Temperature-controlled Structural Diversity of Two Cd(II)Coordination Polymers Based on the Dicarboxylate Ligand①
2022-03-08CHENFngMinZHOUChiChiHEXiongLIYnZHANGXiuQing
CHEN Fng-Min ZHOU Chi-Chi HE Xiong LI Yn② ZHANG Xiu-Qing②
a (College of Chemistry and Bioengineering, Guangxi Key Laboratory of Electrochemical and Magnetochemical Functional Materials, Guilin University of Technology, Guilin 541004, China)
ABSTRACT Two new 3-D Cd(II) coordination polymers with p-phthalic acid (p-BDC) and 4,4΄-dipyridylamine (4,4΄-dpa), namely [Cd2(p-BDC)2(4,4΄-dpa)2]n 1 and {[Cd(p-BDC)(4,4΄-dpa) (H2O)]·4H2O}n 2 were successfully synthesized under hydrothermal conditions at 120 and 140 °C. They were characterized by single-crystal X-ray diffraction, IR, PXRD and TGA. It was further characterized by Hirshfeld surface (HS)analysis for complex 2. The luminescent properties of the complexes have also been investigated.
Keywords: Cd(II) coordination polymers, p-phthalic acid, Hirshfeld surface, Luminescent properties;
1 INTRODUCTION
Recently, the design of coordination polymers (CPs) has attracted considerable attention not only for their interesting topological structures[1]but also for their potential applications in the fields of magnetism, luminescence, gas adsorption, catalysis, electrical conductivity, and so on[2-5]. To date, how to rationally design and synthesize aiming metalorganic complexes with the expected structure and prospective properties is still a big challenge. Thereinto,selecting suitable ligands is crucially to construct complexes with special functionality. However, it remains difficult to predict the exact structures and control the construction of these CPs[6,7]because they might be easily influenced by many factors, such as the geometry of organic ligands,coordination number of metal ions, solvents, pH value of the solution, reaction time, etc[8-11]. Apart from these factors,higher reaction temperature may lead to complicated structures due to the increase in connected number of ligands,the appearance of entanglement, hydroxo metal clusters and so on[12]. Due to different temperatures, a great number of these complexes possessing novel structures and interesting
properties have been effectively prepared under hydrothermal conditions. Inspired by the aforementioned considerations,two novel Cd(II) CPs based onp-phthalic acid and 4,4΄-bipyridylamine ligand, namely, [Cd2(p-BDC)2(4,4΄-dpa)2]n1 and {[Cd(p-BDC)(4,4΄-dpa)(H2O)]·4H2O}n2, have been successfully obtained under hydrothermal conditions at different temperature. Herein, we report the synthesis, crystal structures and luminescent properties of the polymers in this work.
2 EXPERIMENTAL
2. 1 Materials and instruments
All chemicals for syntheses were commercially available(Aldrich, Aladdin, Alfa Aesar or Xilong Scientific) and used as received without further purification. The structures of the complexes have also been confirmed by single-crystal X-ray(Agilent G8910A CCD) diffraction analyses. The Fourier transform infrared spectra were recorded using KBr pellets ranging from 4000 to 500 cm-1on a PerkinElmer spectrum one FT-IR spectrometer. Powder X-ray diffraction (XRD)data were collected on a Bruker D8 Advance X-ray diffractometer with CuKαradiation (λ= 1.5418 Å). The thermal behavior was carried out by a SDTQ 600 apparatus. The photoluminescence spectra for the solid samples were measured at room temperature on a RF-4600 fluorescence spectrophotometer.
2. 2 Preparation of [Cd2(p-BDC)2(4,4΄-dpa)2]n 1
A mixture of Cd(NO3)2·4H2O (0.1542 g, 0.5 mmol),p-BDC (0.0831 g, 0.5 mmol), 4,4΄-dpa (0.0855 g, 0.5 mmol),NaOH (0.0400 g, 1 mmol), and H2O (15 ml) was stirred at room temperature for 10 min. Then, it was sealed in a 25 mL Teflon-lined stainless-steel container. The mixture was heated at 120 °C for 72 h. After slowly cooling to room temperature at a rate of 10 °C·h-1, the mixture was washed with alcohol/distilled water and faint yellow lump-shaped crystals were filtered off and dried at room temperature (Yield: 0.077 g, 28% based on Cd). Anal. Calcd. (%) for C36H26Cd2N6O81:C, 48.24; H, 2.90; N, 9.38. Found (%): C, 48.21; H, 2.89; N,9.34. IR (cm−1, KBr): 3451 (m, O-H), 1562(m, COO-), 1392(m, COO-), 1210 (m, C-H), 1015 (m, C-H), 820 (m, N-M)738 (m, O-M).
2. 3 Preparation of {[Cd(p-BDC)(4,4΄-dpa)(H2O)]·4H2O}n 2
A mixture of Cd(NO3)2·4H2O (0.1542 g, 0.5 mmol),p-BDC (0.0831 g, 0.5 mmol), 4,4΄-dpa (0.0855 g, 0.5 mmol),NaOH (0.0400 g, 1 mmol), and H2O (15 mL) was stirred at room temperature for 10 min. Then, it was sealed in a 25 mL Teflon-lined stainless-steel container. The mixture was heated at 140 °C for 72 h. After slowly cooling to room temperature at a rate of 10 °C·h-1, the mixture was washed with alcohol/distilled water and faint yellow lump-shaped crystals were filtered off and dried at room temperature (Yield: 0.042 g, 16% based on Cd). Anal. Calcd. (%) for C36H46Cd2N6O182:C, 40.16; H, 4.27; N, 7.81. Found (%): C, 40.13; H, 4.25; N,7.79. IR (cm−1, KBr): 3432 (m, O-H), 1566 (m, COO-), 1386(m, COO-), 1216 (m, C-H), 1008 (m, C-H), 814 (m, N-M),758 (m, O-M).
2. 4 X-ray crystal structure determination
Single-crystal X-ray diffraction analyses of 1 and 2 were carried out on an Agilent Technologies G8910A CCD diffractometer equipped with graphite-monochromated MoKαradiation (λ= 0.71073 Å) using anω-scan mode. Theυ-scan technique was employed to measure intensities.Absorption corrections were applied empirically using the SADABS[13]. The crystal structures were solved by direct methods and difference Fourier synthesis and refined by full-matrix least-squares using SHELXL[13]. An absorption correction was applied based on the comparison of multiple symmetry equivalent measurements. The structures were solved by direct methods using SHELXS-97 and refined anisotropically by full-matrix least-squares methods onF2using SHELXL-97 for all non-hydrogen atoms[15,16]. Crystal data as well as details of data correction and refinement for complexes 1 and 2 are summarized in Table 1. Selected bond lengths and bond angles are listed in Tables 2 and 3, and H-bonds for 2 are listed in Table 4.
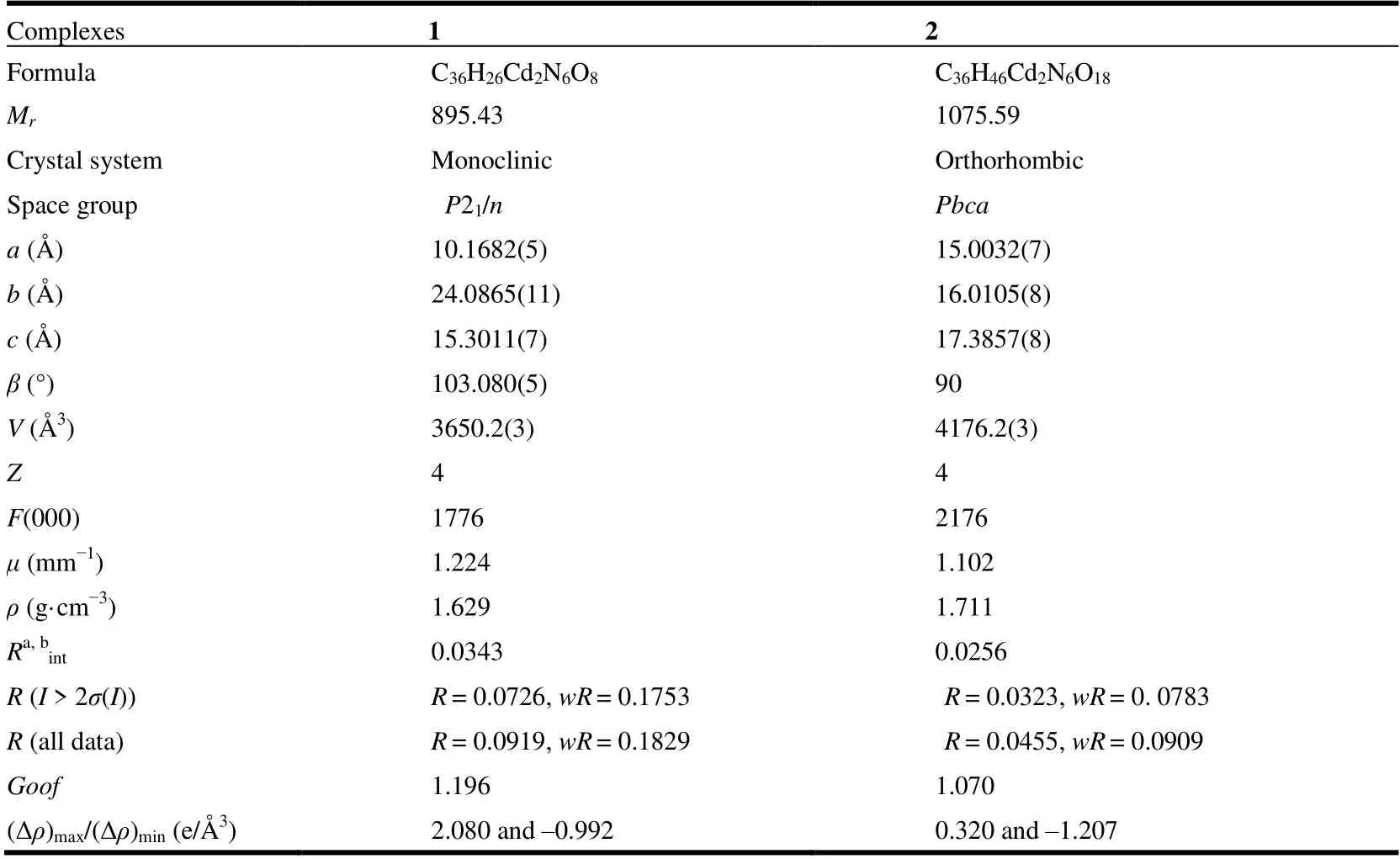
Table 1. Crystallographic Data and Details for 1 and 2

Table 2. Selected Bond Lengths (Å) and Bond Angles (º) for 1
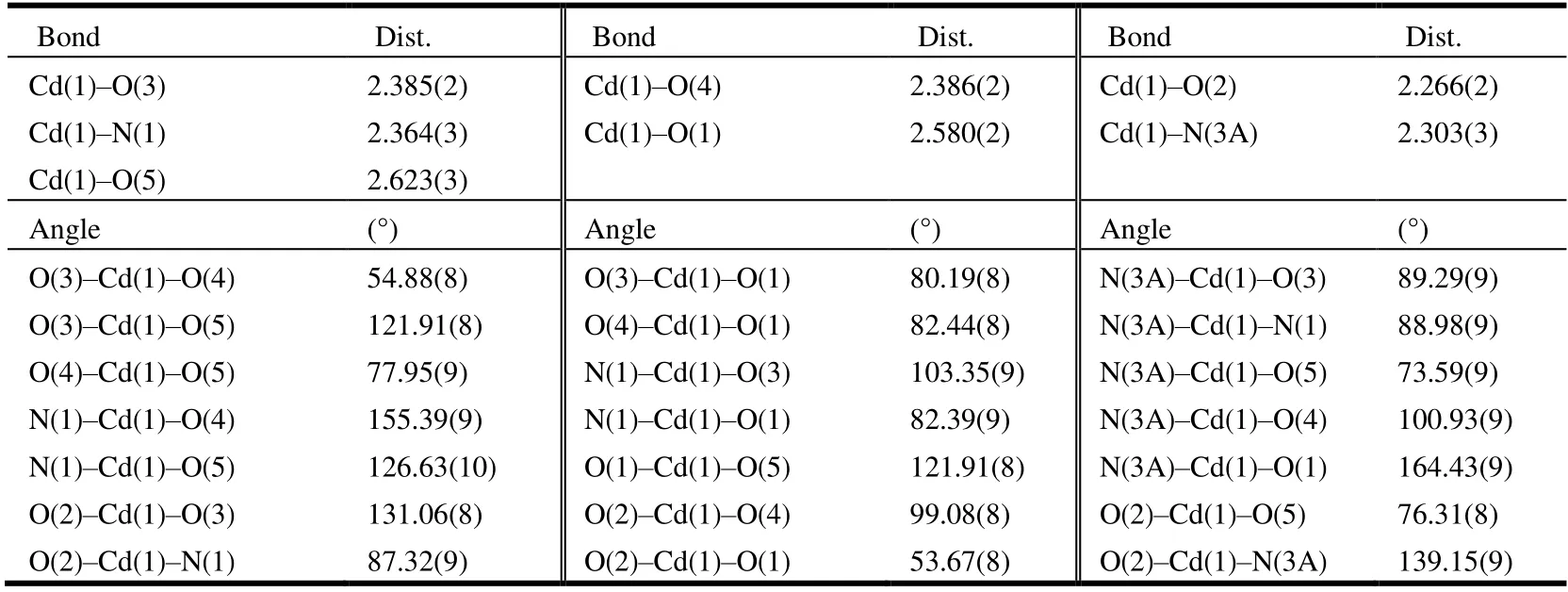
Table 3. Selected Bond Lengths (Å) and Bond Angles (º) for 2
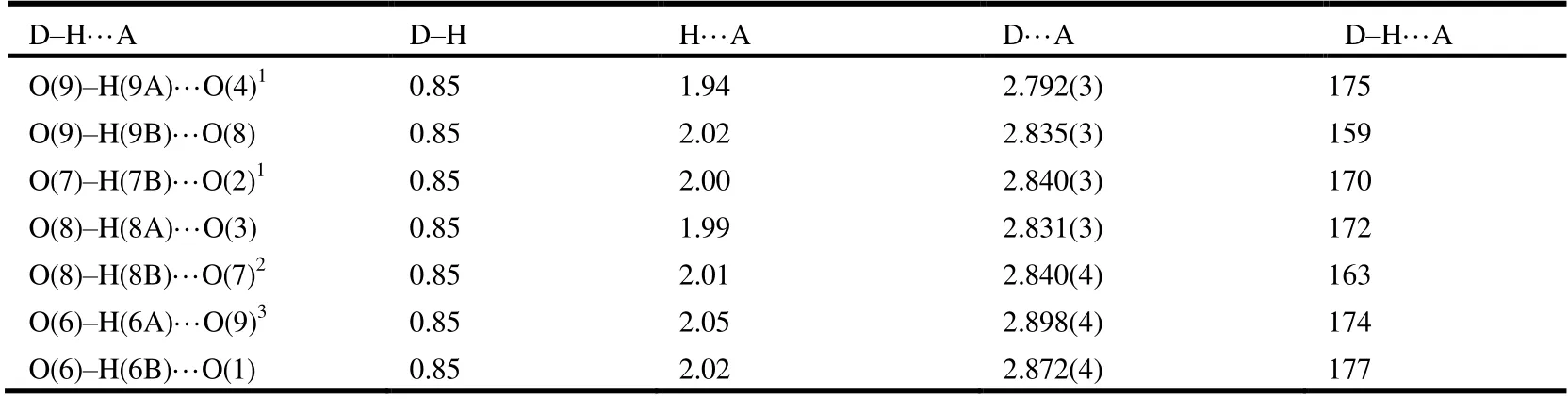
Table 4. Hydrogen Bond Lengths (Å) and Bond Angles (°) for Complex 2
3 RESULTS AND DISCUSSION
3. 1 Crystal structure of [Cd2(p-BDC)2(4,4΄-dpa)2]n 1
Crystallographic analysis reveals that complex 1 crystallizes in the monoclinic systemP21/nspace group. The asymmetric unit of complex 1 contains two Cd(II) cations,two 4,4΄-dpa ligands and twop-BDC2-anions. The two Cd(II)ions lie in two different environments, as shown in Fig. 1a.The Cd(1) is six-coordinated by four oxygen atoms from threep-BDC2-anions and two nitrogen atoms from two 4,4΄-dpa ligands. The Cd(1)–O bond lengths vary from 2.253(2) to 2.464(2) Å, and the Cd(1)–N bond lengths change from 2.267(7) to 2.310(8) Å. Six atoms form a distorted octahedral coordination geometry. The Cd(2) is five-coordinated by three oxygen atoms from threep-BDC2-anions and two nitrogen atoms from two 4,4΄-dpa ligands.The Cd(2)–O bond lengths vary from 2.215(6) to 2.310(6) Å,and the Cd(2)–N bond lengths change from 2.325(7) to 2.333(7) Å. The coordination geometry around the Cd(2) ion can be regarded as an intermediate between square pyramid and trigonal bipyramid as described by theτparameter of 0.61[17]. Two Cd(II) centers are bridged by carboxylate groups from two differentμ4-p-BDC2-anions, generating a binuclear Cd2unit with a Cd···Cd distance of 4.005 Å.Further linkage of these Cd2units via bothμ3- andμ4-p-BDC2-moieties furnishes a 3D structure. The carboxylate groups ofp-BDC2-blocks alternately bridge the adjacent Cd(II) ions to form a 2-D metal-organic net (Fig. 1b).The 2-D layers are further connected by 4,4΄-dpa ligands to form a 3-D structure (Fig. 1c and 1d).
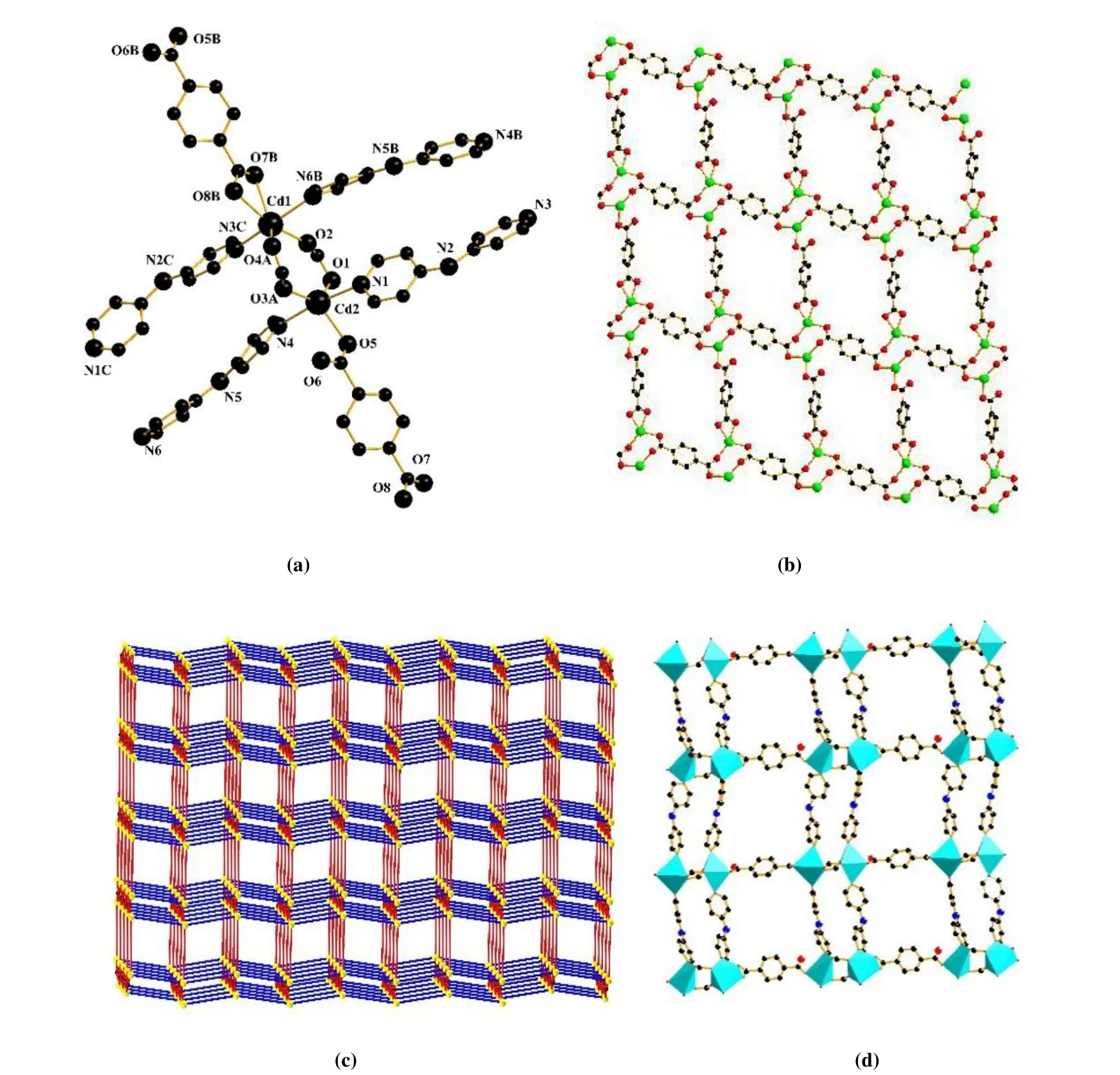
Fig. 1. (a) Coordination environment of Cd(II) in 1 (All hydrogens are omitted for clarity). (b) View of 2-D network in complex 1. (c) Schematic representation of topology for complex 1. (d) View of the 3-D framework for complex 1
3. 2 Crystal structure of{[Cd(p-BDC)(4,4΄-dpa)(H2O)]·4H2O}n 2
The single-crystal X-ray diffraction analysis reveals that complex 2 crystallizes in orthorhombic space groupPbca.The asymmetric unit of 2 contains one crystallographically unique Cd(II) ion, onep-BDC2-anion, one 4,4΄-dpa ligand,one coordinated water molecule, and four lattice water molecules, as shown in Fig. 2. The Cd(II) ion is seven-coordinated in a pentagonal bipyramidal geometry by four oxygen atoms from twop-BDC2-anions, two nitrogen atoms of 4,4΄-dpa ligands, and one oxygen from one coordinated water molecule. The Cd(1)–O bond lengths vary from 2.266(2) to 2.579(2) Å, and the Cd(1)–N bond lengths change from 2.3032(3) to 2.364(3) Å. In complex 2, two different carboxylate groups adopt bidentate chelate coordination modes, which connect Cd(II) ions to form a 3-D structure. As shown in Fig. 3, from the perspective of topology,p-BDC2-and 4,4΄-dpa as linkers connect two adjacent Cd metal atoms,so the structure of 2 could be simplified as a uninodal 4-connected net with a point symbol of (66).

Fig. 2. Coordination environment for Cd(II) in complex 2 (All hydrogens have been omitted for clarity)

Fig. 3. Schematic representation of topology for complex 2
3. 3 Hirshfeld surface analysis for complex 2
Hirshfeld surface analysis is an effective tool for the quantitative study of intermolecular interactions within crystal packings, which provides visual images of intercontacts and molecular shapes in a crystalline material.The Hirshfeld surfaces are mapped via the normalized contact distance (dnorm) relative to both de and di and the van der Waals radius of the atoms, where de is the distance from a point on the surface to the nearest nucleus outside the surface and di is distance from a point on the surface to the nearest nucleus inside the surface[18]. The molecular Hirshfeld surface (dnorm) of the complex can be used to show short intermolecular interactions (Fig. 4). The Hirshfeld surfaces mapped with dnormand full fingerprint plots were made using CrystalExplorer software (Version 3.1). The dnormvalue is negative (red) when intermolecular contacts are shorter than the van der Waals radii, and the dnorm(blue) when longer. The dnormvalue of the white zone is zero and represents contacts equal to the van der Waals radius. The red spots in the picture are mainly caused by the hydrogen bonding between the carboxyl groups of the main ligandp-phthalic acid and free water molecules through O–H···O. In this study, the dnormvalues range from –1.188 to 1.429 Å.
Each 2D fingerprint plot can be split into the respective close contacts, and their contributions can be expressed as percentages. The relative contributions of various interactions for 2 are presented in Fig. 5. The 2D fingerprint plots (Fig. 6)present contacts between two atoms interacting with each other and indicate percentage of contributions from different interaction types. The proportions of H···H and C···H are 40.8% and 5% respectively, while that of O···H is 14%. In the interaction of molecules, H···H and O···H account for a large proportion, so van der Waals forces and hydrogen bonds play a significant role in the three-dimensional stacking structure, of which O–H···O plays a major role in intermolecular hydrogen bonds. This corresponds to the fact the majority of the surface of this molecular crystal is covered with atoms of H.

Fig. 5. Fingerprint plots for H···H (a), H···O (b) and O···H (c) contacts of 2
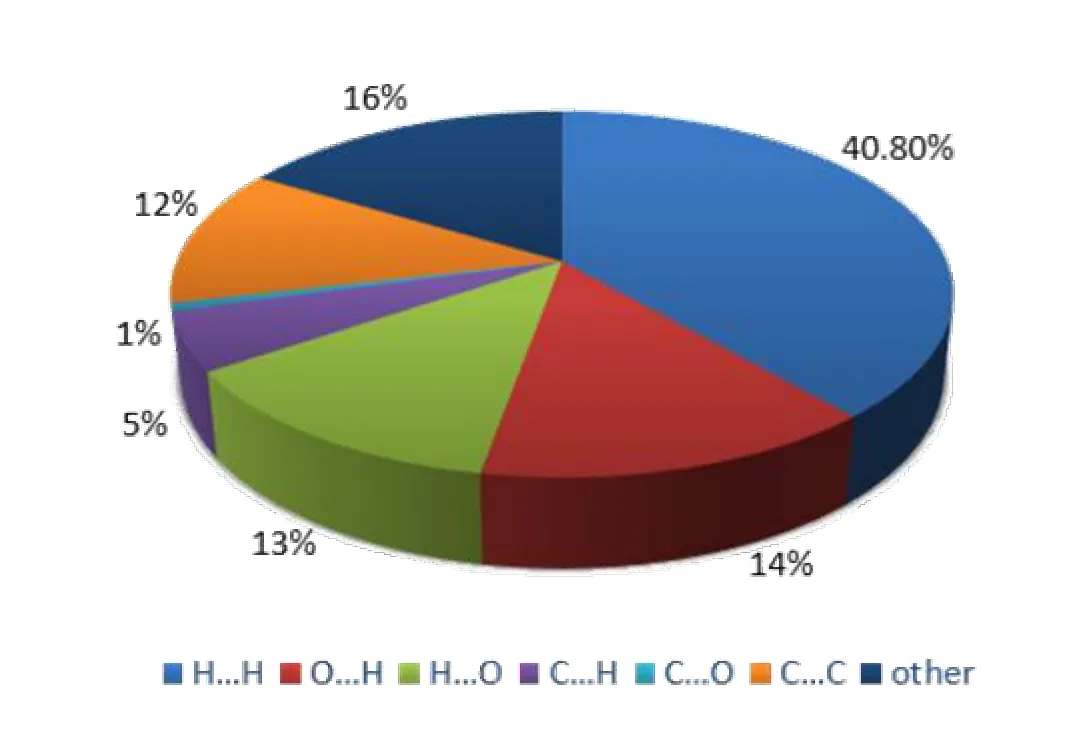
Fig. 6. Hirshfeld surface calculations for 2
3. 4 Powder X-ray diffraction analysis
The powder X-ray diffraction (PXRD) patterns for the coordination polymers were recorded to investigate the crystalline phases of the polycrystalline materials. The simulated and experimental PXRD patterns are shown in Fig. 7.Most of the PXRD peak positions in the simulated and experimental patterns are in good agreement with each other,indicating the pure samples of complexes 1 and 2.

Fig. 7. XRD patterns of complexes 1 and 2
3. 5 Thermal gravimetry analysis
To estimate the stabilities of the complexes, thermogravimetric (TG) analyses of complexes 1 and 2 were carried out under a N2atmosphere from 15 to 800 ℃. The weight loss curves of complexes 1 and 2 are shown in Fig. 8. For 1, the first stage 36.74% weight loss in the range of 379~417 ℃ is contributed to the decomposition ofp-BDC2-ligands (calcd.:37.10%). The second stage of 37.83% weight loss between 417 and 499 ℃ could be attributed to the departure of 4,4΄-dpa ligands (calcd.: 38.19%). Above 500 ℃, the curve area is stable, and the final mass remnant is likely consistent with the deposition of CdO. For 2, the first stage 30.59%weight loss in the range of 385~422 ℃ results from the decomposition ofp-BDC2-ligand (calcd.: 30.91%). The second stage of 32.01% weight loss between 422 and 473 ℃is assigned to the removal of 4,4΄-dpa ligand (calcd.: 31.81%).When reaching 500 ℃, the curve area is stable, and the final mass remnant is likely consistent with the deposition of CdO.
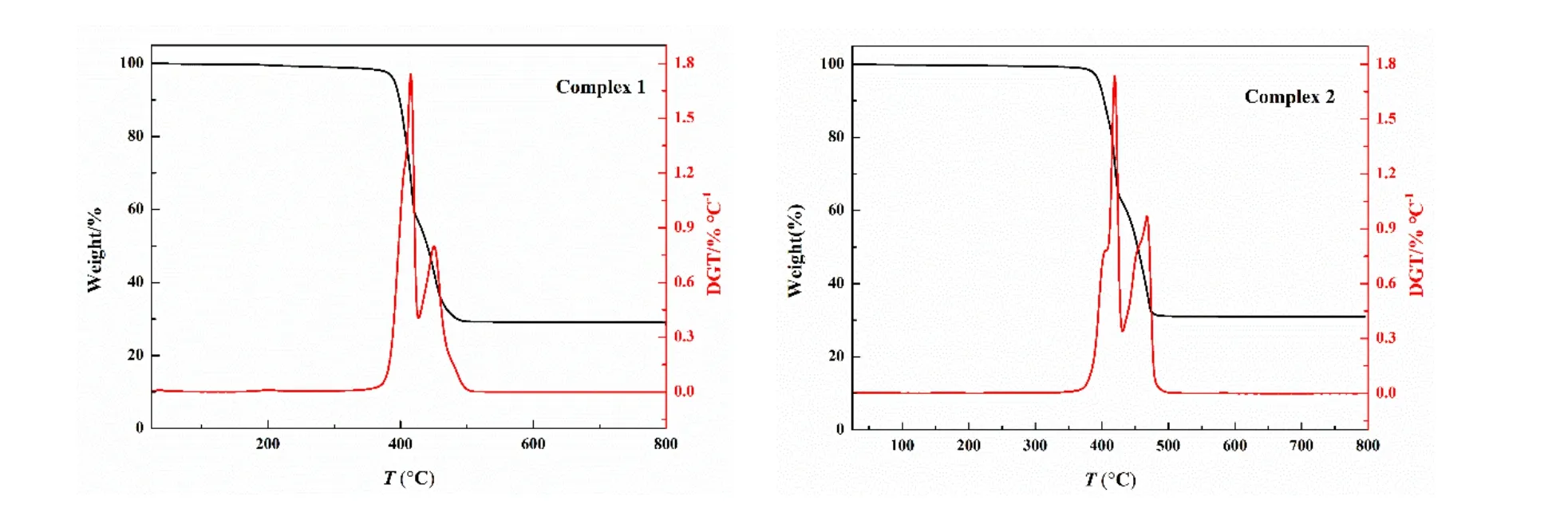
Fig. 8. Thermal behaviours of 1 and 2
3. 6 Luminescent properties
The solid state UV-vis absorption of complexes 1 and 2 was measured (Fig. 9). The maximum absorption peaks are all around 270 nm. It is well known that coordination polymers constructed byd10metal center and conjugated organic linkers are promising candidates for photoactive materials, with potential applications such as chemical sensors and in photochemistry[19-22]. Considering the excellent luminescent properties ofd10transition metalorganic polymers[23,24], the solid-state photoluminescent properties of complexes 1 and 2 were investigated at room temperature. All bands can be assigned to the intraligandπ*→πorπ*→nemission[25]. Since the Cd(II) ions are difficult to oxidize or reduce, the emission of complex 1 and 2 is neither metal-to-ligand charge transfer (MLCT) nor ligand-to-metal charge transfer (LMCT)[26]. As shown in Fig.10, complex 1 exhibits the maximum emission at 385 nm (λex= 270 nm), and complex 2 shows the maximum emission at 385 nm (λex= 270 nm). Compared with 2, the luminous intensity of complex 1 is reduced. The emission discrepancy of 1 and 2 is probably due to the differences of coordination environments of the central metal ions[27-29].

Fig. 9. Solid-state UV-vis absorption spectra of complexes 1 and 2

Fig. 10. Emission spectra of complexes 1 and 2 in the solid state at room temperature
4 CONCLUSION
In this paper, we have synthesized two Cd(II) coordination polymers whose structures and properties were different because of the different temperature. Remarkably, the changes in coordination numbers of metal atoms and coordination modes stimulated by reaction temperature result in the distinct frameworks of 1 and 2, which promote us to make a further research on related functional crystalline solids through such a reliable synthetic procedure. This work demonstrates that the temperature has a significant effect on the structures and properties of coordination polymers. The luminescent properties of complexes 1 and 2 imply that they may be good candidates for luminescent materials.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
杂志排行
结构化学的其它文章
- Synthesis, Crystal Structure and Fungicidal Activity of 3,4-Dichloro-5-(6-chloro-9-(4-fluorobenzyl)-9H-purin-8-yl)isothiazole①
- Synthesis, Crystal Structure and Antifungal Activity of New Furan-1,3,4-oxadiazole Carboxamide Derivatives①
- Syntheses, Crystal Structures, Anticancer Activities and DNA-Binding Properties of the Dibutyltin Complexes Based on Benzoin Aroyl Hydrazone①
- Synthesis, Crystal Structure, Fungicidal Activities and Molecular Docking of Acyl Urea Derivatives Containing 2-Chloronicotine Motif①
- A New Borate-phosphate Compound CsNa2Lu2(BO3)(PO4)2:Crystal Structure and Tb3+ Doped Luminescence①
- Drug Design, Molecular Docking, and ADMET Prediction of CCR5 Inhibitors Based on QSAR Study①
