A Terbium(III)-organic Coordination Polymer:Synthesis, Characterization and Its Luminescence
2022-03-08MIYingHoYANGMingXueKUANGXioFeiLUCnZhong
MI Ying-Ho YANG Ming-Xue KUANGXio-Fei②LU Cn-Zhong,c②
a (State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China)
b (College of Chemistry and Materials Science, Fujian Normal University, Fuzhou 350007, China)
c (University of Chinese Academy of Sciences, Beijing 100049, China)
ABSTRACT A new 3D terbium(III) metal-organic network [Tb(HIMDC)(HCOO)(H2O)]n (complex 1) has been synthesized by Tb3+ ions coordinated with 4,5-imidazole dicarboxylic acid (H3IMDC) and formic acid under solvothermal conditions with the mixed solvents of N,N-dimethylformamide (DMF) and H2O. The unambiguous structural determination has been confirmed by single-crystal X-ray diffraction combined with elemental analysis,infrared (IR) spectrum, thermogravimetric analysis (TGA) and powder X-ray diffraction (PXRD). Complex 1 crystallizes in orthorhombic Fdd2 space group with a = 22.295(2), b = 24.097(3), c = 6.7256(6) Å, V = 3613.3 Å3,Z = 16, Dc = 2.765 g/cm3, F(000) = 2816, μ = 7.855 mm-1, the final R = 0.0173 (I ≥ 2σ(I)), wR = 0.0416 (all data)and GOOF = 1.068. In complex 1, the Tb3+ ions are connected by HIMDC2- anions into 2D metal-organic layers which are further extended into a three-dimensional (3D) architecture through formic acid functional motif. In addition, complex 1 shows intense and characteristic green luminescence.
Keywords: coordination polymer, crystal structure, photoluminescence;
1 INTRODUCTION
Due to the outstanding advantages in the facile synthesis and preparation, flexible structure feature and porosity size tunability[1], coordination polymers have received intense attention in the field of gas storage and separation[2],chemical sensor[3,4], biomedicine[5,6]and photonic devices[7-9].Generally, the choice of suitable organic ligands is critical for constructing coordination polymer with unique structural feature and specific functionality. Among these, 4,5-imidazole dicarboxylic acid contains both coordination-directed N and O atoms and combines the characteristics of imidazole and carboxylic acid motifs, which enable it multiple coordination modes and strong coordination ability towards some transition metals and rare earth cations for assembling some novel supramolecular architecture[10]. Therefore, in recent years, more attention had been paid to the design and synthesis of some metal-organic supramolecules by employing 4,5-imidazole dicarboxylic acid as functional building blocks[11,12]. For example, Yang and coworkers reported early on the lanthanide metal-organic coordination polymer with a spiral structure [Ln2(IMDC)2(H2O)3]∙1.625 H2O (Ln = Eu, Dy)[10]. These compounds possessed a one-dimensional achiral pore and a spiral tubular structure with alternating left and right hands. After that, they also reported a series of Ln-Cd-MOFs with luminescent properties[13]. In addition, Xu etc. constructed four lanthanide coordination polymers throughin situreactions of DMF solvent under solvothermal conditions[14]. The europiumbased coordination polymer exhibited bright red solid-state phosphorescence upon exposure to UV light irradiation under ambient condition. It is noteworthy that the 4,4΄-dipyridine was employed as an auxiliary ligand to construct the coordination network and didn’t participate to the metal center. However, without 4,4΄-dipyridine, the reaction failed to give targeted compound under identical hydrothermal conditions. More recently, Ling and coworkers reported four novel 3Dheterometal-organic compounds by using 2-methyl-1H-4,5-imidazole-dicarboxylic acid as the building unit and the compounds exhibited 3D microporous pillar-layered structural features with isostructure[15]. It is noteworthy that europium-based coordination polymer displayed luminescent sensing for multi-responsive Ag+, Cu+,Zn2+, Co2+, and Ni2+cations and some organic amines.
Based on these results of multiple coordination feature and outstanding functional properties, we tried to extend this intriguing system to assemble some novel structures with unique functional features. Herein, we demonstrate a hybrid supramolecular material through mixed ligands strategy by employing 4,5-imidazole dicarboxylic acid and readily available formic acid as functional motif. Formic acid as a bridging ligand expands the two-dimensional plane formed by 4,5-imidazole dicarboxylic acid and Tb3+into a novel 3D supramolecular architecture (complex 1). Compared with the aforementioned rare earth coordination polymer, complex 1 exhibits non-centrosymmetric space group, which is unique to most of the 4,5-imidazole dicarboxylic acid metal-organic supramolecular systems. Nowadays, non-centrosymmetric and chiral coordination polymers have received extensive attention in the field of asymmetrical catalysis[16], chiral separation[17]and nonlinear optics[18,19]. The synthesis of common non-centrosymmetric and chiral coordination polymers is generally based on chiral ligands[20]or structurally directed regents. The spontaneous assembly of non-centrosymmetric supramolecules by nonchiral motifs provides a new synthetic method for constructing some functional materials. In addition, complex 1 displays intense green luminescence, and the photophysical property and thermal stability have been investigated.
2 EXPERIMENTAL
2. 1 Materials and general methods
All commercially available starting materials were used without any purification. The crystal diffraction data were collected on a Bruker D8 VENTRUE diffractometer with graphite monochromatized MoKαradiation (λ= 0.71073 Å).The structures were obtained by direct methods and refined by full-matrix least-squares methods using the SHELXL-2013 program package. PXRD analyses were collected on a Rigaku Corporation Miniflex600 powder diffractometer at a scanning speed of 5 °/min. Thermogravimetric analyses were carried out at a Mettlertoledo TGA/DSC thermal weight and the synchronous thermal analyzer was measured under a nitrogen atmosphere in the temperature range of 30~1000 °C at a heating speed of 5 K/min. Elemental analyses of C, H,and N were carried out with an Elementar Vario El Cube elemental analyzer. UV-Vis absorption spectra were obtained on a Perkin-Elmer lambda 900 UV-Vis spectrometer.Luminescence spectra were carried out on an Edinburgh Instrument F980 fluorescent spectrometer. The infrared spectra were measured on a Thermo Fisher Scientific Nicolet iS50 Fourier transform infrared spectrometer.
2. 2 Preparation of complex 1
A mixture of 4,5-imidazole dicarboxylic acid (15.6 mg, 0.1 mmol) and Tb(NO3)3·6H2O (90 mg, 0.2 mmol) in a mixed solvent of 2 mL DMF and 4 mL water was poured in a 20 mL Teflon-lined stainless-steel reactor. Then 50 μL of formic acid(concentration 98%) was added to the above solution and sonicated for 15~20 min. Finally, a Teflon-lined stainlesssteel reactor was placed in an oven at a constant temperature of 160 °C for 3 days, and then cooled at a rate of 5 °C/h to room temperature. The needle transparent crystals were obtained by filtration, washed three times with DMF and water, and dried in air. The yield of complex 1 is 60% (based on 4,5-imidazole dicarboxylic acid). EA calcd. (%) for C6H5N2O7Tb: C, 19.16; H, 1.34; N, 7.45. Found (%): C,19.17; H, 1.36; N, 7.49. IR (cm-1): 3453 (w), 3102 (w), 1670(w), 1569 (s), 1504 (m), 1459 (m), 1400 (s), 1353 (s),1246 (m), 1229 (m), 1179 (m), 1094 (w), 963 (m), 865 (m),814 (m), 774 (s), 654 (s).
2. 3 Structure refinements
A colorless needle single crystal with dimensions of 0.141 mm × 0.08mm × 0.026mm was coated with oil, placed on the top of a nylon loop, and then mounted in the diffractometer for X-ray measurement. Single-crystal X-ray diffraction data were collected on a Bruker D8 VENTRUE diffractometer with graphite-monochromatized MoKαradiation (λ=0.71073 Å) at 200 K. Data collection and reduction were used by APEX2. Absorption correction was performed by SADABS program. Using OLEX2[21], the structure was solved by direct methods using the SHELXS program and refined by full-matrix least-squares techniques SHELXL-2013[22]program package onF2. All non-hydrogen atoms were refined with anisotropic thermal parameters. And all hydrogen atoms were added in the idealized positions. In complex 1, a total of 18385 reflections were obtained in the range of 4.98°≤2θ≤56.10° withRint= 0.0529, 2157 of which are independent. The finalR= 0.0173 (I≥ 2σ(I)) andwR= 0.0416 (all data), andGOOF= 1.068. Selected bond lengths and bond angles of complex 1 are shown in Table 1.
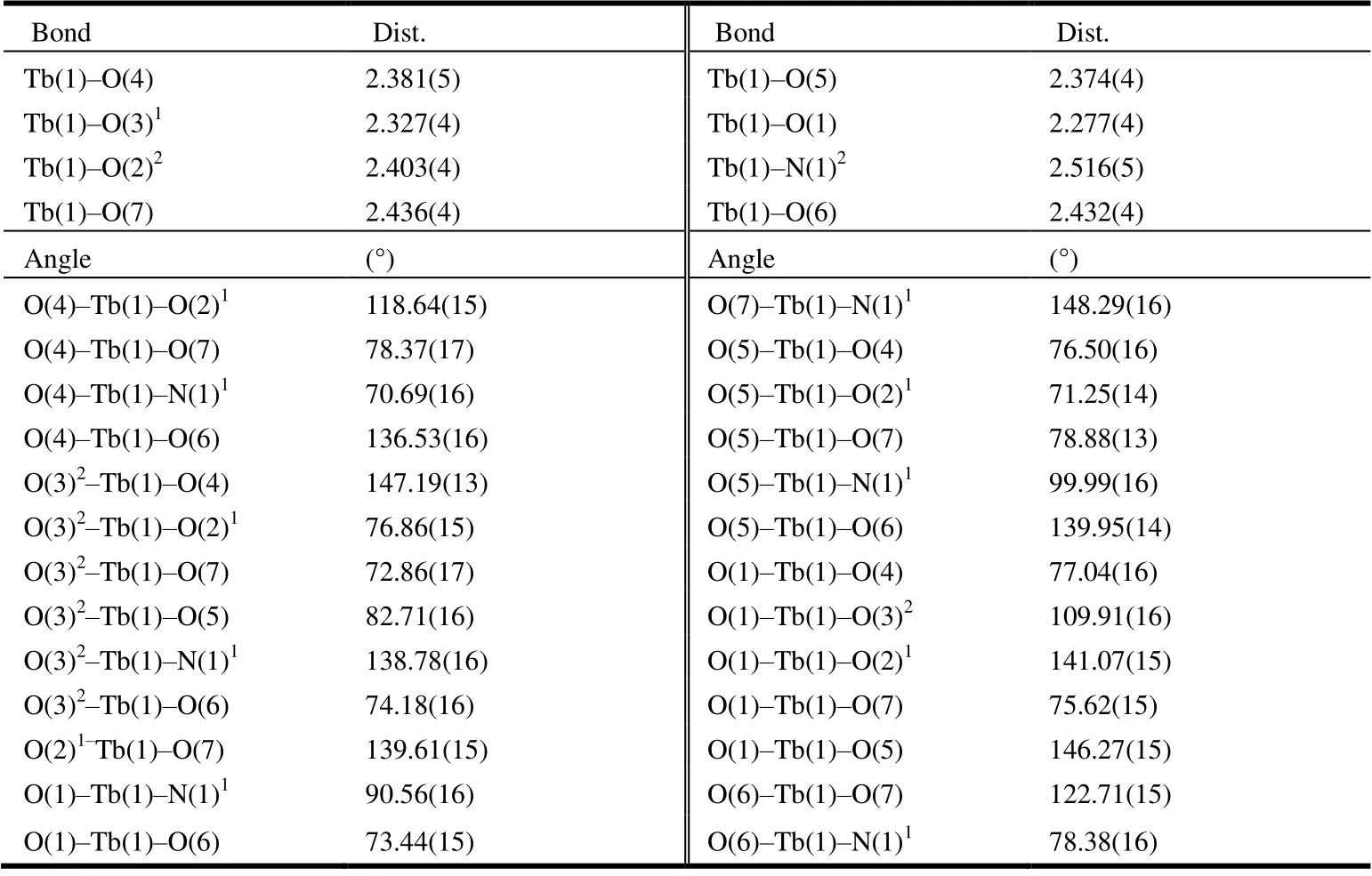
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) of Complex 1
3 RESULTS AND DISCUSSION
3. 1 Synthesis and structural characterization
Complex 1 was synthesized by a solvothermal one-step method, and its structure was determined by single-crystal X-ray diffraction analysis. The structure and bulky phase purity as well as chemical composition were further confirmed by X-ray powder diffraction analysis, infrared spectroscopy, thermogravimetric analysis, and elemental analysis. According to the single-crystal X-ray diffraction, the molecular formula of the asymmetric unit of complex 1 is C6H5N2O7Tb, which could formula as[Tb(HIMDC)(HCOO)(H2O)]n(H3IMDC is 4,5-imidazole dicarboxylic acid). Complex 1 crystallizes in theFdd2 space group of orthorhombic system and shows non-centrosymmetric structural feature. The asymmetric unit contains one Tb3+ion, one HIMDC2-unit contributed by 4,5-imidazole dicarboxylic acid, one HCOO-anion, and one coordinated H2O molecule, as shown in Fig. 1a. The coordination environment of Tb3+ions is shown in Fig. 2(a). In complex 1,each Tb3+ion interacts with four oxygen atoms (O(1), O(2),O(3), O(4)) and one nitrogen atom (N(1)) from HIMDC2-,two oxygen atoms (O(6), O(7)) from HCOO-, and an oxygen atom (O(5)) from H2O, forming an 8-coordinated twisted tetragonal anti-prism. The bond lengths of Tb–O range from 2.277(4) to 2.436(4) Å, and Tb–N is 2.516(5) Å. The bond angles of O–Tb–O vary from 71.25(14)° to 147.19(13)°,while O–Tb–N falls in the 70.69(16) ~ 148.24(16)° range. All bond lengths and bond angles are within the normal ranges.The selected bond lengths and angles of complex 1 are shown in Table 1. In 1, two of the protons from the dicarboxylic acid deprotonated, generating the HIMDC2-multidentate ligand(Fig. 2b). The four O atoms from the carboxylic acid and one N atom contributed by the imidazole ring as well as an O atom from water participate in coordination, thus generating a 2D layer in thebcplane (Fig. 2d). Fig. 2e shows the metal-organic layer from thec-axis. The deprotonated formic acid behaves asμ2-bridged ligand and extends the 2D layer along thea-axis to form a 3D supramolecular architecture.The detailed coordination mode of the HCOO-is shown in Fig. 2c.
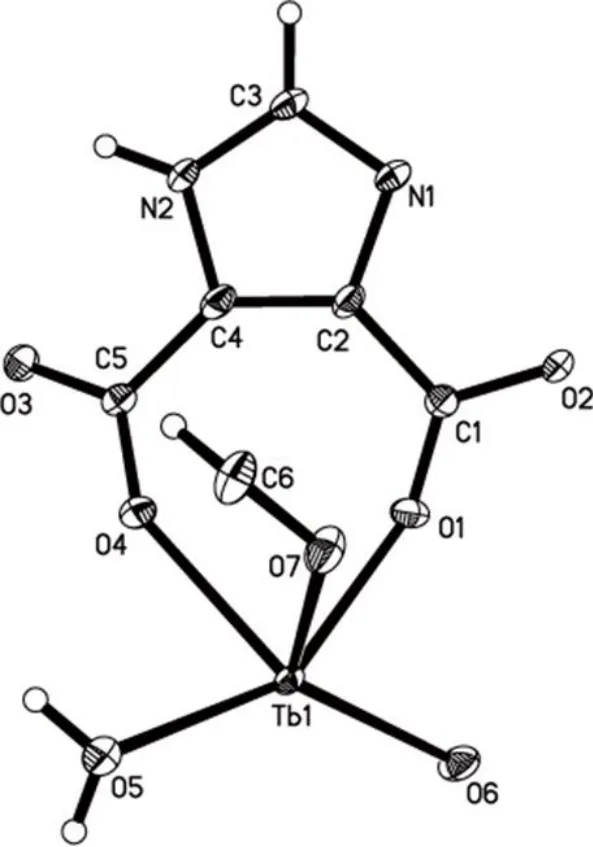
Fig. 1. Asymmetric unit of complex 1

Fig. 2. (a) Coordination environment of Tb3+ ion, (b) Coordination environment of HIMDC2-, (c) Coordination environment of HCOO-, (d) In the a-axis direction, the 2D plane is formed by Tb3+ and HIMDC2-, (e) Along the c-axis, the 2D plane is built by Tb3+ and HIMDC2- (f) 3D network structure bridged by HCOO- motif
3. 2 Powder X-ray diffraction (PXRD)and thermogravimetric analysis of complex 1
In order to confirm the structure analysis and phase purity of the bulky materials, the PXRD was conducted at room temperature. The simulated PXRD pattern from its corresponding single crystal is in good agreement with the experimental as synthesized bulky sample, which demonstrates high purity of the synthesized complex 1 (Fig. 3a). To investigate the thermal stability of 1, thermogravimetic analysis (TGA) was performed under N2atmosphere. As shown in Fig. 3b, 1 could maintain its structure as high as 280 °C, and further heating leads to decomposition of the sample gradually.
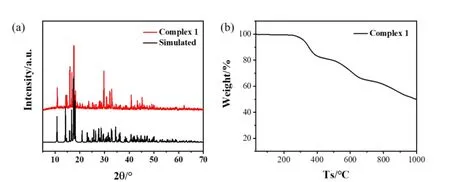
Fig. 3. (a) XRD spectrum of complex 1, (b) Thermogravimetric spectrum of complex 1
3. 3 Photophysical property
The facile preparation of high purity bulky samples with good thermal stability under ambient condition impels us further investigate the photophysic properties. It could be observed that the maximum excitation wavelength is about 282 nm from the fluorescence excitation spectrum of complex 1. However, there are four fluorescence emission peaks emerged at 490, 545, 585, and 623 nm, which correspond to the transition of Tb3+energy levels5D4→7F6,5D4→7F5,5D4→7F4and5D4→7F3in turn[23]. Among them,the emission peak at 545 nm dominates the fluorescent emission, corresponding to the5D4→7F5transition.Thereafter, complex 1 exhibits green light under UV light irradiation (Fig. 4). This phenomenon could be explained by the antenna effect of rare earth complexes. Under ultraviolet light irradiation of complex 1, the organic ligand firstly excited to single state withπ-π* transition, and then jumped to the triple state through the intersystem crossing process.The high level triple state of the ligand transferred the energy to5D4energy level of Tb3+through energy transfer process and sensitized the rare earth ions to the high level exited state.And finally the high level excited state of the Tb3+ions returned to the ground state accompanied with the radiative transition and emission of the green light. Additionally, there is 4 nm blue shift compared with the characteristic emission peak at 549 nm of Tb3+at5D4→7F5transition due to the interference of the metal center and organic motif.
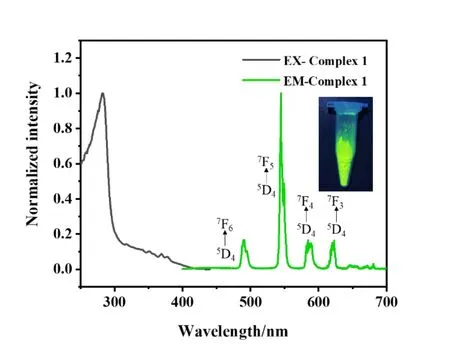
Fig. 4. Fluorescence excitation and emission spectra of complex 1
4 CONCLUSION
In summary, a new terbium(III)-based metal-organic coordination polymer has been constructed through the mixed ligands strategy under solvothermal condition. Single-crystal diffraction analysis demonstrates that complex 1 is a 3D supramolecular material. The 4,5-imidazole dicarboxylic acid and Tb3+ions formed a 2D metal-organic layer and then extended by formic acid, generating a 3D architecture. In addition, complex 1 is highly thermally stable under ambient condition, and also exhibits characteristic green light emission. Moreover, the space group of 1 is noncentrosymmetic. The successful synthesis and characterization provide new insight for the design of new noncentrosymmetric materials for future optoelectronic devices.
杂志排行
结构化学的其它文章
- A Novel 3D CdII-CP Based on a Bi-functional (N-/O-Sites)Triazole-modified Carboxyl Ligand: Structure,Topology and Photoluminescence①
- Structure and Properties of a Cd(II) Metal-organic Framework Based on a Newly Designed Heterotopic Tripodal N-Donor Ligand①
- Effect of Fluorination on the Crystal Structure, Stability and Gas Adsorption Property in Zinc(II)Metal-organic Frameworks①
- Molecular Design and Property Prediction of High Density 4-Nitro-5-(5-nitro-1,2,4-triazol-3-yl)-2H-1,2,3-triazolate Derivatives as the Potential High Energy Explosives①
- Influence of Doped Ions on Persistent Luminescence Materials: a Review①
- Novel 2,4-Diarylaminopyrimidine Derivatives Containing Pyridine Moiety: Design, Synthesis, Crystal Structure and Biological Evaluation①
