An immunoglobulin Y that specifically binds to an in silico-predicted unique epitope of Zika virus non-structural 1 antigen
2022-03-09LeonardoGuevarraJrScottDeanDeSagonTreenaRicaTehMariaKatrinaDianaCruzNikkiCyrillCapistranoAustineJamesStaMariaLaarniGraceCoralesLeslieMichelleDalmacio
Leonardo A. Guevarra Jr., Scott Dean P. De Sagon, Treena Rica D. Teh, Maria Katrina Diana M. Cruz,Nikki Cyrill C. Capistrano, Austine James Z. Sta. Maria, Laarni Grace M. Corales, Leslie Michelle M. Dalmacio
1Department of Biochemistry and Molecular Biology, College of Medicine, University of the Philippines Manila, Philippines
2Department of Biochemistry, Faculty of Pharmacy, University of Santo Tomas, Philippines
3Research Center for Natural and Applied Sciences, University of Santo Tomas, Philippines
ABSTRACT
Objective: To identify unique immunogenic epitopes of Zika virus non-structural 1 (NS1) antigen and produce immunoglobulin Y (IgY)for potential use in he diagnosis of of Zika virus infection.
Methods: Immunogenic epitopes were identified using in silico B-cell epitope prediction. A synthetic peptide analog of the predicted epitope was used to induce antipeptide IgY production in hens which was purified using affinity chromatography. Presence of purified IgY and its binding specificity were performed by gel electrophoresis and ELISA, respectively.
Results: Out of the nine continuous epitopes identified, the sequence at position 193-208 (LKVREDYSLECDPAVI) was selected and used to produce anti-peptide IgY. The produced IgY was found to bind to the synthetic analog of the Zika virus NS1 immunogenic epitope but not to other flaviviruses and random peptides from other pathogens.
Conclusions: In this study, we identified an immunogenic epitope unique to Zika virus that can be used to develop a serodiagnostic tool that specifically detect Zika virus infection.
KEYWORDS: Immunoglobulin Y; IgY; B-cell epitope prediction;Flavivirus; Non-structural 1 antigen; Zika virus
Significance
Flaviviruses such as Zika, dengue and Japanese encephalitis share up to sixty seven percent of amino acid sequences in its non-structural 1 (NS1) protein. The sequences similarity among flaviviruses of this antigen which is a common detection molecule for flavivirus infection, commonly causes cross reactions during diagnosis, hence the inability of serological tests to discriminate, and accurately identify the pathogen causing the infection. This study identifies a unique immunogenic epitope of Zika virus NS1 antigen which has not yet been previously reported. The identified sequence can be used in the development of serologybased diagnostic tool that can specifically detect Zika virus infection.
1. Introduction
Zika virus (ZIKV) infection has emerged as a public health concern because of its potential threat to become a global pandemic[1]. Since its discovery in 1947, sporadic outbreaks of this flavivirus have been observed in several countries which include Micronesia (2007), French Polynesia (2013), Brazil (2015), in the Americas, and in most countries in Southeast Asia including the Philippines, where about 57 cases of infections were reported in 2017, of which seven were pregnant women[2,3].
ZIKV is an enveloped, positive sense, single-stranded RNA virus translated as a single polyprotein containing three structural proteins such as capsule (C), envelope (E), and precursor membrane (prM)and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3,NS4A, NS4B, and NS5)[4]. Belonging to the Flaviviridae family,other epidemiologically significant arboviruses in this family include dengue virus (DENV), Japanese encephalitis virus (JEV), West Nile virus (WNV) and yellow fever virus (YFV)[5]. Since ZIKV infections present similarly with other arboviral diseases such as dengue, the early and accurate diagnosis of ZIKV is imperative to appropriately manage the disease[6].
Several diagnostic techniques such as culture-based detection methods and molecular diagnostics have been developed for ZIKV[7]. Particularly, serological assays have focused on the NS1 peptide secreted by the virus as the marker of current infection. However, the high degree of cross-reactivity of NS1 across flaviviruses even within the same genus and serocomplex complicates the accurate detection of ZIKV[8]. To circumvent the observed cross-reactivity in serological diagnosis of ZIKV,in silico approaches have been adapted in designing the capture peptide. This makes use of database analysis resources and sequence alignment algorithms to search for unique sequences of the detecting peptide that are specific to the pathogen[9]. Thus, the study utilized a similar approach in identifying an immunogenic epitope unique to ZIKV and DENV serotype 2 NS1 antigen[10].
One of the tools for antibody production for diagnostic purposes that have emerged in the past years is IgY technology[11]. This has previously been studied for the diagnostics and therapeutics of parasitic infections and, more recently, the development of monoclonal IgY antibodies against the SARS-CoV-2 spike protein[12,13]. IgY technology pertains to the immunization of hens and the subsequent isolation of specific IgY antibodies from the egg yolk[14]. This technology offers several key advantages over the use of mammalian antibodies for ZIKV diagnostics. These include larger specific antibody yield over rabbit sera, lack of crossreactivity with human rheumatoid factors (RFs) and human antimouse antibodies (HAMA), lack of hetero-agglutinins, inability to activate the complement system, and large phylogenetic distance from mammals[15,16]. These features allow IgY technology to address the limitations of current mammalian-based antibody diagnostic kits in the market today. Therefore, this study aimed to identify unique immunogenic sequences of ZIKV NS1 to produce IgY antibodies that specifically bind to in silico predicted ZIKV NS1 immune epitope.
2. Materials and methods
2.1. Epitope mapping
The ZIKV NS1 antigen with the NCBI PDB Accession Number of 5K6K_A was retrieved from the National Center for Biotechnology Information Protein Database (NCBI-PDB). The candidate immunogenic epitopes were chosen based on antigenicity, surface accessibility, and hydrophilicity using the B-cell epitope prediction analysis resource tool of Immune Epitope Database (IEDB). The cutoff values for antigenicity, surface accessibility, and hydrophilicity were 1.021, 1.785, and 1.000, respectively; the values of which were based on the mean of each parameter over the entire sequence as determined by the prediction tool. The candidate immunogenic epitopes were then run in the Protein Basic Local Alignment Search Tool (BLAST) program from NCBI to discriminate sequences similar with other flaviviruses and to identify epitopes unique to ZIKV NS1.
2.2. Hen immunization and IgY isolation
The peptide sequence of the top predicted immunogenic epitope was sent to Genetel Laboratories (WI, USA) for synthesis and chicken immunization. Two hens were immunized with the synthetic peptide analog, the in silico predicted immunogenic epitope of ZIKV NS1, while another two which served as the control groups were immunized with PBS. Immunizations were performed at days 1, 14,35, and 63. IgY were isolated from pooled egg yolks collected from 20 to 25 eggs of each hen. Protocols pertaining to the use of animals in this study were approved and complied with the guidelines set by the Institutional Animal Care and Utilization Committee (IACUC) of the UP-NIH.
2.3. Affinity purification of anti-ZIKV peptide IgY
The affinity purification of anti-ZIKV peptide IgY followed the methods described by Camenisch et al. with modifications[16].Synthesized ZIKV peptide (2.5 mg) was dissolved in 50 μL of distilled water. It was mixed thoroughly using a vortex and incubated at room temperature for two hours. After incubation, it was mixed with 5 mL coupling buffer (0.1 mol/L NaHCO3with 0.5 mol/L NaCl, pH 8.3).
Following the manufacturer’s instruction, the swelling of Sepharose gel was performed. One gram (1 g) of freeze-dried CNBr-activated Sepharose 4B Fast Flow (Sigma, USA) was used and dissolved in 5 mL of 1 mmol/L HCl. The peptide solution and swollen Sepharose were combined and incubated at room temperature with gentle shaking. The gel was transferred to an Econo-column chromatography (BioRad), blocked with 0.1 mol/L Tris-HCl at pH 8.0, and alternately washed three times with 10 mL of pH 8.0 Trisbuffered saline (TBS) (0.1 mol/L Tris-HCl buffer containing 0.5 mol/L NaCl, pH 8.0) and 10 mL of pH 4.0 acetate-buffered saline(ABS) (0.1 mol/L NaCH3COO with 0.5 mol/L NaCl, pH 4.0).
The column with crude IgY isolates was washed with 50 mL of 0.1 mol/L PBS at pH 7.4. The peptide specific IgY was then eluted with 10 mL of 0.2 mol/L glycine at pH 2.2 containing 0.15 mol/L NaCl at 2 mL intervals. To ensure that the IgY fractions were neutralized,eluents were collected in tubes containing 1 mL of TBS pH 8.0.After elution, the column was regenerated immediately by washing with 10 mL TBS and 10 mL PBS. The column was stored in PBS containing 0.05% NaN3at 4°℃.
Fractions with similar concentrations were then pooled and lyophilized for a short time to reduce the amount of liquid in the pooled fractions. The lyophilized fractions were then used for the purity assessment, molecular weight determination, anti-peptide activity determination, and binding specificity testing.
2.4. Protein concentration determination of semi-purified IgY fractions by Bradford total protein analysis
The total protein concentration of eluates was determined using Bradford Total Protein Analysis Microplate technique. From each fraction, 50 μL of aliquots were obtained and pipetted onto the wells of a microplate. Then, to each aliquot, 150 μL of 1× Bradford Dye(0.1 mg/mL Coomassie Brilliant Blue G-250, 5% methanol, 10% of 85% phosphoric acid, and distilled water) was dispensed. The plates were incubated for five minutes, after which absorbance was measured at 595 nm using a microplate reader.
The total protein concentration determination of samples was done in triplicates and the presence of blanks (Elution buffer, 10× PBS, 1× PBS)(5 μL) in each experiment was ensured. The mean absorbance reading was computed and used for analysis. A standard curve using bovine serum albumin (BSA) ranging from 0.031 25 mg/mL to 1 mg/mL was generated for every run and the total protein concentration of each fraction was computed using linear regression analysis.
2.5. Confirmation of the presence of IgY via SDS-PAGE
Denaturing Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) was performed to confirm the presence of the heavy and light chains of the anti-ZIKV peptide IgY. Mini Protean 3 Cell (Bio-Rad) was used in performing the SDS-PAGE using 12% resolving gel and 4% stacking gel. Standard chicken IgY(Abcam) and Kaleidoscope Pre-stained Molecular Weight Markers(Bio-Rad) were used as purity and molecular weight standards.Fourteen micrograms (14 μg) of standard IgY and 0.7 μg of the fractions were loaded for each run. Both fractions and standards were run at 180 volts for 60 minutes. Visualization of the proteins was carried out by staining using Coomassie Brilliant Blue R-250 Staining solution (Bio-Rad) for 30 minutes, washing with distilled water, and destaining using Coomassie R-250 Destaining Solution(Bio-Rad).
2.6. Anti-ZIKV peptide IgY binding affinity determination
The binding affinity of the generated anti-ZIKV peptide IgY was assessed using indirect ELISA. The wells of a 96-well microtiter plate were coated with 50 μL of serially diluted synthetic ZIKV NS1 peptide solution ranging from 100 μg/mL to 0.01 μg/mL with coating buffer as a diluent and incubated for 12 to 16 hours at 4 ℃. After incubation, the wells were washed with washing buffer (0.01 mol/L PBS, 0.05% Tween 20). Blocking buffer (100 μL of 1% ovalbumin in washing buffer) was added in each well and incubated for 2 hours at 37 ℃. The unbound proteins were removed by washing the plates with washing buffer. Fifty microliters (50 μL) of affinity purified anti-ZIKV peptide IgY were introduced to each well and incubated for 1 hour at 37 ℃. After incubation, plates were washed and incubated with 50 μL of horseradish peroxidase (HRP)-conjugated anti-chicken IgY for 1 hour at 37 ℃. The wells were then washed and 100 μL of TMB stabilized substrate for HRP was added in 20 second intervals to each well and incubated for 5 minutes at room temperature. The reaction was stopped using 50 μL 2 mol/L H2SO4using the same time intervals, and the plates were read at 450 nm using a microplate reader.
2.7. Cross-reactivity of anti-ZIKV peptide IgY
Cross-reactivity of anti-ZIKV peptide IgY was also evaluated using indirect ELISA and tested against chikungunya virus (CHIKV)linear epitope E2EP3, dengue virus (DENV) NS1, Japanese encephalitis virus (JEV-1, JEV-2, JEV polypeptide), and five random peptides (Fap2-1, Fap2-2, Bacteroides (B.) fragilis fragilysin, OmpC peptide 1, and B. fragilis CProt). These peptides were obtained from an existing collection in the laboratory. Table 1 presents the amino acid sequences of these peptides.

Table 1. Amino acid sequences of peptides used in the cross-reactivity assay.
The wells of a 96-well microtiter plate were coated with 50 μL of 5 μg/mL peptide in coating buffer and incubated for 16 to 18 hours at 4 ℃. The same washing technique and reagents for the binding affinity analysis were used in the cross-reactivity assay.
2.8. Statistical analysis
Spectrophotometric assays were done in triplicates. The mean absorbance readings of the fractions in both binding affinity and cross-reactivity were computed. Cut-off value was obtained from serum blanks using the formula by Frey et al. described below[17].
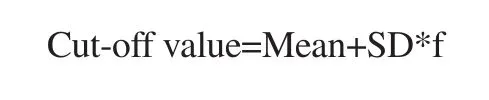
Where Mean=mean absorbance of the blank, SD=standard deviation of the absorbance of the blank, f=multiplier which is dependent number of replicates at 95% confidence interval.
This was used to determine if there is a positive or negative binding affinity per experiment wherein an absorbance value higher than the cut-off value is interpreted as positive activity. The confidence interval used for this experiment is 95%.
3. Results
3.1. Immune epitope prediction
Based on the epitope prediction done, nine continuous epitopes of the ZIKV NS1 antigen have been identified. Table 2 lists all the predicted epitopes. Among the in silico-predicted epitope, the sequence at position 193-208 (LKVREDYSLECDPAVI) was the one with the highest antigenicity and surface accessibility and third highest in hydrophilicity scores. The Kolaskar & Tongaonkar Antigenicity Prediction, Emini Surface Accessibility Prediction and Parker Hydrophilicity Prediction scores of this continuous epitope are 1.078 (cut-off value= 1.021), 3.092 (cut-off value=1.000), and 2.686 (cut-off value=1.785) respectively, hence our choice for this study. This sequence also shares no similarity with other flaviviruses except for West Nile virus (WNV) which is only 56%.

Table 2. B-cell epitope prediction scores of peptide sequences from the Zika virus non-structural 1 antigen.
3.2. Affinity purification of IgY
The elution profile typical of affinity chromatography was observed.An early eluting peak and a second peak were seen (Figure 1). A remarkable resolution of the two peaks was also noted through the sharp decline from the first peak followed by its gradual decline up to the 50th mL mark beyond which the second peak emerged. No significant spikes were observed in between the two peaks.
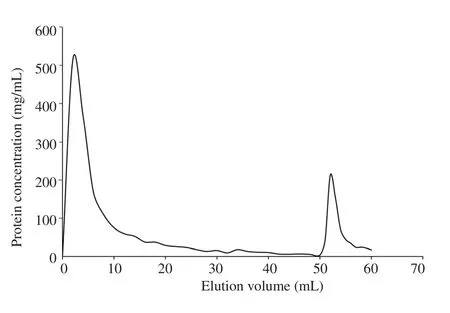
Figure 1. Affinity chromatography elution profile of crude IgY samples based on Bradford total protein determination assay. Each fraction was collected at 2 mL up to the elution of the IgY using the elution buffer. The semi-purified peptide specific IgY was collected in Fraction 27 as indicated by the second peak.
Additionally, in the denaturing SDS-PAGE performed to confirm the presence of IgY in the affinity-purified fractions, two distinct bands with molecular weights of 65 and 27 kDa were observed (Figure 2).

Figure 2. Denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) result of the affinity-purified anti-ZIKV peptide IgY fraction. Lane 1 corresponds to the affinity-purified IgY fraction;Lane 2 corresponds to the standard IgY.
3.3. Binding affinity of semi-purified anti-ZIKV peptide IgY
The binding affinity of the semi-purified anti-ZIKV peptide IgY was assessed by plotting the absorbance to the IgY concentration across the different ZIKV peptide concentrations (Figure 3). Across all ZIKV peptide concentrations, increasing the semi-purified anti-ZIKV IgY antibody led to an increase in the absorbance detected by ELISA. The lowest semi-purified anti-ZIKV IgY concentration whose absorbance values of all antigen concentrations was above the cut-off was at 0.04 mg/mL. Interestingly, at 0.01 mg/mL IgY concentration, the lower ZIKV peptide concentration of 0.01 μg/mL was able to generate a higher absorbance signal than that of the 0.1 μg/mL antigen concentration. This observation was not consistent with the general trend of the curves observed in Figure 3.

Figure 3. Binding affinity of semi-purified anti-ZIKV peptide IgY antibody to the synthetic ZIKV peptide analog. Concentration-dependent increase in the absorbance reading at 450 nm versus IgY concentration was observed in the indirect ELISA performed. Values presented are means of two technical replicates.
3.4. Cross-reactivity of Affinity-purified anti-ZIKV IgY
The cut-off value for a positive result was computed to be 0.202.The affinity purified anti-ZIKV peptide IgY fraction, with an absorbance value of 0.238, was the only one with value higher than the cut-off value (Figure 4), suggesting that the IgY produced binds specifically to ZIKV NS1. IgY from control hens were not reactive with any of the peptides tested.

Figure 4. Cross-reactivity of affinity-purified anti-ZIKV peptide IgY from immunized hens (experimental) and crude IgY from control hens (control) against various peptides determined using indirect ELISA. Peptides used were from Fap2-1 (RPep1), Fap2-2 (RPep2), JEV-1 (JEVPep1), JEV-2 (JEVPep2), ZIKV NS1(ZIKVNS1), CHIKV linear epitope E2EP3 (CHIKVPep), DENV NS1 (DENVNS1Pep), JEV polypeptide (JEVPep3), Bacteroides fragilis fragilysin (RPep3),OmpC peptide 1 (RPep4), and Bacteroides fragilis CProt (RPep5). The dashed line indicates the cut-off value for a positive result, which is 0.202.
4. Discussion
Flavivirus detection is complicated by immunological crossreactivity[18]. This has been exhibited by human antibody responses against ZIKV and DENV envelope proteins where serological cross-reaction has been documented[19]. Additionally, the use of the NS1 protein as the target antigen of detection has proven to be useful since its circulating levels in the blood increase during an acute infection. However, its highly conserved nature among the flaviviruses contribute to the cross-reactivity observed in flavivirus diagnosis[8]. Although this can confer protective advantage to those previously infected with DENV[19], the accurate diagnosis at point of care remains a challenge. This prompts the further design and development of more accurate ZIKV diagnostic tools[7].
The present study employed the Immune Epitope Database (IEDB)for the identification of unique immunogenic epitopes. The IEDB contains an extensive archive of experimentally determined epitopes,and it also offers analysis resources for epitope prediction[20].Particularly, B-cell epitope prediction has been used in providing the molecular basis in the serologic discrimination of the NS1 protein between ZIKV and DENV in a previous study[10]. It was also used in the determination of the potential of these predicted B-cell epitopes of envelope proteins of similar flaviviruses, specifically JEV and WNV, in developing epitope-based vaccines[21]. More recently, B-cell and T-cell epitopes have been identified in the surface glycoprotein of the SARS-CoV-2, the pathogen responsible for COVID in the search for potential candidates for vaccine development against the current pandemic[22]. These studies show how an immunoinformatic approach has yielded useful information that directed the development of more accurate diagnostic tools and more effective vaccine designs while also reducing the burden of epitope mapping[9].
The in silico analyses in the study were able to predict continuous epitopes which had good antigenicity, surface accessibility, and hydrophilicity scores. However, some of these were found to have high sequence similarity with other flaviviruses such as DENV,WNV, and JEV, as well as with other human proteins. This highlights the importance of subjecting candidate epitopes to sequence alignment and similarity analysis first to identify epitopes which are unique to ZIKV.
In particular, the predicted epitope at position 193-208(LKVREDYSLECDPAVI) was selected as the most important epitope due to its remarkable scores in the three B-cell epitope prediction tools. These scores can be attributed to the abundance of polar amino acids which is an important consideration for the expected immunogenicity of the detecting peptide[23]. This prediction of immunogenicity of these epitopes relies on the parameters set in the B-cell epitope prediction tool which are antigenicity,hydrophilicity, and surface accessibility[24]. These parameters have been used since most continuous epitopes are in loops or regions of the antigen that protrude to the external environment[25]. Although structure-based methods significantly outperform sequence-based methods, the latter are more easily synthesized, purified, stored, and handled[26, 27]. This can confer practical advantages in its application to a rapid diagnostic tool similar with a previous study[28].
In the accurate diagnosis of ZIKV, the production of capture antibodies specific to the predicted epitope is crucial in the further development of a detection tool. IgY technology offers several key advantages over the use of mammalian antibodies for ZIKV diagnostics. These include larger specific antibody yield over rabbit sera, lack of cross-reactivity with human rheumatoid factors(RFs) and human anti-mouse antibodies (HAMA), lack of heteroagglutinins, inability to activate the complement system, and large phylogenetic distance from mammals[29]. These advantages of using avian immunoglobulin allow development of low-cost, highthroughput diagnostic device that can detect diseases with high specificity and sensitivity.
After peptide synthesis and IgY production, antibody purification is the next bottleneck in the development of antibody-based serological detection kits[30]. Among existing purification methods, affinity chromatography remains one of the most efficient and versatile methods for purifying antibodies[31]. This technique involves the specific interaction between an antigen and antibody and the reversibility of this binding to allow selective elution through the column[32]. This was exemplified in the current study where the early eluting peak identified in the affinity purification Figure 1 corresponds to the non-binding proteins to the conjugated ZIKV antigen peptide in the column. Meanwhile, the second eluting peak indicates the antibodies that have specificity to the ZIKV antigen and thus were selectively purified from the sample. The confirmation of anti-ZIKV peptide IgY was performed through a Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) where it allows visualization of the light and heavy chains of the IgY antibody corresponding to their respective molecular weights[33].
Once purified, the binding affinity and cross-reactivity of the anti-ZIKV peptide IgY were assessed through indirect enzyme-linked immunosorbent assay (ELISA). Indirect ELISA has been extensively used in the clinical sciences as a means of diagnostic evaluation as well as in the basic sciences where it allows for the detection and quantification of specific antigens or antibodies in a sample[34].
Elucidation of binding affinity determines the ability of the isolated IgY to detect the ZIKV antigen above the calculated cut-off value as a possible tool for ZIKV diagnostics. As determined by indirect ELISA in this study, the concentration-dependent increase in the anti-ZIKV peptide IgY-peptide interaction is indicative of its binding affinity. This is because as more antibodies bind to the peptide, the signal detected increases[35]. However, the higher absorbance reading detected at a lower ZIKV antigen concentration of 0.01 μg/mL compared to 0.1 μg/mL can be attributed to the limitation of the ELISA setup in discriminating between specific and non-specific binding due to background signal interference[36]. This can also be attributed to its binding affinity and/or the enzyme label used[37]. Thus, the limits of detection of these IgY isolates must be determined separately to conclusively assess the sensitivity of these antibodies[38]. This will ensure the applicability of a future diagnostic tool in discriminating low circulating levels of the ZIKV NS1 antigen.
Additionally, although the use of synthetic peptides has been established as a good model for the immunogenicity of antigens and their translation into a diagnostic test, the detection of the whole NS1 protein in sera must be studied independently[39]. This is because of steric hindrances that can confound the binding affinity results obtained in this study[40]. Interestingly, the synthetic peptide analog can be further modified at the amino and carboxyl termini of the peptide through acetylation and amidation to further increase its immunogenicity and therefore, its binding affinity[41,42]. This can result in a more robust binding of the IgY with the ZIKV NS1 protein.
The high degree of sequence similarity of the NS1 protein across Flaviviridae viruses makes it difficult for current ZIKV diagnostic tools to discriminately detect ZIKV from other flaviviruses. This is evidenced by previous reports observing cross-reactivity of sera previously infected with other flaviviruses on diagnostic tests intended for ZIKV[19]. In fact, because of the sequence similarity among flaviviruses, the pathogenesis of ZIKV infection can even be exacerbated by previous flavivirus infection through antibodydependent enhancement (ADE)[43,44]. In the present study, the IgY antibodies produced against the predicted ZIKV NS1 epitope were not found to be cross-reactive with peptides from other flaviviruses and microorganisms. This predicted epitope, therefore, holds potential as a basis for producing IgY antibodies that can be used for specific ZIKV detection.
In summary, the study was able to identify a promising immunogenic epitope of the ZIKV NS1 protein using in silico B-cell epitope prediction, as well as to produce and evaluate IgY against it. IgY produced and affinity-purified based on the most promising epitope was found to be capable of binding its peptide analog assessed through indirect ELISA. The IgY was also found to have no cross-reactivity with other peptides tested, suggesting that the IgY produced binds specifically to ZIKV NS1. Altogether, this study demonstrated a potential application of combining IgY technology with in silico B-cell epitope prediction for future development of ZIKV diagnostics.
Conflict of interest statement
The authors declare no conflict of interest in this study.
Authors’ contributions
All authors contributed to the study conception and design. LAG,LGCM and LMD initiated the conceptualization of the study.SDPDS, TRDT, MKDMC, NCCP and AJZSM prepared the materials, performed the experiments, and analyzed and interpreted the data. SDPDS and TRDT prepared the final draft, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- SARS-CoV-2 variants: A continuing threat to global health
- Plasmodium cynomolgi: An emerging threat of zoonotic malaria species in Malaysia?
- Liposomes as immunological adjuvants and delivery systems in the development of tuberculosis vaccine: A review
- Endophthalmitis caused by Bacteroides fragilis after pars plana vitrectomy and treatment approach
- Disease progression after discontinuation of corticosteroid treatment in a COVID-19 patient with ARDS
