=8.5 Hz,2H),7.40(d,J=8.1 Hz,2H),7.32(d,J=8.1 Hz,2H),7.17(t,J=8.1 Hz,1H),5.53-5.46(m,1H),3.27(dd,J= 17.6,14.3 Hz,1H),2.64(dd,J= 17.6,4.3 Hz,1H),2.58(d,J= 2.3 Hz,3H),2.48(s,3H);13C NMR(125 MHz,CDCl3)δ 169.2,149.4,145.8,135.9,135.2,134.0,129.7,129.5,128.9,54.0,36.1,24.0,21.7;HRMS(EI)Calcd for C18H17Cl2N2O3S[M+H]+:411.0337,Found 411.0333;IR(KBr)ν(cm-1):2924,1721,1560,1547,1439,1275,1142,1084.
2-Methyl-3-tosyl-6-(4-(trifluoromethyl)phenyl)-5,6-dihydropyrimidin-4(3H)-one(4k):白 色 固 体(27.6 mg,66%).1H NMR(500 MHz,CDCl3)δ 8.00(d,J= 8.3 Hz,2H),7.62(d,J= 8.1 Hz,2H),7.50(d,J= 8.1 Hz,2H),7.36(d,J= 8.2 Hz,2H),4.73(d,J=12.4 Hz,1H),2.80(dd,J=17.3,3.8 Hz,1H),2.61(d,J= 1.6 Hz,3H),2.52-2.43(m,1H),2.46(s,H);13C NMR(125 MHz,CDCl3)δ 168.6,151.5,145.9,144.2,135.7,130.0(q,JC-F=32.5 Hz),129.6,129.0,126.9,125.8(q,JC-F= 3.7 Hz),124.0(q,JC-F= 270.5 Hz),55.8,40.4,24.3,21.7;19F NMR(470 MHz,CDCl3)δ -62.55;HRMS(EI)Calcd for C19H18CF3N2O3S[M+H]+:411.0990,Found 411.0983;IR(KBr)ν(cm-1):1736,1663,1369,1327,1171,1126,1069,852,665.
6-(4-Fluorophenyl)-2-methyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(4l):白色固体(23.7 mg,66%).1H NMR(500 MHz,CDCl3)δ 8.00(d,J= 8.3 Hz,2H),7.36(d,J= 8.2 Hz,2H),7.32(dd,J=8.5,5.4 Hz,2H),7.04(t,J=8.6 Hz,2H),4.64(d,J=12.0 Hz,1H),2.75(dd,J=17.3,3.8 Hz,1H),2.59(d,J= 1.7 Hz,3H),2.49-2.41(m,1H),2.46(s,3H);13C NMR(125 MHz,CDCl3)δ 169.0,162.2(d,JC-F= 244.7 Hz),151.0,145.7,136.0(d,JC-F= 3.1 Hz),135.8,129.6,128.9,128.1(d,JC-F=8.1 Hz),115.6(d,JC-F= 21.4 Hz),55.6,40.7,24.3,21.7;19F NMR(470 MHz,CDCl3)δ -114.70;HRMS(EI) Calcd for C18H18FN2O3S [M+H]+:361.1022,Found 361.1014;IR(KBr)ν(cm-1):1732,1667,1510,1362,1227,1167,660.
2.5 化合物8a-8i的合成及表征
2-Phenyl-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8a):白色固体(19.6 mg,60%),已报道化合物[2],1H NMR(600 MHz,CDCl3)δ 7.85(d,J=8.3 Hz,2H),7.59(d,J= 7.2 Hz,2H),7.48(t,J= 7.4 Hz,1H),7.40(t,J= 7.7 Hz,2H),7.31(d,J= 8.1 Hz,2H),3.74(t,J= 6.4 Hz,2H),2.55(t,J= 6.4 Hz,2H),2.45(s,3H);13C NMR(150 MHz,CDCl3)δ 170.5,154.5,145.7,136.1,135.9,130.7,129.34,129.31,128.3,127.3,43.3,33.9,21.7.
2-(o-Tolyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8b):白色固体(23.1 mg,67%).1H NMR(500 MHz,CDCl3)δ 7.54(d,J= 8.3 Hz,2H),7.31(td,J=7.7,1.4 Hz,1H),7.20(d,J=8.2 Hz,2H),7.15(d,J= 7.7 Hz,1H),7.13-7.07(m,2H),3.83(t,J=6.4 Hz,2H),2.63(t,J=6.4 Hz,2H),2.42(s,3H),2.16(s,3H);13C NMR(125 MHz,CDCl3)δ 170.2,153.2,145.5,136.4,135.1,134.8,130.5,129.6,129.1,129.0,128.6,125.2,43.3,34.1,21.7,19.1;HRMS(EI)Calcd for C18H19N2O3S[M+H]+:343.1116,Found 343.1112;IR (KBr) ν(cm-1):1740,1369,1173,1086,669,596.
2-(m-Tolyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8c):白色固体(20.5 mg,60%).1H NMR(500 MHz,CDCl3)δ 7.85(d,J= 8.4 Hz,2H),7.40-7.35(m,1H),7.33-7.30(m,3H),7.28(d,J= 4.5 Hz,2H),3.75(t,J= 6.3 Hz,2H),2.56(t,J= 6.4 Hz,2H),2.45(s,3H),2.33(s,3H);13C NMR(125 MHz,CDCl3)δ 170.6,154.6,145.6,137.9,136.0,135.9,131.5,129.3,128.2,127.8,124.6,43.4,34.1,21.7,21.3;HRMS(EI)Calcd for C18H19N2O3S[M+H]+:343.1116,Found 343.1107;IR(KBr)ν(cm-1):1736,1368,1171,1150,1086,669,598.
2-(p-Tolyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8d):白色固体(27.5 mg,80%),已报道化合物[2],1H NMR(500 MHz,CDCl3)δ 7.87(d,J= 8.4 Hz,2H),7.51(d,J=8.2 Hz,2H),7.32(d,J=8.2 Hz,2H),7.21(d,J=8.0 Hz,2H),3.70(t,J=6.4 Hz,2H),2.52(t,J= 6.4 Hz,2H),2.45(s,3H),2.40(s,3H);13C NMR(125 MHz,CDCl3)δ 170.6,154.4,145.6,141.1,136.0,133.4,129.3,129.0,127.3,43.2,34.0,21.7,21.5.
2-(4-Methoxyphenyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8e):白色固体(20.2 mg,56%),已报道化合物[2].1H NMR(500 MHz,CDCl3)δ 7.88(d,J=8.3 Hz,2H),7.59(d,J=8.8 Hz,2H),7.32(d,J=8.2 Hz,2H),6.91(d,J= 8.8 Hz,2H),3.86(s,3H),3.68(t,J= 6.3 Hz,2H),2.52(t,J= 6.3 Hz,2H),2.45(s,3H);13C NMR(125 MHz,CDCl3)δ 170.7,161.7,154.0,145.6,136.0,129.34,129.28,129.0,128.6,113.7,55.4,43.2,34.1,21.7.
2-(4-Chlorophenyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8f):白色固体(8.7 mg,24%),已报道化合物[2].1H NMR(500 MHz,CDCl3)δ 7.86(d,J=8.4 Hz,2H),7.56(d,J=8.6 Hz,2H),7.38(d,J=8.6 Hz,2H),7.33(d,J=8.2 Hz,2H),3.72(t,J= 6.4 Hz,2H),2.54(t,J= 6.4 Hz,2H),2.46(s,3H);13C NMR(125 MHz,CDCl3)δ 170.3,153.5,145.9,136.9,135.7,134.7,129.4,129.3,128.7,128.6,43.4,33.8,21.8.
2-(4-Bromophenyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8g):白色固体(6.6 mg,16%),已报道化合物[2],1H NMR(500 MHz,CDCl3)δ 7.86(d,J=8.3 Hz,2H),7.54(d,J=8.5 Hz,2H),7.49(d,J=8.5 Hz,2H),7.33(d,J= 8.2 Hz,2H),3.74-3.68(m,2H),2.55-2.50(m,2H),2.46(s,3H);13C NMR(125 MHz,CDCl3)δ 170.0,153.4,145.7,135.5,134.9,131.3,129.2,129.0,128.7,125.0,43.1,33.6,21.5.
2-(4-Iodophenyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8h):白色固体(9.1 mg,20%).1H NMR(600 MHz,CDCl3)δ 7.86(d,J=8.3 Hz,2H),7.75(d,J= 8.5 Hz,2H),7.37-7.31(m,4H),3.71(t,J=6.4 Hz,2H),2.53(t,J=6.4 Hz,2H),2.46(s,3H);13C NMR(150 MHz,CDCl3)δ 170.3,153.8,146.0,137.5,135.7,129.5,129.3,128.9,97.4,43.4,33.8,21.8;HRMS(EI)Calcd for C17H16IN2O3S[M+H]+:454.9926,Found 454.9918;IR(KBr)ν(cm-1):1736,1366,1190,1169,1142,1086.
2-(4-Fluorophenyl)-3-tosyl-5,6-dihydropyrimidin-4(3H)-one(8i):白色固体(14.5 mg,42%).1H NMR(500 MHz,CDCl3)δ 7.86(d,J= 8.4 Hz,2H),7.61(dd,J= 8.7,5.3 Hz,2H),7.33(d,J= 8.1 Hz,2H),7.09(t,J= 8.6 Hz,2H),3.71(t,J= 6.4 Hz,2H),2.54(t,J= 6.4 Hz,2H),2.46(s,3H);13C NMR(125 MHz,CDCl3)δ 170.4,164.2(d,JC-F=249.5 Hz),153.5,145.9,135.8,132.3(d,JC-F= 3.4 Hz),129.5(d,JC-F= 8.9 Hz),129.4,129.2,115.5(d,JC-F= 22.0 Hz),43.3,33.9,21.7;19F NMR(470 MHz,CDCl3) δ -109.01;HRMS(EI) Calcd for C17H16FN2O3S[M+H]+:347.0866,Found 347.0859;IR(KBr)ν(cm-1):2920,1734,1645,1375,1175,1142,1084.
3 结语
通过经典的铜催化点击化学反应,合成了化合物4a,并用单晶衍射实验证实了化合物4a的确切结构. 开发的由N-乙酰基-1-苯基炔丙基胺或N-苯甲酰基-炔丙基胺和磺酰叠氮化合物制备二氢嘧啶-4-酮的方法反应条件温和,收率高,底物的普适性好.二氢嘧啶-4-酮本身具有多种药理活性,本合成方法可为其在药物化学中的应用提供有力的帮助.
