Reports of clinical trial protocols of coronavirus disease 2019:a systematic review
2022-03-09RuiJinQiuXuXuWeiMengZhuZhaoChangMingZhongChenZhaoJiaYuanHuMinLiYaHuangSongJieHanTianMaiHeJingChenHongCaiShang
Background
Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection reported since December 2019.The disease was named coronavirus disease 2019(COVID-19)by the World Health Organization on February 12,2020.It is rapidly spreading around the world.According to the website of the National Health Commission of the People’s Republic of China,83,017 confirmed cases have been reported from all areas of China until 0000 hours,May 31,2020.Of these,78,307 cured patients have been discharged and 4,634 patients have died.[1].Per the World Health Organization website,140,332,386 confirmed cases of COVID-19 have been reported in the world,of whom,3,004,088 patients had died by 1,000 hours CET,April 18,2021[2].
SARS-CoV-2 is probably zoonotic origin.Though the genetically closest-known lineage(96% identity)has been found in horseshoe bats[3],there is no sufficiently evidence to prove it as the immediate ancestor of SARS-CoV-2[4].After the first whole genome sequence was published[5],thousands of genomes have been sequenced and reported.A research analyzed 7,666 SARS-CoV-2 genome assemblies,the results showed the emergence of genomic diversity and recurrent mutations[4].These problems hinder the research of vaccines and drugs for COVID-19.There is still no specific treatment for COVID-19.
COVID-19 is considered as “damp pestilence” in traditional Chinese medicine,which belongs to the category of warm disease[6].Warm disease was first recorded in the
,for Qin Yue-ren in Eastern Han(25 C.E.-220 C.E.)[7],which is explained as acute infectious diseases with fever of modern medicine.As for the therapies of warm disease,were systematically described in
,for Wu Tang,born 1798 C.E.[8]In China,the government encourages traditional Chinese medicine(TCM);herbal medicine,moxibustion,Baduanjin,etc.to form an important part of clinical practice.The National Health Commission of the People’s Republic of China and National Administration of Traditional Chinese Medicine released the version 6.0 of
(informal version)on February 18,2020[9].The guideline recommends TCM therapy methods including herbal medicine formulas and proprietary Chinese medicine according to TCM syndromes.
Not long afterward10, my classmate Brian said he wanted to introduce me to a young woman named Susan Maready. I was sure I d heard that name before, but couldn t remember how or where. Since Brian was married, and therefore I wouldn t be breaking my rules about being fixed11 up by single guys, I accepted his offer to meet Susan.
Currently,an increasing number of clinical trials on COVID-19 are being conducted.After searching for clinical trial protocols in the Chinese Clinical Trial Registry and ClinicalTrials.gov,we realized that different researchers chose different outcomes.It is very important for clinical trials to provide evidence in treating COVID-19.However,the heterogeneity of outcomes makes it impossible to conduct future meta-analyses,which may reduce the value of clinical trials and prove wasteful.
We aim to establish a core outcome set(COS)for clinical trials pertaining to COVID-19.A COS is an agreed standardized set of outcomes that should be measured and reported,as a minimum,in all clinical trials in specific areas of health or health care(https://www.comet-initiative.org/).When researchers reported all of outcomes included in the COS,these clinical trials will be included in systematic reviews/meta-analyses to provide evidence to clinical practice.The study has been registered in Core Outcome Measures in Effectiveness Trials database[10].The first clinical trial of COVID-19 was registered on January 23,2020[11].When we registered the COS study,there were about 50 clinical trials already registered[10].On February 25,2020,the number of registered trials increased to 297.
Two reviewers(RQ and MZ)independently extracted information.The information included the primary investigators’ name,study type,type of disease,primary sponsor,number of settings,sample size,population’s age,course of treatment,interventions,outcomes,outcomedefinition/measurement instruments,and measurement time frame.Any disagreement was resolved by discussion.
This is the first step to develop a COS for COVID-19.We conducted a review of outcome reporting from the registered clinical trials of COVID-19 on February 14,2020.Because TCM and Western medicine essentially play the same role in the treatment of COVID-19 in China,we included both types of intervention in this review.
We rapidly developed a COS for COVID-19 according to the long list of outcomes in March.Different stakeholders participated in two rounds of Delphi survey and a consensus meeting.However,an increasing number of clinical trials registered in different registry platforms from all over the world.Therefore,we updated the systematic review.The outcomes that did not include in the long list of outcomes or did not propose by stakeholders in the Delphi survey[12].
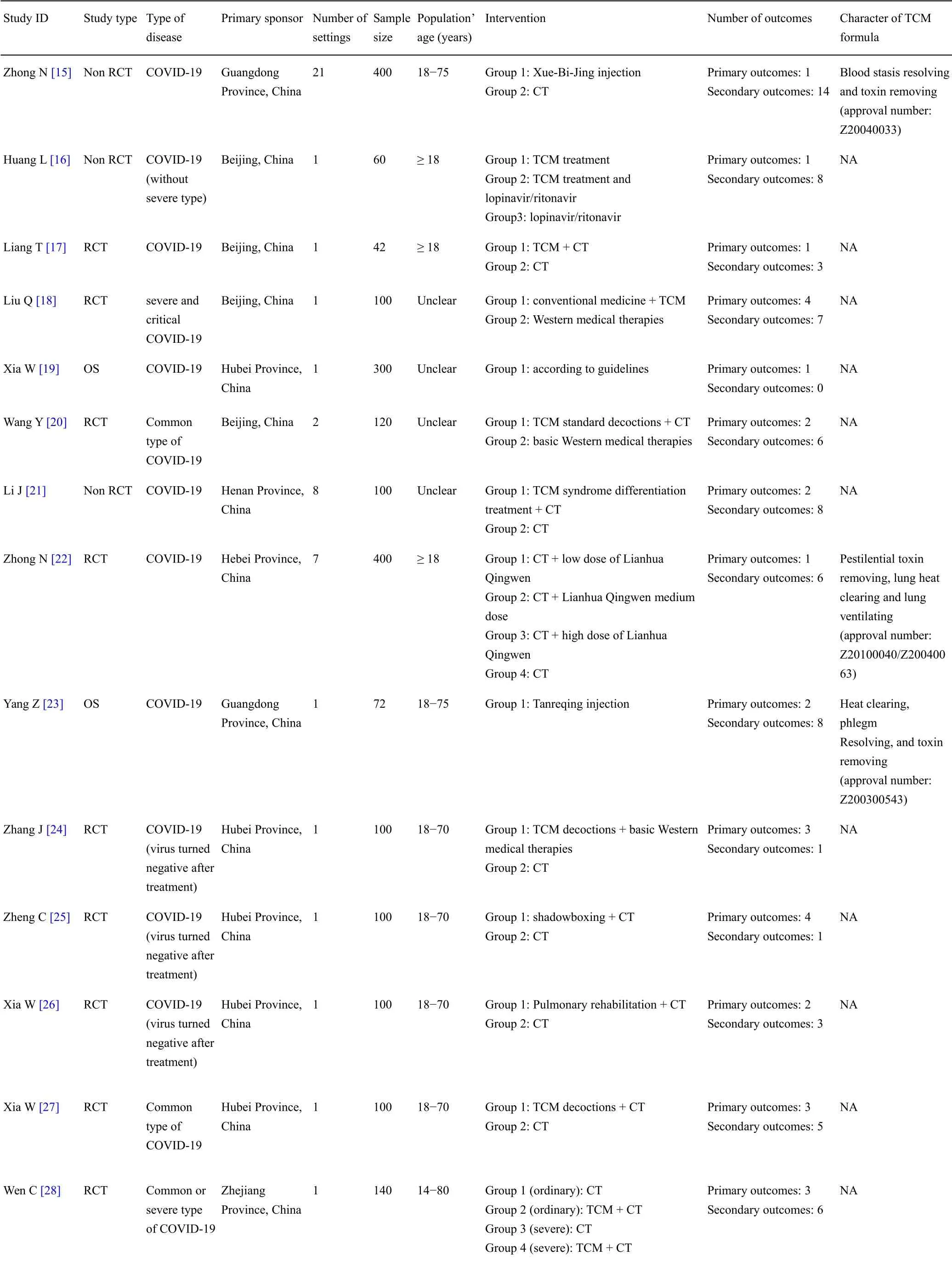
But Grannonia, who in one and the same moment saw herself merry and sad, cheerful and despairing, rich and beggared, complained bitterly over this robbery of her happiness, this poisoning of her cup of joy, this unlucky stroke of fortune, and laid all the blame on her parents, though they assured her that they had meant no harm
After all, six hundred years is an eternity14! Ah, dear king, replied the young man, your offer is very tempting15! But at the end of six hundred years we should have to die, so we should be no better off! No, I must go on till I find the country where there is no death at all
Methods
Search strategy
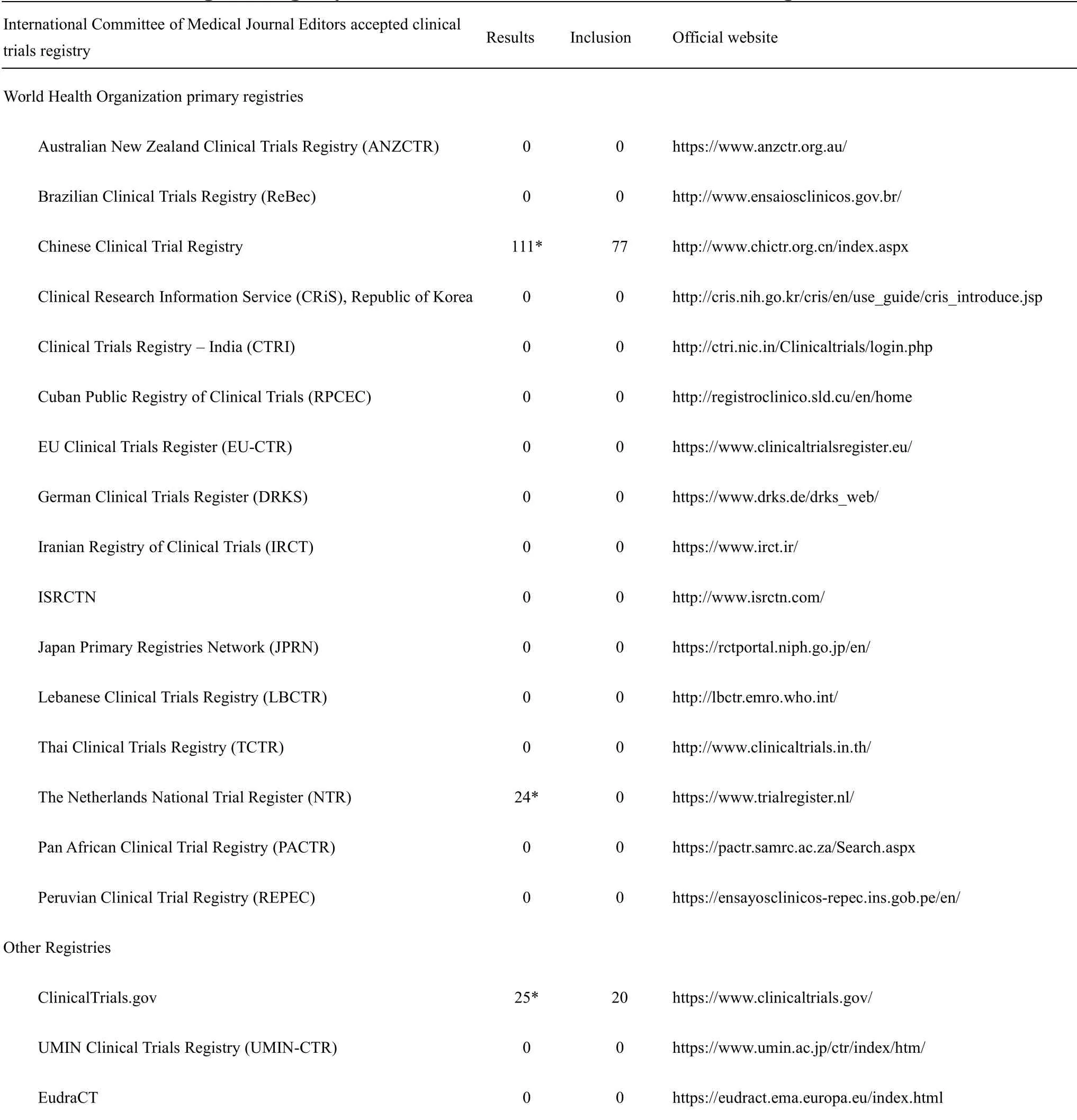
All the databases ofInternational Committee of Medical Journal Editors accepted clinical trial registry platforms[13]were considered.Search terms for Chinese Clinical Trial Registry included the following:“COVID-19”,“2019-novelCoronaVirus(2019-nCoV)”,“Novel Coronavirus Pneumonia(NCP)”,“Severe Acute Respiratory Infection(SARI)”or “Severe Acute Respiratory Syndrome - Corona Virus- 2(SARS-CoV-2)”.Search terms for Netherlands National Trial Register(NTR)included“nCoV”,“Coronavirus”,“SARS”,“SARI”,“NCP” or“COVID”.Search terms for other databases included“2019-nCoV OR Novel Coronavirus OR New Coronavirus OR SARS-CoV-2 OR SARI OR NCP OR Novel Coronavirus Pneumonia OR COVID-19 OR Wuhan pneumonia”.The first search was conducted on February 14,2020.We update the search on May 31,2020,studies registered from February 15,2020 to May 31,2020 were considered.
Then the lid sprang up to the right, and the princess came out, straight over him, and rushed round the church, howling and shrieking33 Sentry, where are you? Sentry, where are you? She went towards the altar, and right up to it, but there was no one there; then she screamed again,My father has set no sentry in, War and Pest will now begin
Inclusion criteria
In the 16 outcome domains of protocols of TCM clinical trials,4 outcome domains(adverse events/effects,hepatobiliaryoutcomes,mortality/survival,and psychiatric outcomes)consisted of only one outcome.These outcomes were reported between 1 and 9 times,and the median outcome reporting time was 6.5.Respiratory,thoracic and mediastinal outcomes included the largest number of outcomes(i.e.,31);chest imaging was reported more frequently than other outcomes.The number of outcomes in different outcome domains in protocols of TCM clinical trials are shown in Supplementary File 1 Figure S4.
That Friday after work, as I drove over to pick her up I was a bit nervous. When I arrived at her house, I noticed that she, too, seemed to be nervous about our date. She waited in the door with her coat on.
The interventions include any type of TCM or Western therapy.
Patients’ conditions was classified as mild(the clinical symptoms are mild,and there is no obvious manifestation of pneumonia on chest imaging),ordinary(fever,respiratory symptoms,pneumonia manifestation can be identified from chest imaging),severe(respiratory distress,respiratory rate ≥ 30 times/min;the oxygen saturation is ≤93% at rest;arterial blood oxygen partial pressure/fraction of inspired oxygen(PaO2/FiO2)≤300 mmHg),or critical(respiratory failure occurs,and mechanical ventilation is required;shock;complicated with other organs failure,ICU admission is needed).
The study types include randomized controlled trial(RCT)and observational study.
When girls flower into womanhood, they are always a bit shocked to discover they are Cinderella or Snow White, and the man they thought was Prince Charming really turned out to be Prince Clod.
Exclusion criteria
Studies on discharged patients.
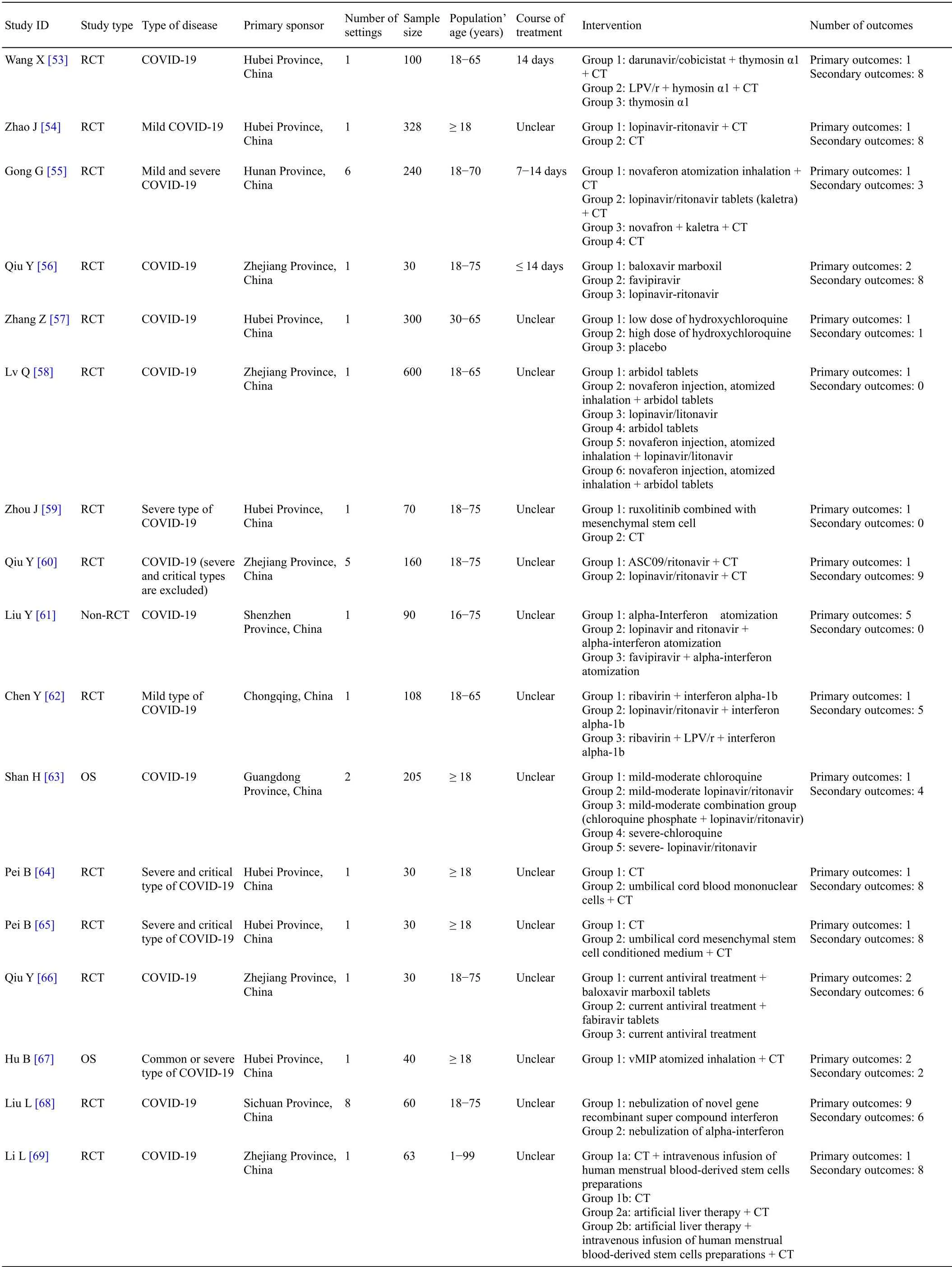
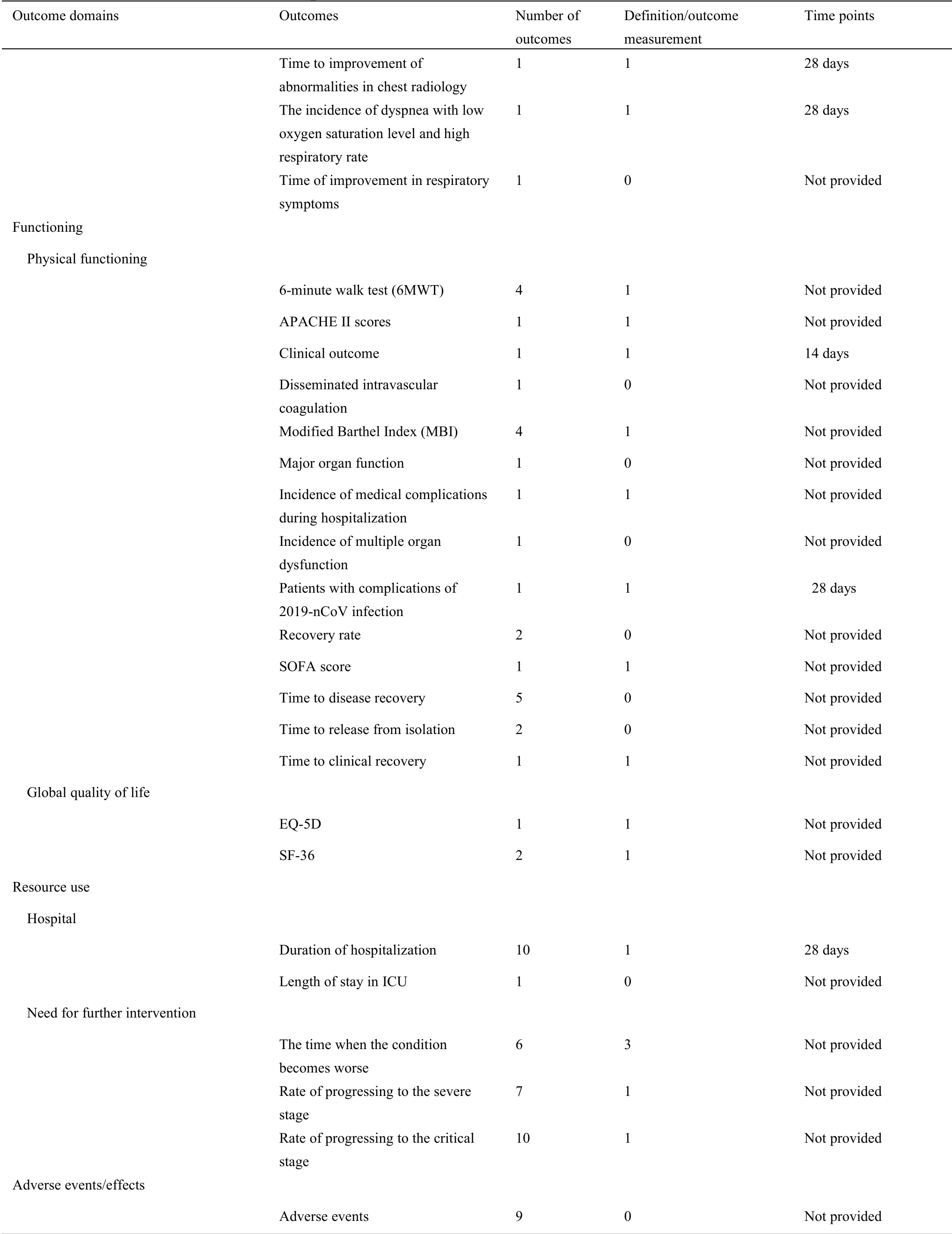
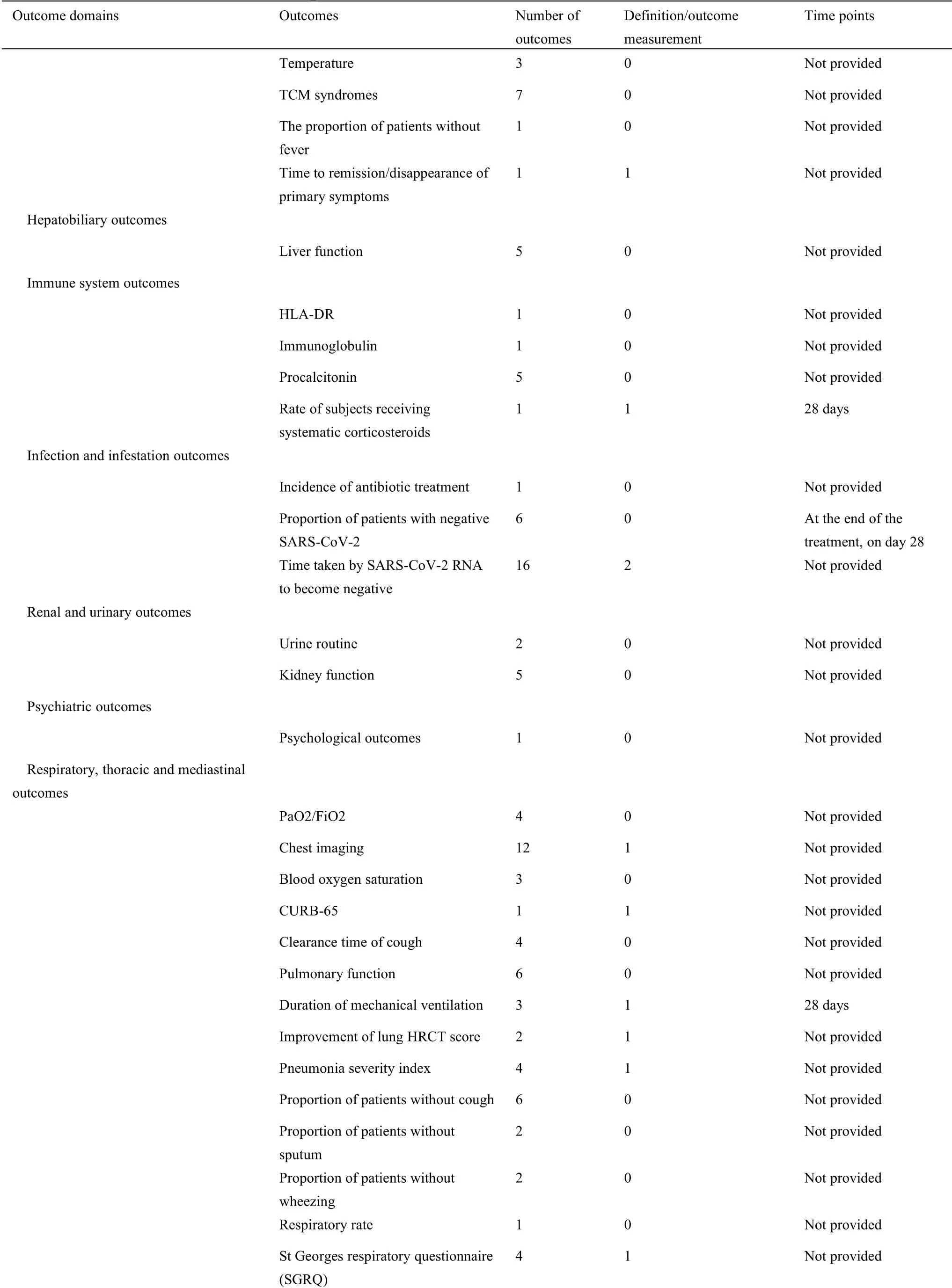
For 34 protocols of TCM clinical trials,there were 76 different outcomes from 16 outcome domains after merging and grouping outcomes(Table 4).Almost half of outcomes were reported only once(34/76,44.74%).The most frequently reported outcome was “time of SARS-CoV-2 RNA turns to negative”,which was reported 16 times.Only 3(3/76,3.95%)outcomes were reported more than 10 times.Only 27(27/76,35.53%)outcomes were provided one or more outcome measurement instruments/definitions.Only 10 outcomes(10/76,13.16%)were provided one or more measurement time frame.The summary of outcome reporting for protocols of TCM clinical trials is shown in Supplementary File 1 Figure S3.
Studies on complications of COVID-19.
Study identification
Two reviewers(RQ and XW)independently assessed all the registered protocols.Any disagreement was resolved by discussion.
Data extraction
Developing a COS generally involves four steps:(1)conduct a systematic review to develop a long list of outcomes;(2)interview patients to acquire outcomes that are important for them;(3)carry out Delphi survey in different stakeholders;(4)hold a consensus meeting to define the final COS.
Merging outcomes and grouping under outcome domains
Two researchers(RQ and CZ)independently merged the overlapping outcomes according to the definition of outcomes.If the researchers could not provide a definition of outcome,they discussed the issue and arrived at a consensus when necessary.For example,“PaO2/FiO2”,“oxygenation index”,“oxygen index”,and “the difference of PaO2/FiO2 between two groups” were combined as “PaO2/FiO2”.Many protocols presented composite outcomes.When definitions were provided or when all these single outcomes in the composite one could be measured in one test,it was listed in the review.If a single outcome which belongs to a composite outcome was reported by one or more protocols,the composite outcome was removed from the review.However,in future Delphi surveys,we plan to list the composite outcome to consult with and include the participants’opinion.
After the original outcomes were aggregated,two researchers(RQ and CZ)grouped individual outcomes into the appropriate outcome domain together and arrived at a consensus.The taxonomy of outcome domains was developed by researchers from the Core Outcome Measures in Effectiveness Trials initiative[14].
Tears of happiness poured down Susan s cheeks. She was so lucky for he had given her a gift more powerful than sight, a gift she didn t need to see to believe—the gift of love that can bring light where there is darkness.
Statistical analysis
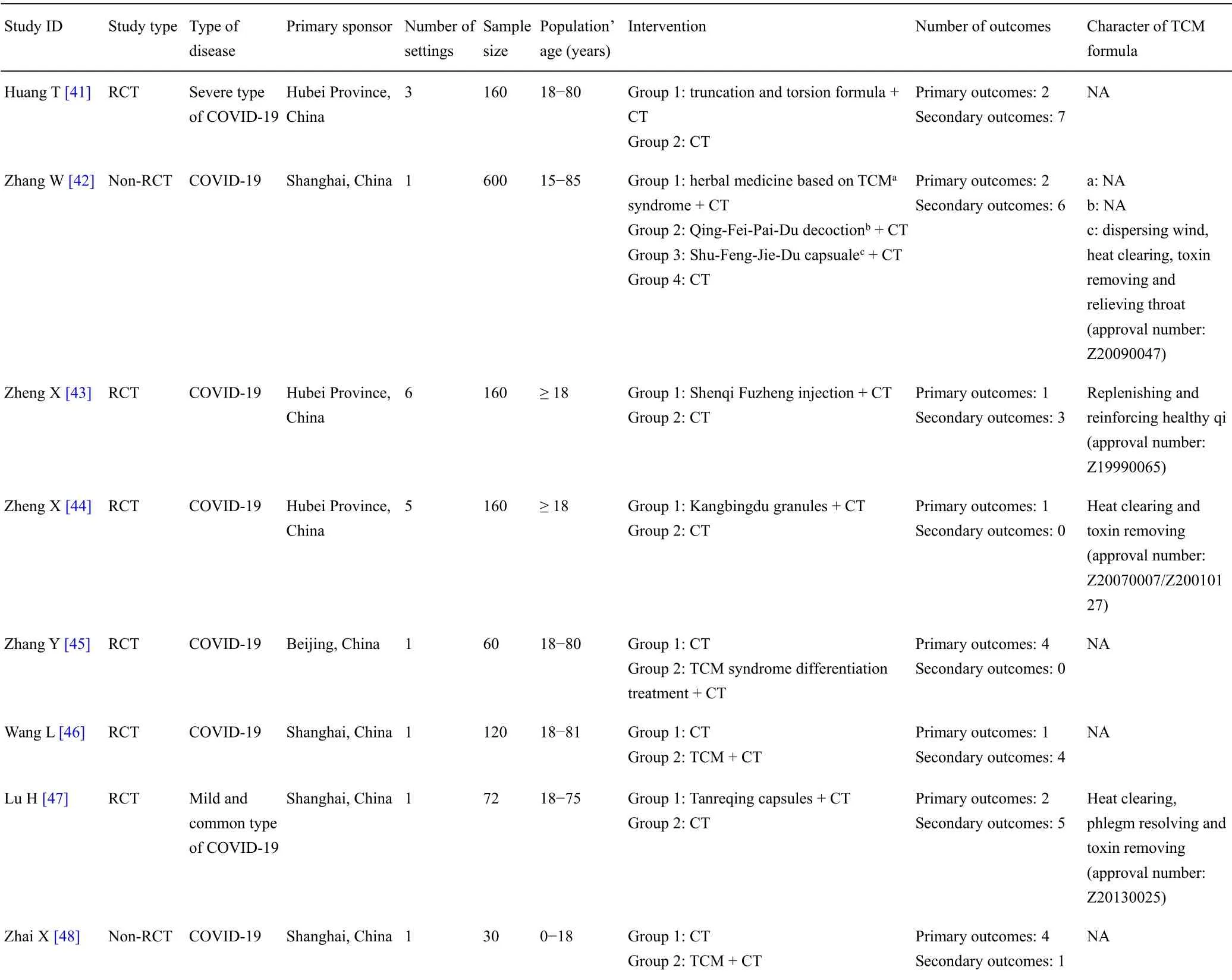
The results were analyzed by descriptive analysis.The frequencyofoutcomes,definition/outcome measurement and time points were calculated.
Patient and public involvement
This is a systematic review.As the COVID-19 is highly infectious,patients and public were not involved in the design or planning of the study for their own safety.
“I must travel,” he had said to her; “I must see this beautiful princess; my parents desire it; but they will not oblige me to bring her home as my bride
Results
Characteristics of included literature for the first search
In this review,a total of 160 protocols from 19 different clinical trials registry platforms were searched on February 14,2020.After reading the titles and study details,63 non-relevant or ineligible study protocols were excluded.In the end,97 eligible study protocols were included from the Chinese Clinical Trial Registry and ClinicalTrials.gov.The search results and inclusion numbers are detailed in Table 1.
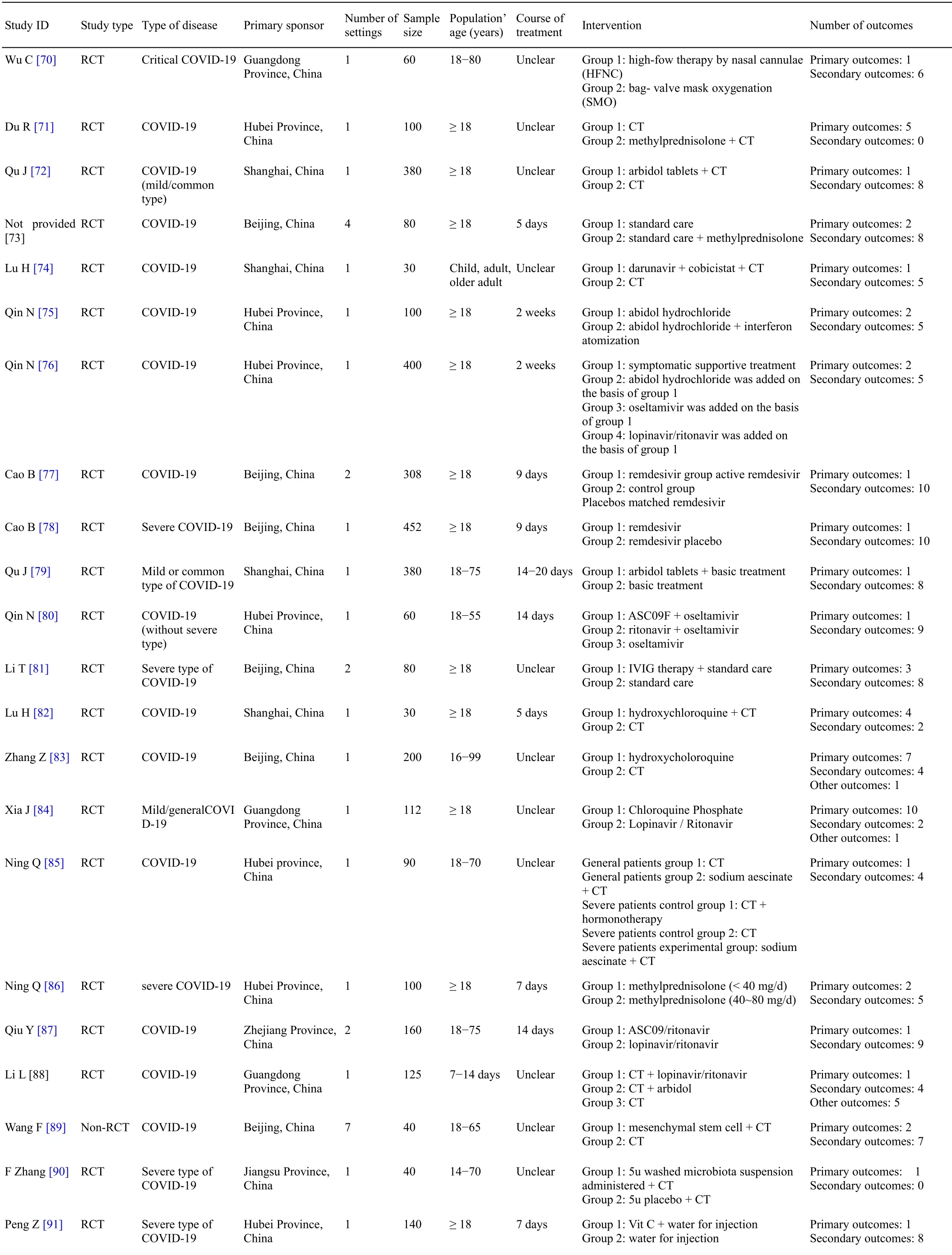
In the included protocols,34 clinical trials were for TCM therapy and the other 63 clinical trials were for Western medicine therapy.All of 97 clinical trials will be conducted in China.These clinical trials include 75 RCTs(53 for Western medicine and 22 for TCM)and 22 non-RCTs(10 for Western medicine and 12 for TCM).The first clinical trial for Western medicine was registered on January 23,2020,while the first clinical trial for TCM was registered on January 27,2020.The general characteristics of the included protocols for the first search are shown in Table 2 and Table 3.



Based on the information of the primary sponsor,we found that the clinical trials were registered from 13 different provinces of China.Researchers from Hubei Province registered more clinical trials(31/97,31.96%)than those from other provinces.The distribution of clinical trials is shown in Supplementary File 1 Figure S1.
Characteristics of included literature for the second search
A total of 2,741 protocols from 19 different clinical trials registry platforms were searched from February 15,2020 to May 31,2020.After reading the titles and study details,1,714 non-relevant or ineligible study protocols were excluded.In the end,1,027 eligible study protocols were included from different registry platforms.
For 63 protocols of Western medicine clinical trials,there are 126 different outcomes from 17 outcome domains after merging and grouping(Table 5).Almost half of outcomes were reported only once(62/126,49.21%).The most frequently reported outcome was“proportion of patients with negative SARS-CoV-2”,which was reported 40 times.Only 11(11/126,8.73%)outcomes were reported more than 10 times.Twenty-seven outcomes were provided one or more outcome measurement instruments.Forty(40/126,31.75%)outcomes were provided one or more measurement time frame.The summary of outcome reporting for protocols of TCM clinical trials is shown in Supplementary File 1 Figure S6.
The list of outcomes from the first search
For protocols of TCM clinical trials,the number of primary outcomes were from 1(13/34,38.24%)to 13(1/34,2.94%),the number of secondary outcomes were from 1(7/34,20.59%)to 14(1/34,2.94%).For individual clinical trial,the number of total outcomes were from 1(2/34,5.88%)to 15(1/34,2.94%).The number of outcomes in protocols of TCM clinical trials is shown in Supplementary File 1 Figure S2.
15.So good and so mannerly: The woman states the exact reason why she is giving a fairy gift to the youngest daughter. It is intended to be a reward for good behavior. The tale is overtly38 didactic, explaining the rewards for good behavior and the penalties for bad behavior. In some variants, the emphasis is less on good vs. bad behavior and more on industry vs. laziness, such as in Mother Holle.Return to place in story.
Studies on psychological intervention.

The study populatio n should include patients with confirmed COVID-19 infection.
For protocols of Western medicine clinical trials,the number of primary outcomes were from 1(39/63,61.90%)to 10(1/63,1.59%),the number of secondary outcomes were from 0(8/63,12.7%)to 15(1/63,1.59%).For individual clinical trial,the number of outcomes were from 1(4/63,6.35%)to 16(1/63,1.59%).The number of outcomes in protocols of Western medicine clinical trials is shown in Supplementary File 1 Figure S5.
In the included protocols,80 clinical trials were for TCM therapy and 947 clinical trials were for Western medicine therapy.They are from 7 different registry platforms:ClinicalTrials.gov(n = 668),Chinese Clinical Trial Registry(n = 198),EU Clinical Trials Register(n = 131),Australian New Zealand Clinical Trials Registry(n = 12),Japan Primary Registries Network(n = 9),German Clinical Trials Register(n =5),ISRCTN(n =4).From April 2020,some registered clinical trials were withdrawn because of no sufficient patients to be recruited and the majority of included protocols were conducted by researchers outside of China for the second search.The details of characteristics of the included protocols for the second search are shown in Supplementary File 2.
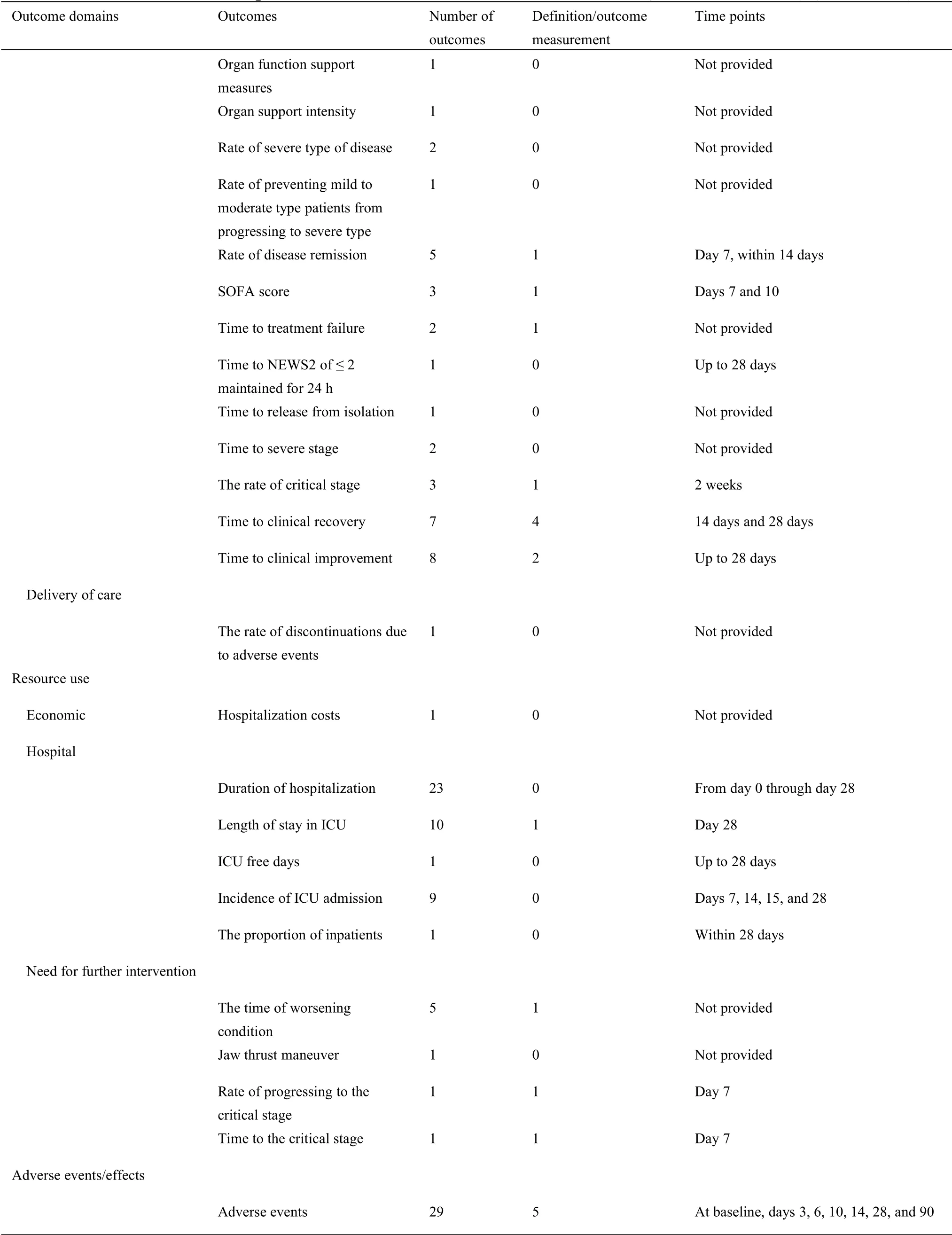
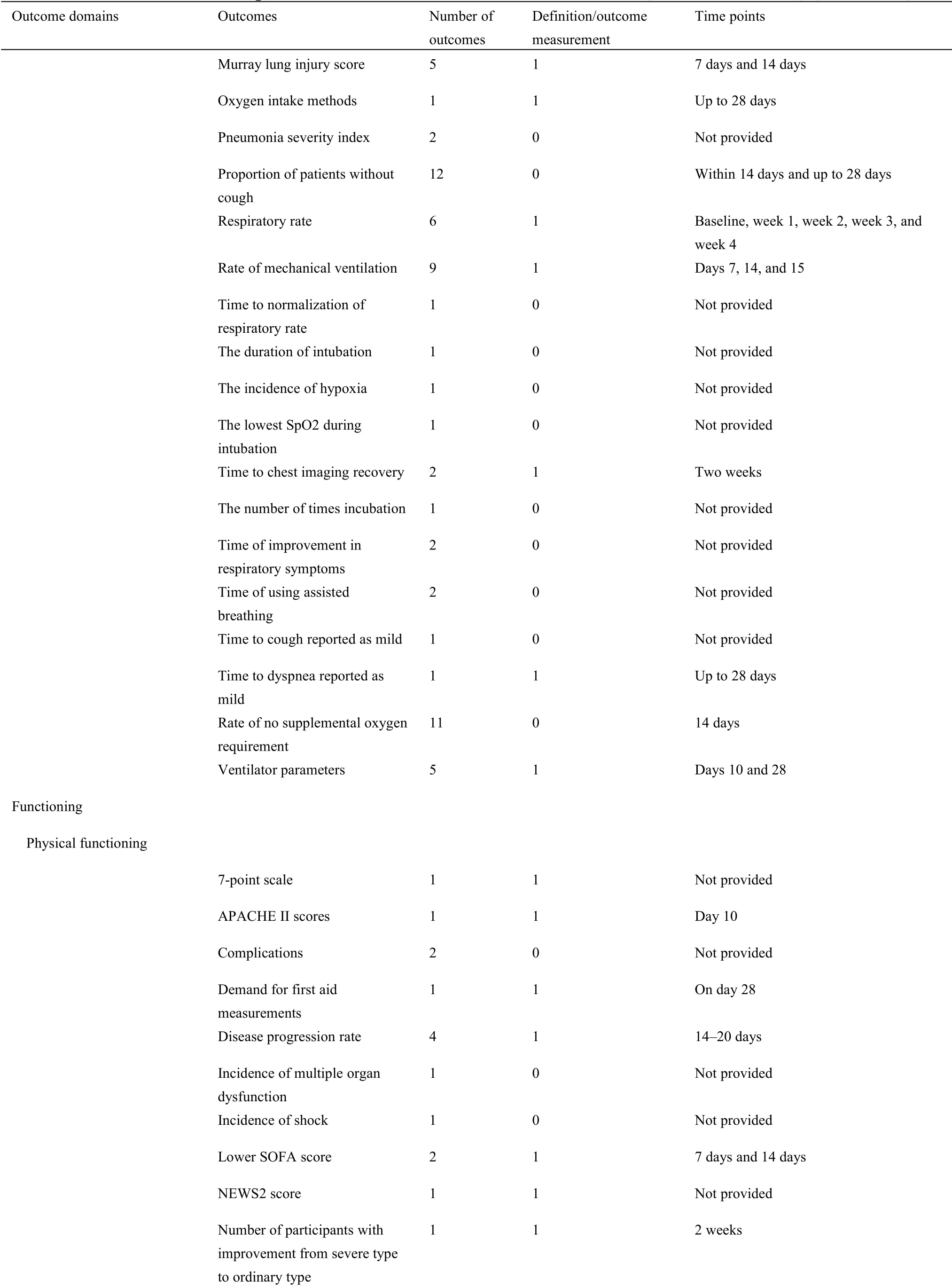
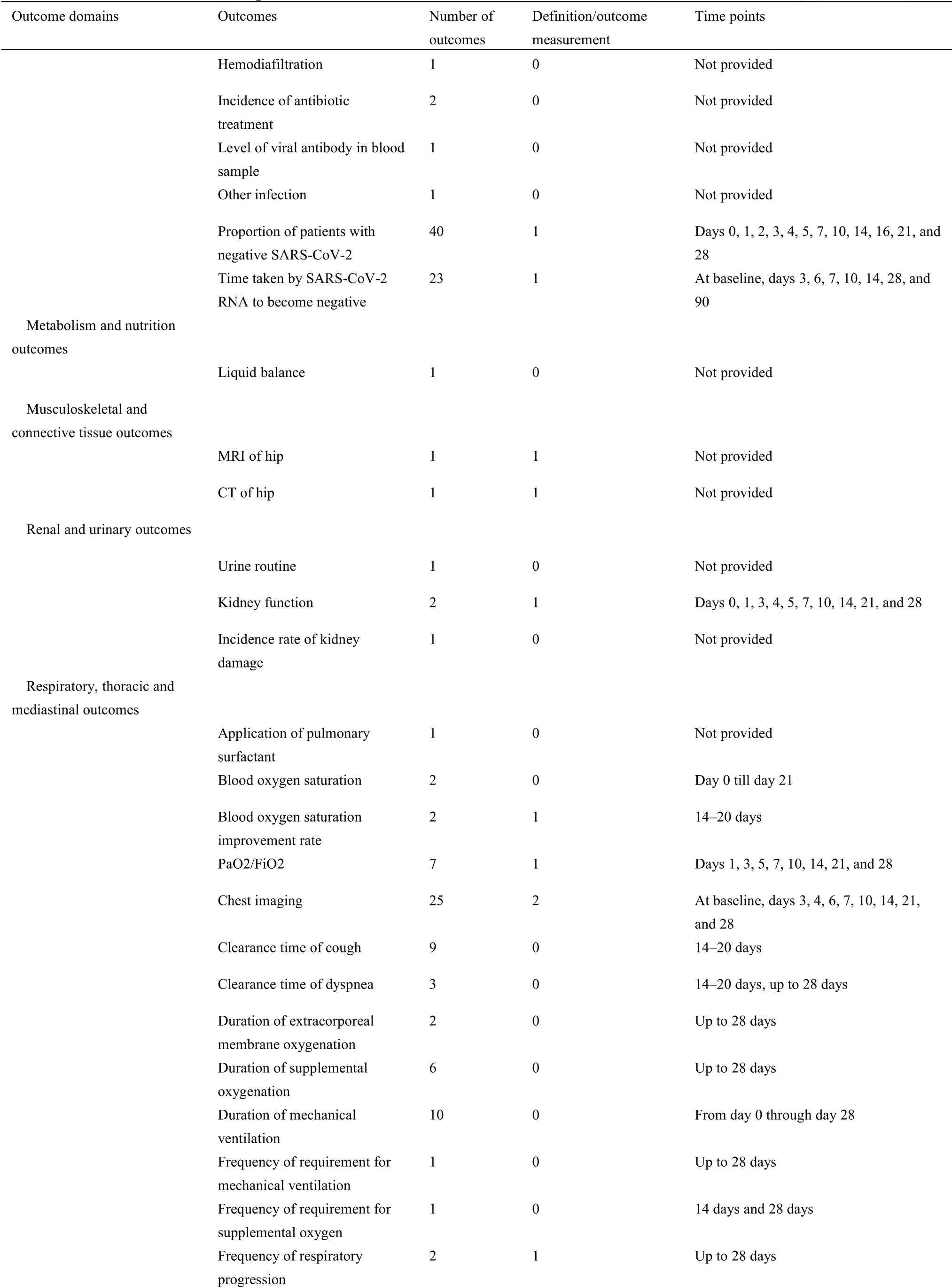
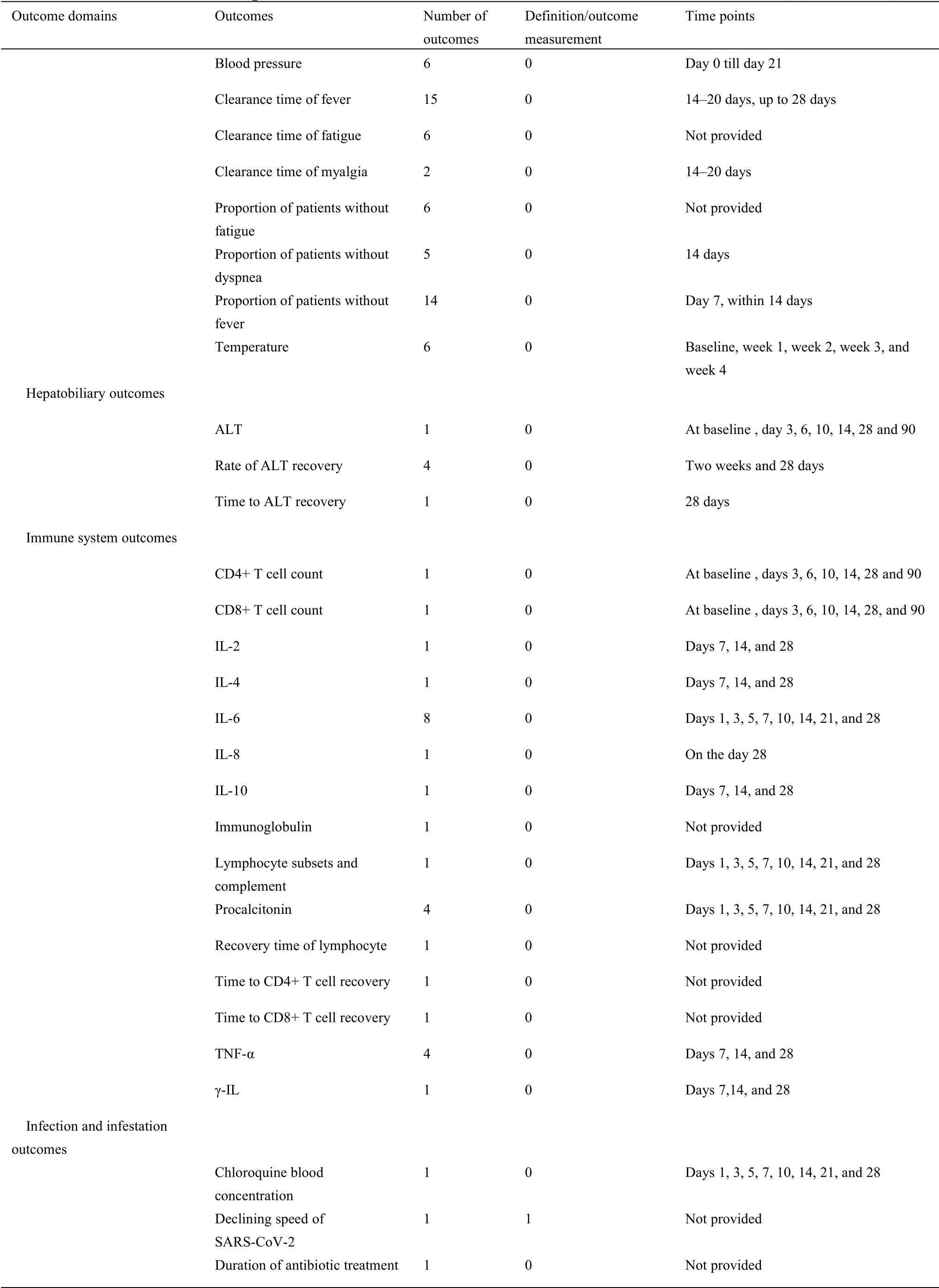
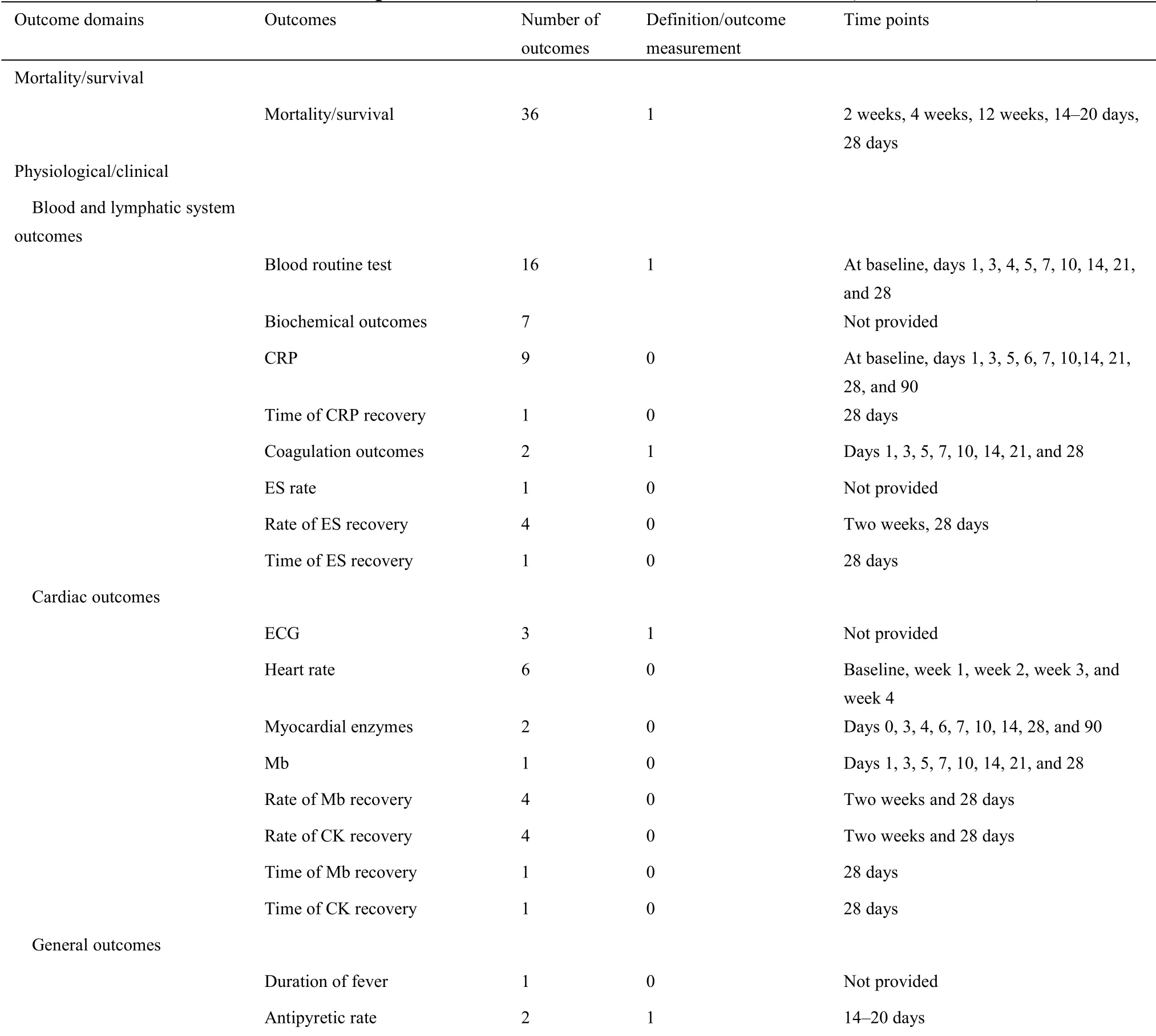
In the 17 outcome domains of protocols of Western medicine clinical trials,5 outcome domains(adverse events/effects,delivery of care,economic,metabolism and nutrition outcomes,and mortality/survival)consisted of only one outcome.These outcomes were reported between 1 and 36 times,and the median outcome reporting time was 1.Respiratory,thoracic,and mediastinal outcomes included the largest number of outcomes(i.e.,31);chest imaging was reported more frequently than other outcomes.The number of outcomes in different outcome domains in protocols of Western medicine clinical trials are shown in Supplementary File 1 Figure S7.
There were >40 duplicated outcomes between the clinical trials protocols of TCM and western medicine protocols.After removing duplicated outcomes,a questionnaire of outcomes for COVID-19 was developed.Some participants proposed 6 new outcomes that did not include in the protocols of clinical trials that searched for the first time,which were “quality of sleep”,“decline of temperature”,“area of temperature declining and time”,“tongue coating and pulse”,“diet”,“viral load”[12].
The list of outcomes from the second search
Though the number of eligible protocols of clinical trials from the second search were about 10 times that of the first search,the majority outcomes from the second search were duplicated with the results of the first search or participants’ proposal in the Delphi survey.In the end,we found that there were 43 new outcomes(Table 6).In addition,there are 47 different outcome measurement instruments to measure mental health,quality of life,or physiological function that were not reported in the first search(Supplementary File 1 Table S8).

Discussion
This systematic review is the first to evaluate the outcome reporting of TCM and Western medicine protocols of clinical trials for treating COVID-19.The aim of the systematic review is to provide a long list of outcomes for COS development.
Simon bought them both, paid as small a price as he could for them, and leading them home with him, he told Nina to prepare a good meal, as he was going to invite some friends to dinner
The first search was conducted on February 14,2020.The results showed significant variations in outcome reporting.Considerable heterogeneity was observedforoutcomemeasurement instruments/outcomedefinitionsandoutcome measurement time.However,many primary investigators did not provide outcome measurement instruments/outcomedefinitionsoroutcome measurement time.Therefore,it is difficult to predict the results of the clinical trials.However,it is obvious that these problems may result in the exclusion of some studies from systematic reviews/meta-analyses owing to the heterogeneity of outcomes or outcome measurements.It is waste.
In this review,we found that there were more than 40 duplicated outcomes between the TCM and Western medicine clinical trial protocols in the first search.Regardless of whether a clinical trial involves TCM or Western medicine,etiological tests,chest imaging,respiratorysymptoms,temperature,mortality/survival,and adverse events are very important outcome parameters.These outcomes are relevant to the prognosis of disease and safety of therapy.According to the
[9],discharged patients should meet the following criteria:normal body temperature should last for more than three days;significant improvement in respiratory symptoms;obvious improvement in acute exudative lesions confirmed by chest imaging;and two SARS-CoV-2 nucleic acid tests(with an interval of more than 24 h)should show negative results.We believe that etiological tests,chest imaging,respiratory symptoms,temperature should be included in the COS of COVID-19.For patients with severe or critical type of disease,it is important to assess mortality/survival.Whilst,it is much more important to assess the progression of disease for patients with mild or ordinary type.
After analyzed the results of the first search,we conducted Delphi survey and consensus meeting.A COS for COVID-19 was developed,including a total of20outcomes,including recovery/improvement/progression/death,SARS-CoV-2 nucleic acid tests,viral load,CRP,temperature,respiration,lymphocyte,virus antibody,pulmonary imaging,blood oxygen saturation,PaO2/FiO2,arterial blood-gas analysis,mechanical ventilation,oxygen intake,pneumonia severity index,prevalence of preventing mild-to-moderate disease progressing to severe disease,clinical symptom score,which should be reported in clinical trials of TCM or Western medicine for different types of disease[12].Not all of outcomes that are important for both clinical trials of TCM and Western medicine were included in the final COS.For example,clinical symptom score was included in the COS for assessing clinical trials of TCM to substitute respiratory symptoms for all clinical trials.Though adverse events reporting is important,the stakeholders believe that it due to different interventions.In the end,the COS for COVID-19 did not include adverse events.
Besides the COS based on this systematic review,other researchers also developed COS for COVID-19.Because of different stakeholder groups,development methods,and geographical area of researchers,the COSs are different from each other.The Core OutcomeMeasuresinEffectivenessTrials management group organized videoconference to help different COS developers achieve to consensus and developedameta-COS(http://www.comet-initiative.org/Studies/Details/1538).The outcomes in the meta-COS include all-cause mortality at hospital discharge,type of respiratory support(oxygen by mask or nasal prongs;oxygen by noninvasive ventilation or high flow;intubation &mechanical ventilation;ECMO).That does not mean that other outcomes are not important.Researchers are also encouraged to choose any other outcomes for their research questions.
All COSs for COVID-19 were developed at early time,when the progression and pathological characteristics were unclear.Therefore,we updated the search.We analyzed new outcomes that did not include in the long list of outcomes for the first search,so that researcher can use it when it is necessary to update the COS for COVID-19.However,the majority new outcomes are biomarkers that are related to specificinterventions,orspecificoutcome measurement instruments that are used to measure quality of life,mental health or physical function.For these outcomes,we believe that re-positive nucleic acid and readmission rate are important.SARS-CoV-2 nucleic acid test did not include in the meta-COS that were achieved to consensus by all COS developers,which is believed that it is an outcome which shows the existing of disease rather than disease severity or progression.
The limitation of the systematic review is that studies on discharged patients were excluded.The scope of the COS for COVID-19 is for hospitalized patients.According to the
,discharged patients are cured or in the rehabilitation period,which are different from hospitalized patients.It is difficult to achieve consensus when different types of patients were considered.In addition,there are few clinical trials for discharged patients.When there are an increasing number of clinical trials for discharged patients,it is necessary to develop a specific COS.
The witch was enchanted17 at this sight, and eagerly helped her brother to set down and open the chest, which was full of the ghastly food she had been longing18 for
Conclusion
The outcome reporting of clinical trials for treating COVID-19 is diversity.The researchers should cautiously choose outcomes in clinical trials.
1.China Health Commission of the People's Republic of China.The latest situation of new coronavirus pneumonia,up to 24 May 31st.http://www.nhc.gov.cn/xcs/yqtb/202006/d5af6cfe b9814e03ad34b76b0fc41842.shtml.Published June 1 2020.Accessed April 25 2021.
2.World Health Organization.Coronavirus disease(COVID-19)weekly epidemiological update and weeklyoperationalupdate.https://www.who.int/emergencies/diseases/novelcoronavirus-2019/situation-reports.Accessed April 25 2021.
3.Zhou P,Yang X,Wang X,et al.A pneumonia outbreak associated with a new coronavirus of probablebatorigin.
.2020;579(7798):270-273.
4.Dorp L,Acman M,Richard D,et al.Emergence of genomic diversity and recurrent mutations in SARS-CoV-2.
.2020;83:104351.
5.Wu F,Zhao S,Yu B,et al.A new coronavirus associated with human respiratory disease in China.
.2020;579(7798):265-269.
6.Wang Y,Qi W,Ma J,et al.Clinical features and syndrome differentiation of novel coronavirus pneumonia in traditional Chinese medicine.
.2020;61(04):281-285.
7.Qin YR.
.Guangzhou,China:Guangdong Economy Press;2012.
8.Wu T.
.Beijing,China:People's Medical Publishing House;2005.
9.China Health Commission of the People's Republic of China.New Coronavirus pneumonia diagnosisandtreatmentplan.http://www.nhc.gov.cn/yzygj/s7653p/202002/833 4a8326dd94d329df351d7da8aefc2/files/b218cfeb 1bc54639af227f922bf6b817.pdf.Published Feb 18 2020.Accessed Feb 25 2020.
10.Shang H,Qiu R.http://www.comet-initiative.org/Studies/Details/1507.Accessed Feb 20 2020.
11.HuangC.Arandomized,open-label,blank-controlled trial for the efficacy and safety of lopinavir-ritonavir and interferon-alpha 2b in hospitalization patients with novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=486 84.Updated March 09 2020.Accessed April 20 2020.
12.Qiu R,Zhao C,Liang T,et al.Core outcome set for clinical trials of COVID-19 based on traditional Chinese and Western medicine.
.2020;11:781.
13.ICMJE.Which trials registries are acceptable to theICMJE?http://www.icmje.org/about-icmje/faqs/clinical-tri als-registration/.Accessed Feb 14 2020.
14.Dodd S,Clarke M,Becker L,et al.A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery.
.2018;96:84-92.
15.Zhong N,Song Y,Qiu H,Li Y,Liu X.A prospective comparative study for Xue-Bi-Jing injection in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=487 68.Accessed Feb 14 2020.
16.Huang L.Clinical controlled trial for traditional Chinese medicine in the treatment of novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=488 24.Accessed Feb 14 2020.
17.Liang T,Wang T,Hao X,et al.Chinese herbal medicine for severe nevel coronavirus pneumonia(COVID-19):a randomized controlled trial.http://www.chictr.org.cn/showproj.aspx?proj=488 86.Accessed Feb 14 2020.
18.Liu Q,Miao Q,Zhang B.A randomized controlled trial of integrated TCM and Western medicine in the treatment of severe novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 11.Accessed Feb 14 2020.
19.Xia W,An C,Zhang B.A single arm study for combination of traditional Chinese and Western medicine in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 13.Accessed Feb 14 2020.
20.Wang Y,Li X,Zhang B.Combination of traditional Chinese medicine and Western medicine in the treatment of common type novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 04.Accessed Feb 14 2020.
21.Li J,Li S.A single arm study for evaluation of integrated traditional Chinese and Western medicine in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=488 84.Accessed Feb 14 2020.
22.Zhong N,Zhang B,Li J,et al.A randomized,open-label,blank-controlled trial for Lian-Hua Qing-Wen capsule/granule in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=488 89.Accessed Feb 14 2020.
23.Yang Z,Wen M.A real world study for the efficacy and safety of large dose tanreqing injection in the treatment of patients with novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=488 81.Accessed Feb 14 2020.
24.Zhang J.Traditional Chinese medicine for pulmonary fibrosis,pulmonary function and quality of life in patients with novel coronavirus pneumonia(COVID-19)in convalescent period:a randomizedcontrolledtrial.http://www.chictr.org.cn/showproj.aspx?proj=489 31.Accessed Feb 14 2020.
25.Zheng C.The effect of shadowboxing for pulmonary function and quality of life in patients with novel coronavirus pneumonia(COVID-19)inrehabilitationperiod.http://www.chictr.org.cn/showproj.aspx?proj=489 30.Accessed Feb 14 2020.
26.Xia W.The effect of pulmonary rehabilitation for pulmonary function and quality of life in patients with novel coronavirus pneumonia(COVID-19)inrehabilitationperiod.http://www.chictr.org.cn/showproj.aspx?proj=489 29.Accessed Feb 14 2020.
27.Xia W.A randomized controlled trial for integrated traditional Chinese medicine and Western medicine in the treatment of common type novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 27.Accessed Feb 14 2020.
28.Wen C.Chinese medicine prevention and treatment program for novel coronavirus pneumonia(COVID-19):aperspective,double-blind,placebo,randomised controlled trial.http://www.chictr.org.cn/showproj.aspx?proj=488 60.Accessed Feb 14 2020.
29.Xie C.Recommendations of integrated traditional Chinese and Western medicine for novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=490 14.Accessed Feb 14 2020.
30.Xie C.Recommendations of integrated traditional Chinese and Western medicine for diagnosis and treatment of novel coronavirus pneumonia(COVID-19)inSichuanProvince.http://www.chictr.org.cn/showproj.aspx?proj=487 92.Accessed Feb 14 2020.
31.Wen C.Chinese medicine prevention and treatment program for novel coronavirus pneumonia(COVID-19):a perspective,sing-arm trial.http://www.chictr.org.cn/showproj.aspx?proj=490 80.Accessed Feb 14 2020.
32.Liu Q.An open,prospective,multicenter clinical study for the efficacy and safety of Reduning injection in the treatment of ovel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 04.Accessed Feb 14 2020.
33.Wang D,Zhao J.A randomized,open-label,blank-controlled,multicentertrialfor Shuang-Huang-Lian oral solution in the treatment of ovel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=490 51.Accessed Feb 14 2020.
34.Zhang Z.An observational study for Xin-Guan-1 formula in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 27.Accessed Feb 14 2020.
35.Xiao X.Treatment and prevention of traditional Chinese medicines(TCMs)on 2019-nCoV infection.https://clinicaltrials.gov/ct2/show/NCT04251871?cond=nCoV&draw=1&rank=7.Accessed Feb 14 2020.
36.Zhang N.Effect evaluation and prognosis of Chinese medicine based on novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=492 87.Accessed Feb 14 2020.
37.Mao W.Clinical study for traditional Chinese medicine in the prevention and treatment of novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 54.Accessed Feb 14 2020.
38.Liu D.A randomized,open,parallel-controlled clinical trial on the efficacy and safety of Jingyebaidu granulesin treatingnovel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 01.Accessed Feb 14 2020.
39.Huang L,Li Z.The efficacy of traditional Chinese medicine on novel coronavirus pneumonia(COVID-19)patients treated in square cabin hospital:a prospective,randomized controlled trial.http://www.chictr.org.cn/showproj.aspx?proj=494 08.Accessed Feb 14 2020.
40.Lv D.Babaodan capsule used for the adjuvant treatment of Severe novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=494 15.Accessed Feb 14 2020.
41.Huang T,Fang B,Feng J,et al.A multicenter,randomized,controlled trial for integrated chinese and Western medicine in the treatment of novel coronavirus pneumonia(COVID-19)based on the'TruncatedTorsion'strategy.http://www.chictr.org.cn/showproj.aspx?proj=493 80.Accessed Feb 14 2020.
42.Zhang W.Clinical study for traditional Chinese medicine combined with Western medicine in treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=494 22.Accessed Feb 14 2020.
43.Zheng X.A multicenter,randomized,open,controlled trial for the efficacy and safety of Shen-Qi Fu-Zheng injection in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=492 20.Accessed Feb 14 2020.
44.Zheng X.A multicenter,randomized,open and controlled trial for the efficacy and safety of Kang-Bing-Du granules in the treatment of novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 38.Accessed Feb 14 2020.
45.Zhang Y,Shang J.Traditional Chinese medicine cooperative therapy for patients with novel coronaviruspneumonia(COVID-19):a randomizedcontrolledtrial.http://www.chictr.org.cn/showproj.aspx?proj=494 52.Accessed Feb 14 2020.
46.Wang L.Clinical study for the integration of traditional Chinese and Western medicine in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=494 53.Accessed Feb 14 2020.
47.Lu H,Chen X.Clinical trial for Tanreqing capsules in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=494 25.Accessed Feb 14 2020.
48.Zhai X.Clinical trial for integrated Chinese and Western medicine in the treatment of children with novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 87.Accessed Feb 14 2020.
49.Chen Y.Adjunctive corticosteroid therapy for patients with severe novel coronavirus pneumonia(COVID-19):a randomized controlled trial.http://www.chictr.org.cn/showproj.aspx?proj=487 77.Accessed Feb 14 2020.
50.Zhao D.Clinical study for the remedy of M1 macrophages target in the treatment of novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 07.Accessed Feb 14 2020.
51.Jiang H.A real-world study for lopinavir/ritonavir(LPV/r)andemtritabine(FTC)/tenofovir alafenamide fumarate tablets(TAF)regimen in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 19.Accessed Feb 14 2020.
52.Jiang S.Study for the efficacy of chloroquine in patients with novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 68.Accessed Feb 14 2020.
53.Wang X,Ke H.A randomised,open,controlled trial for darunavir/cobicistat or lopinavir/ritonavir combined with thymosin a1 in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 92.Accessed Feb 14 2020.
54.Zhao J.A randomized,open-label study to evaluatetheefficacyandsafetyof lopinavir-ritonavir in patients with mild novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=489 91.Accessed Feb 14 2020.
55.Gong G.A randomized,open label,parallel controlled trial for evaluating the efficacy of recombinant cytokine gene-derived protein injection in eliminating novel coronavirus in patients with novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=488 09.Accessed Feb 14 2020.
56.Qiu Y.Randomized,open-label,controlled trial for evaluating of the efficacy and safety of baloxavirmarboxil,favipiravir,and lopinavir-ritonavir in the treatment of novel coronavirus pneumonia(COVID-19)patients.http://www.chictr.org.cn/showproj.aspx?proj=490 15.Accessed Feb 14 2020.
57.ZhangZ.Therapeuticeffectof hydroxychloroquine on novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=488 80.Accessed Feb 14 2020.
58.Lv Q.A multicenter,randomized,open-label,positive-controlled trial for the efficacy and safety of recombinant cytokine gene-derived protein injectioncombinedwithabidole,lopinavir/litonavir in the treatment of novel coronavirus pneumonia(COVID-19)patients.http://www.chictr.org.cn/showproj.aspx?proj=490 65.Accessed Feb 14 2020.
59.Zhou J.A prospective,single-blind,randomized controlled trial for ruxolitinib combined with mesenchymal stem cell infusion in the treatment of patients with severe novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=490 88.Accessed Feb 14 2020.
60.Qiu Y.A randomized,open-label,multi-centre clinical trial evaluating and comparing the safety and efficiency of ASC09/ritonavirand lopinavir/ritonavir for confirmed cases of novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=490 75.Accessed Feb 14 2020.
61.Liu Y.Clinical study for safety and efficacy of favipiravir in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=490 42.Accessed Feb 14 2020.
62.Chen Y.Comparison of efficacy and safety of three antiviral regimens in patients with mild to moderatenovelcoronaviruspneumonia(COVID-19):a randomized controlled trial.http://www.chictr.org.cn/showproj.aspx?proj=487 82.Accessed Feb 14 2020.
63.ShanH.Aprospective,open-label,multiple-center study for the efficacy of chloroquine phosphate in patients with novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 45.Accessed Feb 14 2020.
64.Pei B.Safety and efficacy of umbilical cord blood mononuclear cells in the treatment of severe and criticallynovelcoronaviruspneumonia(COVID-19):a randomized controlled clinical trial.http://www.chictr.org.cn/showproj.aspx?proj=417 60.Accessed Feb 14 2020.
65.Pei B.Safety and efficacy of umbilical cord blood mononuclear cells conditioned medium in the treatment of severe and critically novel coronaviruspneumonia(COVID-19):a randomizedcontrolledtrial.http://www.chictr.org.cn/showproj.aspx?proj=490 62.Accessed Feb 14 2020.
66.Qiu Y.A randomized controlled trial for the efficacy and safety of baloxavir marboxil,favipiravir tablets in novel coronavirus pneumonia(COVID-19)patients who are still positive on virus detection under the current antiviraltherapy.http://www.chictr.org.cn/showproj.aspx?proj=490 13.Accessed Feb 14 2020.
67.Hu B,Li W.Efficacy and safety of aerosol inhalation of vMIP in the treatment of novel coronavirus pneumonia(COVID-19):a single armclinicaltrial.http://www.chictr.org.cn/showproj.aspx?proj=492 15.Accessed Feb 14 2020.
68.Liu L.Multicenter randomized controlled trial for novel recombinant high-efficiency compound interferon in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=492 24.Accessed Feb 14 2020.
69.Li L,Xu X,Xiang C.Clinical study for human menstrual blood-derived stem cells in the treatment of acute novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 46.Accessed Feb 14 2020.
70.Wu C.Nasal high-fow preoxygenation assisted fibre-optic bronchoscope intubation in patients with critical novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=490 74.Accessed Feb 14 2020.
71.Du R.A randomized,open-label study to evaluate the efficacy and safety of low-dose corticosteroids in hospitalized patients with novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=490 86.Accessed Feb 14 2020.
72.Qu J.Clinical study of arbidol hydrochloride tablets in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 65.Accessed Feb 14 2020.
73.Unclear.Glucocorticoid therapy for critically ill patients with severe acute respiratory infections caused by noval coronovirus 2019-nCoV:a prospective,randomizedcontrolledtrial.https://clinicaltrials.gov/ct2/show/record/NCT042 44591.Accessed Feb 14 2020.
74.Lu H.Efficacy and safety of darunavir and cobicistat for treatment of pneumonia caused by 2019-nCoV(DACO-nCoV).https://clinicaltrials.gov/ct2/show/NCT04252274?cond=nCoV&draw=1&rank=6.Accessed Feb 14 2020.
75.Qin N.A prospective,randomized controlled clinical study of interferon atomization in the 2019-nCoVpneumonia.https://clinicaltrials.gov/ct2/show/NCT04254874?cond=nCoV&draw=2&rank=3.Accessed Feb 14 2020.
76.Qin N.A prospective,randomized controlled clinical study of antiviral therapy in the 2019-nCoV pneumonia .https://clinicaltrials.gov/ct2/show/NCT04255017?cond=nCoV&draw=2&rank=2.Accessed Feb 14 2020.
77.Cao B.Mild/moderate 2019-nCoV remdesivir RCT.https://clinicaltrials.gov/ct2/show/NCT04252664?cond=nCoV&draw=2&rank=1.Accessed Feb 14 2020.
78.Cao B.Severe 2019-nCoV remdesivir RCT.https://clinicaltrials.gov/ct2/show/NCT04257656?cond=nCoV&draw=2&rank=7.Accessed Feb 14 2020.
79.Qu J.Clinical study of arbidol hydrochloride tablets in the treatment of pneumonia caused by novelcoronavirus.https://clinicaltrials.gov/ct2/show/NCT04260594?cond=nCoV&draw=2&rank=6.Accessed Feb 14 2020.
80.Qin N.A randomized,open,controlled clinical study to evaluate the efficacy of ASC09F and ritonavirfor2019-nCoVpneumonia.https://clinicaltrials.gov/ct2/show/NCT04261270?cond=nCoV&draw=2&rank=5.Accessed Feb 14 2020.
81.Li T.The efficacy of intravenous immunoglobulin therapy for severe 2019-nCoV infected pneumonia.https://clinicaltrials.gov/ct2/show/NCT04261426?cond=nCoV&draw=2&rank=2.Accessed Feb 14 2020.
82.Lu H.Efficacy and safety of hydroxychloroquine for treatment of pneumonia caused by 2019-nCoV(HC-nCoV).https://clinicaltrials.gov/ct2/show/NCT04261517?cond=nCoV&draw=3&rank=13.Accessed Feb 14 2020.
83.Zhang Z.Efficacy of therapeutic effects of hydroxycholoroquine in novel coronavirus pneumonia(COVID-19)patients(randomized open-labelcontrolclinicaltrial).http://www.chictr.org.cn/showproj.aspx?proj=493 17.Accessed Feb 14 2020.
84.XiaJ.Efficacyofchloroquineand lopinavir/ritonavirinmild/generalnovel coronavirus(CoVID-19)infections:a prospective,open-label,multicenter randomized controlled clinicalstudy.http://www.chictr.org.cn/showproj.aspx?proj=492 63.Accessed Feb 14 2020.
85.Ning Q,Han M.A randomized,parallel controlled trial for the efficacy and safety of sodium aescinate injection in the treatment of patients withpneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=492 97.Accessed Feb 14 2020.
86.Qin N.The efficacy of different hormone doses in 2019-nCoVseverepneumonia.https://clinicaltrials.gov/ct2/show/NCT04263402?id=NCT04263402&draw=2&rank=1.Accessed Feb 14 2020.
87.Qiu Y.Evaluating and comparing the safety and efficiencyofASC09/ritonavirand lopinavir/ritonavir for novel coronavirus infection.https://clinicaltrials.gov/ct2/show/NCT04261907?id=NCT04261907&draw=2&rank=1.Accessed Feb 14 2020.
88.Li L.The efficacy of lopinavir plus ritonavir and arbidol against novel coronavirus infection(ELACOI).https://clinicaltrials.gov/ct2/show/NCT04252885?id=NCT04252885&draw=2&rank=1.Accessed Feb 14 2020.
89.Shi L,Wang F.Patients infected with 2019 novel coronavirus.https://clinicaltrials.gov/ct2/show/NCT04252118?id=NCT04252118&draw=2&rank=1.Accessed Feb 14 2020.
90.Zhang F.Washed microbiota transplantation for patientswith2019-nCoVinfection.https://clinicaltrials.gov/ct2/show/NCT04251767?cond=%E2%80%9C2019-nCoV+OR+Novel+Cor onavirus+OR+New+Coronavirus+OR+NCP+OR+Novel+Coronavirus+Pneumonia+OR+COVID-1 9+OR+SARS-CoV-2+OR+Wuhan+pneumonia%E2%80%9D&draw=2&rank=10.Accessed Feb 14 2020.
91.Peng Z.Vitamin C infusion for the treatment of severe2019-nCoVinfectedpneumonia.https://clinicaltrials.gov/ct2/show/NCT04264533?cond=%E2%80%9C2019-nCoV+OR+Novel+Cor onavirus+OR+New+Coronavirus+OR+NCP+OR+Novel+Coronavirus+Pneumonia+OR+COVID-1 9+OR+SARS-CoV-2+OR+Wuhan+pneumonia%E2%80%9D&draw=2&rank=12.Accessed Feb 14 2020.
92.Chen X.Treatment of acute severe 2019-nCoV pneumonia with immunoglobulin from cured patients.https://clinicaltrials.gov/ct2/show/NCT04264858?cond=2019-nCoV+OR+Novel+Coronavirus+OR+New+Coronavirus+OR+NCP+OR+Novel+Coro navirus+Pneumonia+OR+COVID-19+OR+SARS-CoV-2+OR+Wuhan+pneumonia&draw=2&rank=13.Accessed Feb 14 2020.
93.Zhang Z.Clinical study of nebulized Xiyanping injection in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=492 22.Accessed Feb 14 2020.
94.Liu Z.Convalescent plasma for the treatment of severe novel coronavirus pneumonia(COVID-19):a prospective randomized controlled trial.http://www.chictr.org.cn/showproj.aspx?proj=490 81.Accessed Feb 14 2020.
95.Kang Y.Cohort study of novel coronavirus pneumonia(COVID-19)critical ill patients .http://www.chictr.org.cn/showproj.aspx?proj=492 95.Accessed Feb 14 2020.
96.Hu P.A multicenter,randomized,open label,controlled trial for the efficacy and safety of ASC09/ritonavircompoundtabletsand lopinavir/ritonavir(kaletra)and arbidol tablets in the treatm ent of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 52.Accessed Feb 14 2020.
97.Mao H.A study for the efficacy of hydroxychloroquine for mild and moderate COVID-19infectiousdiseases.http://www.chictr.org.cn/showproj.aspx?proj=493 69.Accessed Feb 14 2020.
98.Huang W.Clinical study on the safety and effectiveness of hydroxychloroquine sulfate tablets in the treatment of patients with novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=494 00.Accessed Feb 14 2020.
99.Huang W.Clinical study for the effect and safety of hydroxychloroquine sulfate tablets in the treatment of patients with severe novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=494 04.Accessed Feb 14 2020.
100.Xu X.A multicenter,randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=494 09.Accessed Feb 14 2020.
101.Lin J.A randomized,open,controlled trial for diammoniumglycyrrhizinateenteric-coated capsules combined with vitamin C tablets in the treatment of common novel coronavirus pneumonia(COVID-19)in the basic of clinical standard antiviral treatment to evaluate the safety andefficiency.http://www.chictr.org.cn/showproj.aspx?proj=491 31.Accessed Feb 14 2020.
102.HuangX.Arandomized,open-label,blank-controlled,multicentertrialfor polyinosinic-polycytidylic acid injection in the treatment of novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 42.Accessed Feb 14 2020.
103.Wu W.Clinical application of ECMO in the treatment of patients with very serious respiratory failure due to novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 78.Accessed Feb 14 2020.
104.Xia J.Immunomodulatory therapy for severe novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=491 61.Accessed Feb 14 2020.
105.Xu C.Clinical study for anti-aging active freeze-dried powder granules in the treatment of acute novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 55.Accessed Feb 14 2020.
106.Xu C.Clinical study for umbilical cord blood mononuclear cells in the treatment of acute novel coronaviruspneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 74.Accessed Feb 14 2020.
107.Xu C.Clinical study for cord blood mesenchymal stem cells in the treatment of acute novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 89.Accessed Feb 14 2020.
108.Xu C.Clinical study of cord blood NK cells combined with cord blood mesenchymal stem cells in the treatment of acute novel coronavirus pneumonia(COVID-19).http://www.chictr.org.cn/showproj.aspx?proj=493 84.Accessed Feb 14 2020.
109.Xie J.Immunoregulatory therapy for 2019-nCoV.https://clinicaltrials.gov/ct2/show/NCT04268537?cond=%222019-nCoV%22+OR+%22Novel+Cor onavirus%22+OR+%22New+Coronavirus%22+O R+%22SARS-CoV-2%22OR+%22SARI%22OR+%22NCP%22+OR+%22Novel+Coronavirus+Pn eumonia%22+OR+%22COVID-19%22++OR+%22Wuhan+pneumonia%22&draw=2&ra.Accessed Feb 14 2020.
110.PengZ.Umbilicalcord(UC)-derived mesenchymal stem cells(MSCs)treatment for the 2019-novelcoronavirus(nCOV)pneumonia.https://clinicaltrials.gov/ct2/show/NCT04269525?cond=%222019-nCoV%22+OR+%22Novel+Cor onavirus%22+OR+%22New+Coronavirus%22+O R+%22SARS-CoV-2%22OR+%22SARI%22OR+%22NCP%22+OR+%22Novel+Coronavirus+Pn eumonia%22+OR+%22COVID-19%22++OR+%22Wuhan+pneumonia%22&draw=3&ra.Accessed Feb 14 2020.
