Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment
2022-03-05MatildaFlorentinMichaelKostapanosAthanasiaPapazafiropoulou
INTRODUCTION
Diabetes mellitus (DM) is a worldwide health problem with epidemic proportions and a huge economic burden. The global prevalence of DM in 2019 was estimated to be 9.3% (463 million people) with a projection to rise to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045[1]. DM is a major cause of blindness, chronic kidney disease (CKD), stroke, lower extremity amputations and death from coronary heart disease and heart failure (HF)[2].
Until a few years ago the main focus of the management of patients with DM was the adequate or even strict glycemic control, mainly based on the fact that a glycated hemoglobin (HbA1c) of < 7% has been associated with a reduction in microvascular complications[3]. However, intensive glycemic control not only does not appear to reduce all-cause mortality and macrovascular endpoints in patients with DM type 2(DM2), but it may increase the relative risk (RR) of severe hypoglycemia up to 30%[3,4]. Therefore, the glycemic target needs to be individualized and associated risk factors and co-morbidities be appropriately managed[5].
Another issue which emerged over a decade ago, due to concerns about agents such as rosiglitazone, is the cardiovascular (CV) safety of antidiabetic agents[6,7]. Ever since the regulatory authorities, such as the U.S. Food and Drug Administration (FDA)[8]and the European Medicines Agency (EMA)[9], require large CV outcomes trials(CVOTs) for all new treatments for DM2. Incretin-based therapies,
, glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1 RA) and dipeptidyl peptidase 4 (DPP-4)inhibitors, and subsequent drug classes have, thus, been approved after their efficacy was established in CVOTs.
She didn t come on the 9:18 either, nor on the 9:40, and when the passengers from the 10:02 had all arrived and left, Harry was looking pretty desperate. Pretty soon he came close to my window so I called out and asked him what she looked like.918,940。1002,。,,。
Importantly, about 6 years ago a novel class of drugs, namely sodium-glucose transporter 2 (SGLT2) inhibitors (SGLT2-i), was demonstrated to reduce major adverse CV events (MACE) and mainly hospitalizations for HF[10]. Of note, a recent metaanalysis demonstrated that SGLT2-i significantly improve CV outcomes including CV and all-cause mortality in patients with HF without excess risk of serious adverse events[11], while their capacity to slow the progression of CKD and/or albuminuria or even improve renal function has already been established[12-14].
Some GLP-1 RA were also found to decrease MACE, as well as secondary outcomes(
, HF and progression of renal disease) in patients with established CV disease(CVD) or CKD. Furthermore, recent evidence demonstrated that these drugs reduce the risk of nonfatal stroke in patients with DM2[15].
The CAROLINA study was another non-inferiority study that compared linagliptin with glimepiride as an active comparator[45]. It included patients with DM2 and suboptimal glycemic control (HbA1c 6.5%-8.5%) and high CV risk. The latter was defined as the presence of established CVD or microvascular complications, the presence of multiple CV risk factors or age > 70 years. These patients were randomized to linagliptin 5 mg/d
glimepiride 1-4 mg/d with investigator-led option to add other antidiabetic agents titrated to achieve sufficient glycemic control. The primary composite endpoint and the non-inferiority margins were the same as in the CARMELINA study. After 6.3 years (median) no significant difference between groups was noted in the glycemic control. Similarly, linagliptin was non-inferior to glimepiride in the primary composite endpoint which occurred in 11.8%
12.0%,respectively [HR = 0.98 (95.47% CI: 0.84-1.14);
< 0.001 for noninferiority;
= 0.76 for superiority]. The same was relevant also for the individual components of the primary endpoint[45].
MECHANISM OF ACTION AND CHARACTERISTICS OF DPP-4 INHIBITORS
In 2006 the first DPP-4 inhibitor, sitagliptin, was approved for the treatment of diabetes[16,17]. These drugs inhibit DPP-4,
, the enzyme that degrades incretins,subsequently prolonging their half-life[18]. Two such hormones have been identified in humans; glucose-dependent insulinotropic peptide or gastric inhibitory polypeptide(GIP) and GLP-1. The latter may achieve glucose lowering
various actions.Specifically, GLP-1 enhances glucose-dependent insulin secretion[19], activates insulin biosynthesis and gene transcription, thus restoring the cellular supplies of insulin for subsequent release[20], while it suppresses glucagon secretion[21,22] and food intake[23,24] and slows gastric emptying[25].
In DM2 there is a reduction in GLP-1 secretion[26], an effect which in part accounts for the impaired “incretin effect” in patients with diabetes[27]. The “incretin effect”stands for the observation that insulin response to glucose is amplified when insulin is delivered orally
intravenously[28]. By inhibiting the enzyme which is responsible for the degradation of incretin hormones,
, DPP-4, DPP-4 inhibitors prevent the proteolytic breakdown and inactivation of GLP-1 and GIP[29,30]. Typically, these drugs decrease serum DPP-4 activity by > 80%, which translates in doubling of intact,biologically active GLP-1 concentration[31] along with a significant reduction in postprandial glucose levels[31,32] and an approximately 0.8% decrease in HbA1c[33].Importantly, DPP-4 inhibitors do not increase the risk of hypoglycemia, which is a major concern and an unfavorable prognostic factor in patients treated with antidiabetic agents. This occurs as native GLP-1, whose action is prolonged by DPP-4 inhibitors, stimulates glucose-dependent insulin secretion from pancreatic β-cells[34].
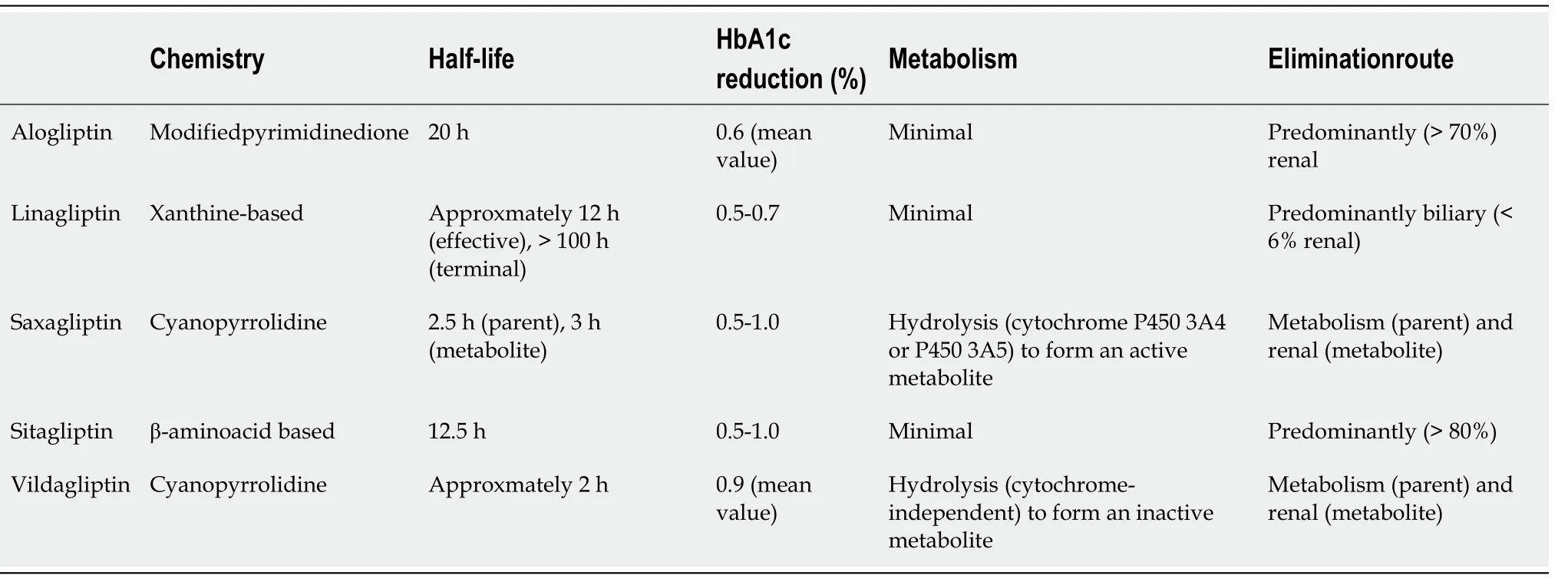
Dissimilarities in the chemical structure of the different DPP-4 inhibitors affect their pharmacokinetic properties, formulation and daily dosing (Table 1). The relatively long half-lives of sitagliptin, linagliptin and alogliptin allow for once-daily dosing.Saxagliptin, which has a short half life, may also be administered once daily due to the presence of its active metabolite, BMS-510849, which inhibits DPP-4[35-37]. In contrast,vildagliptin has a short half-life and, thus, requires twice-daily dosing[38]. As far as route of elimination is concerned, sitagliptin and alogliptin are primarily excreted renally, whereas saxagliptin undergoes both renal and hepatic clearance. In contrast,linagliptin is predominately (approximately 90%) secreted unchanged in the feces[39],while vildagliptin is metabolized
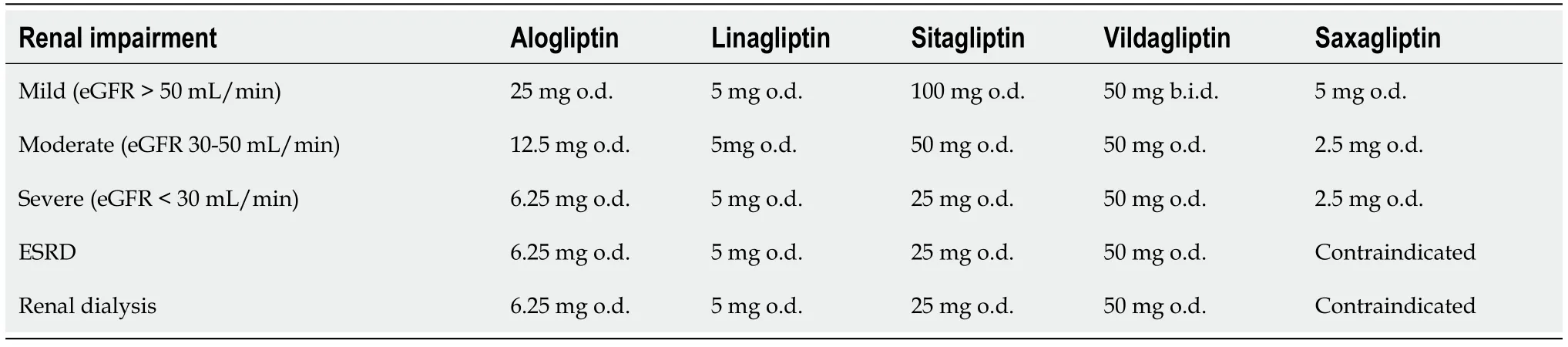
at least four pathways before excretion[38,40].Regarding CKD, all DPP-4 inhibitors may be given to patients at all CKD stages in reduced doses in order to avoid increased drug exposure[38,40], with the exception of linagliptin which does not require dose modification. Furthermore, saxagliptin is contraindicated in end-stage renal disease (ESRD) and in dialysis[38] (Table 2). This agent is also prone to drug-drug interactions as it is metabolized
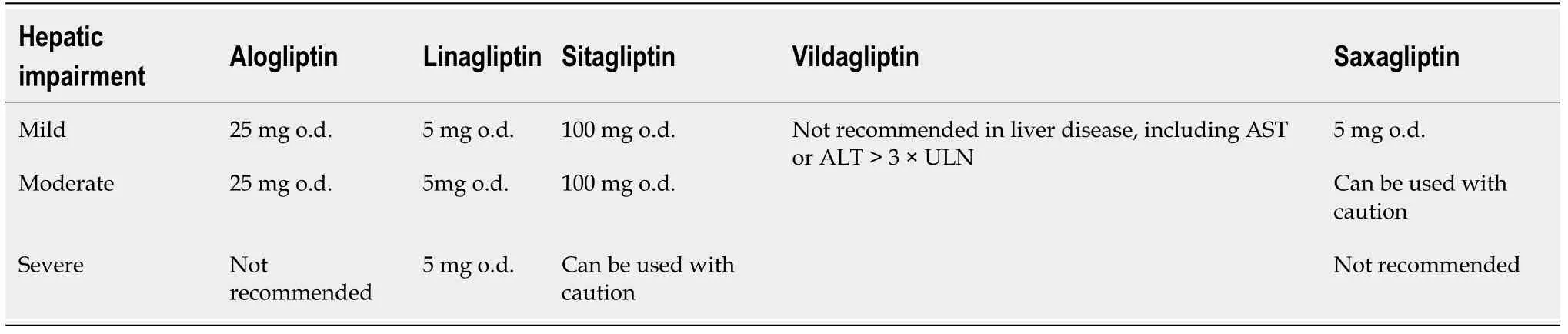
cytochrome P450(CYP450). Hence, patients co-administered saxagliptin and CYP3A4/5 inhibitors should reduce saxagliptin dose[38,41]. Table 3 summarizes the doses which are appropriate for all stages of hepatic impairment for each DPP-4 inhibitor.
DDP-4 INHIBITORS IN CVOTS
Since over 10 years ago concerns have been raised as to the CV safety of certain antidiabetic drugs[42]. Subsequently, the FDA requires evidence of CV safety before approval of any new antidiabetic agent. In this context, no drugs that could be associated with an unacceptable level of CV risk in clinical trials would be approved for the management of DM2. Incretin-based therapies, including DDP-4 inhibitors,were the newer antidiabetic agents added to the DM2 treatment armamentarium at the time of this statement[42].
The old man did his best to comfort her by repeating all he had said before, but she begged him afresh to tell her truly where his heart was and at last he told her
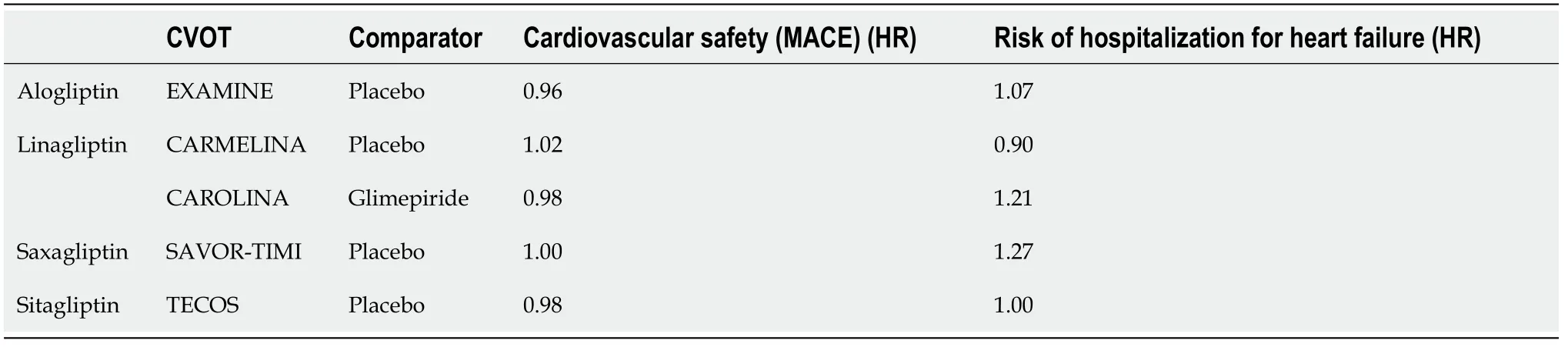
No safety signals were identified in the aforementioned clinical trials in the risk of acute pancreatitis or pancreatic cancer. These two clinical entities were regarded important safety issues until up to a few years ago, as there were several relevant reports and signals from clinical studies with these drugs[51]. However, a recent metaanalysis of randomized controlled trials demonstrated that the available data do not support an association of DPP-4 inhibitors with pancreatitis or pancreatic cancer. We should note that the evidence regarding pancreatic cancer is more limited and, thus,insufficient to draw definitive conclusions[52]. The excess of hospitalizations for HF associated with saxagliptin in the SAVOR-TIMI53 trial was not observed with the other DDP-4 inhibitors in CVOTs except a non-significant rise in the EXAMINE trial with alogliptin. In this context, regulatory authorities have added a warning in the labels of saxagliptin and alogliptin for the increased risk of HF[53]. The results of the VIVIDD study were reassuring as for the drug class. However, this matter should be investigated more in future longitudinal studies as the relatively short follow-up of these CVOTs may not be sufficient to detect a relevant safety signal.
The trial evaluating cardiovascular outcomes with Sitagliptin (TECOS) trial included 14671 patients with DM2 with an HbA1c between 6.5 and 8.0% when treated with stable doses of one or two oral agents (
, metformin, pioglitazone or sulfonylurea) or insulin (with or without metformin) and established CVD[43]. These patients were randomized to sitagliptin 50-100 mg/d
placebo on top of standard treatment. The primary endpoint of this study was the composite of CV death,nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina.This was a non-inferiority trial with upper safety boundary of 1.3 RR. During the 3 years of follow-up (median) sitagliptin was associated with mild though significant hypoglycemic effect; by lowering mean HbA1c by 0.29% points [95% confidenceinterval (CI): -0.32 to -0.27] compared with placebo. In the intention-to-treat analysis sitagliptin was non-inferior to placebo in the primary composite endpoint [hazard ratio (HR) 0.98; 95%CI: 0.88-1.09;
< 0.001 for non-inferiority]. The same was relevant for all secondary CV endpoints in this trial. Interestingly, acute pancreatitis or pancreatic cancer events did not differ significantly between the sitagliptin and the placebo group. Also, sitagliptin was not associated with any excessive risk of hospitalizations for HF compared with placebo[43].
Furthermore, no differences between groups were noted in overall deaths and in hospitalizations for HF. As expected, the incidence of hypoglycemic events was lower in the linagliptin than in the glimepiride group: incidence rate difference, -8.7 [95%CI: -9.4 to -8.0; HR, 0.23 (95%CI: 0.21-0.26);
< 0.001]. Also, more weight gain was noted in the glimepiride group, with a mean between group difference of -1.54 kg (95%CI: -1.80 to -1.28). However, no difference in fasting plasma glucose, lipids and blood pressure was noted between groups. The results of this study established the role of linagliptin as a non-inferior to sulfonylureas second-line option (after metformin) for the management of DM2[45].
Overall, the large-scale randomized placebo-controlled trials with DDP-4 inhibitors established their CV and overall safety for the management of high-risk patients with DM2. However, no evidence of superiority was demonstrated in CV outcomes as compared with controls or sulfonylurea treatment. To date, there are no published head-to-head comparison CVOTs between DDP-4 inhibitors and antidiabetic drugs with established CV efficacy such as SGLT2-i or GLP1-RA. Overall, the modest hypoglycemic effects alongside the neutral effect of DDP-4 inhibitors on the lipid profile, blood pressure and body weight make DDP-4 inhibitors less promising for CVD prevention compared with the SGLT2-i and GLP-1 RA[49]. Indeed, in a network meta-analysis (236 trials; 176,310 patients) the use of SGLT2-i or GLP1-RA was associated with lower mortality compared with DPP-4 inhibitors or placebo or no treatment. Treatment with DPP-4 inhibitors was not associated with lower mortality compared with placebo or no treatment[50].
After 2.2 years (median) follow-up the overall difference in HbA1c over the full study duration was -0.36% (95%CI: -0.42% to -0.29% based on least-square means). The primary composite outcome occurred in 5.77/100 person-years
5.63/100 personyears in the linagliptin
placebo group respectively; absolute incidence rate difference was 0.13 (95%CI: -0.63 to 0.90 per 100 person-years) (HR = 1.02; 95%CI: 0.89-1.17;
< 0.001 for non-inferiority). Similar were the findings for the key secondary renal endpoint of composite of adjudication-confirmed ESRD, death due to renal failure, or a sustained decrease of at least 40% in eGFR from baseline. No difference in the total mortality rates was noted between groups, too. Similarly, no difference between groups was observed in the components of the key secondary renal endpoint except for progression of albuminuria which occurred less frequently in the linagliptin
the placebo group: 21.4/100 person-years
24.5/100 person-years respectively;absolute incidence rate difference, -3.18; 95%CI: -5.44 to -0.92) (HR = 0.86; 95%CI: 0.78-0.95;
= 0.003). Regarding safety, the incidence of pancreatitis episodes and pancreatic cancer was higher in the linagliptin compared with the placebo group though the number of cases was very limited in both groups to reach safe conclusions. No statistically significant different between groups was noted in hospitalizations for HF.
Then the whole thing dawned on the King, and groaning12 deeply he muttered to himself So this is what I did not know about, and the tears rolled down his cheeks
These findings consequently changed the guidelines for the management of hyperglycemia in patients with DM2[5]. Therefore, the role of drugs which were used as second line agents (after metformin) in the therapeutic algorithm has been adjusted.DPP-4 inhibitors fall into this category. In this paper, we discuss the characteristics and CVOTs of this class of drugs as well as their current role in the therapeutic armamentarium of DM2.
Will you be off at once? So he was frightened and went out; but he felt quite faint, and trembled and shook, and his knees and legs began to give way under him
Non-inferiority of alogliptin (6.25-25 mg/d adjusted according to eGFR)
placebo was evaluated in 5380 high-risk participants with DM2 of the Examination of Cardiovascular Outcomes with Alogliptin
Standard of Care (EXAMINE) study[46].These patients had a recent (within 15-90 d) hospitalization for an acute coronary syndrome and suboptimal glycemic control (HbA1c 6.5%-11.0% at screening or 7.0%-11.0% if the antidiabetic regimen included insulin). The primary endpoint was the composite of CV death or nonfatal myocardial infarction or stroke and the noninferiority margins were similar to the studies above. After 17.5 mo (median) alogliptin was associated with a mild though significant hypoglycemic effect compared with placebo; mean difference in HbA1c between groups -0.36% points (95%CI: -0.43 to -0.28;
< 0.001). No significant changes between groups were noted in body weight changes or changes in lipoprotein levels. At the end of follow-up the primary endpoint occurred in similar rates in both groups: 11.3%
11.8% in the alogliptin
placebo group, respectively (HR = 0.96; upper boundary of the one-sided repeated CI, 1.16;
<0.001 for non-inferiority;
= 0.32 for superiority). No difference between groups was noted in the individual components of this endpoint or in the overall or CV mortality.No safety signal regarding the risk of acute pancreatitis or pancreatic cancer was noted in this study. Changes in eGFR throughout the study were similar between groups.
Similar was the design of the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53(SAVOR-TIMI53) trial[47]. This was a phase 4 randomized placebo-controlled trial including 16492 patients with DM2 with suboptimal glycemic control (6.5%-12.0%)and high CV risk (in secondary prevention or in primary prevention with multiple CV risk factors). These patients were randomized to saxagliptin 2.5-5 mg/d (adjusted based on eGFR)
placebo for 2.1 years (median). The primary endpoint was the same as in the EXAMINE trial, whilst a secondary major composite endpoint of CV death,myocardial infarction, stroke, hospitalization for unstable angina, coronary revascularization, or HF was assessed too. Saxagliptin was associated with significantly reduced HbA1c compared with placebo throughout the study (difference by 0.2% points at the end of follow-up) and with more patients achieving glycemic targets. However, no significant difference between groups was noted either in the primary or in the secondary major endpoint at the end of follow-up: HR = 1.00; 95%CI: 0.89-1.12;
=0.99 for superiority;
< 0.001 for non-inferiority for the primary endpoint and HR =1.02; 95%CI: 0.94-1.11;
= 0.66 for the secondary endpoint. Interestingly, among the individual components of these endpoints saxagliptin was associated with an increased risk of hospitalization for HF compared with placebo (HR = 1.27; 95%CI:1.07-1.51;
= 0.007). As mentioned above no similar signal was identified with sitagliptin and linagliptin in the TECOS and CARMELINA trial, respectively.
This matter is of particular significance since worsening of HF has been associated with excessive mortality in patients with DM2. To further assess this question the Vildagliptin in Ventricular Dysfunction Diabetes (VIVIDD) trial included 254 patients with symptomatic HF [New York Heart Association (NYHA) class II and III] with reduced left ventricular ejection fraction (LVEF < 40%) and a HbA1c of 6.5%-10%[48].These patients were randomized to vildagliptin 50 mg twice daily
placebo for 52 wk. Vildagliptin was non-inferior to placebo in mean changes of LVEF assessed after ≥22 wk on treatment (adjusted mean change 4.95% ± 1.25%
4.33% ± 1.23% in the vildagliptin
placebo group, respectively). This was not accompanied by any differences between the 2 groups regarding other HF outcomes, including NYHA classification status and hospitalizations for HF.
Life is full of shit... lots of it. And there are many a times when you may feel stuck/bored and it seems that there s nothing left to hold your interests or anything that doesn t piss you off. Well, there is such a thing as true love. It s there, it s indescribable and few are blessed with it. We are one of the lucky couples.
However, vildagliptin was associated with significant increases in the end-diastolic LV volume as well as a non-significant trend to increased end-systolic one. The latter could be attributed to pre-treatment differences between groups in this regard.Namely, mean baseline end-diastolic volumes and brain natriuretic peptide were higher in the vildagliptin than in the placebo group. Hence, patients randomized to vildagliptin may have been more susceptible to such changes. However, the clinical relevance of this finding was uncertain and was not accompanied with any worse HF outcomes.
EDWARD is 14 years old, SAM is 12. Both visited our garden centre one day. After some talking, we knew each other’s name, age, nationality, living town and so on. We have became friends each other.
Linagliptin was evaluated in a non-inferiority multicenter randomized placebocontrolled clinical trial. The Cardiovascular And Renal Microvascular Outcome study with Linagliptin (CARMELINA) study included 6979 patients at high risk for CVD[established CVD and significant albuminuria; urine albumin creatinine ratio (UACR)> 200 mg/g] or renal disease [low estimated glomerular estimated glomerular filtration rate (eGFR) and micro- or macro-albuminuria] and suboptimal glycaemic control (baseline HbA1c 6.5%-10%)[44]. These patients were randomized to linagliptin 5 mg/d
placebo. The primary composite endpoint was the time to first occurrence of CV death or nonfatal myocardial infarction or stroke. The non-inferiority marginswere the same as in the TECOS trial.
SAFETY
Consequently, randomized placebo-controlled clinical trials were designed to assess the CV safety of DDP-4 inhibitors. These studies mostly included high-risk patients with DM2. They had a non-inferiority design since the research question to be addressed at the time was safety rather than additional CV benefits, which were demonstrated only later with SGLT2-i and GLP-1 RA. To date, every DDP-4 inihibitor available for clinical use has been assessed in at least one of these trials (Table 4).
Furthermore, as previously mentioned, this drug class does not increase the risk of hypoglycemia and is neutral in terms of weight gain, two issues important for patients with DM2, while other side effects are minor and reversible (
, gastrointestinal adverse effects, flu-like symptoms).
CURRENT USE OF DPP-4 INHIBITORS
DPP-4 inhibitors were the first therapeutic choice after metformin initiation only up to a few years ago as they improve glycemic control without producing hypoglycemia or weight gain[54]. However, the inability to show a beneficial effect in morbidity and mortality as well as the significant findings of the large-scale CVOTs of the newer antidiabetic agents (
, SGLT2-i and GLP-1 RA) have moved DPP-4 inhibitors lower in the algorithm of hyperglycemia management[5]. The above-mentioned change in the prescription of antidiabetic agents during the last years is reflected by the results of a recent study in Greece[55]. The percentage of patients treated with a DPP-4 inhibitor, a GLP-1 RA or a SGLT2-i in 2018 was 43.4%, 18.5% and 16.5%, respectively[55].
Miraculously4 he managed to reach his friend, hoisted5() him onto his shoulder, and brought him back to their company s trench. As the two of them tumbled in together to the bottom of the trench, the officer checked the wounded soldier, then looked kindly6 at his friend. I told you it wouldn t be worth it, he said. Your friend is dead, and you are mortally wounded.
Her child, her own child, which she had never loved, lay now buried in the sea, and might rise up, like a spectre, from the waters, and cry, Hold fast; carry meto consecrated ground!
However, previous studies reflect the large use of DPP-4 inhibitors as a second choice of antidiabetic agents almost a decade ago. A large epidemiology study in the United States in a cohort of patients aged 18 years to 100 years who were newly initiated on oral hypoglycemic monotherapy between January 1, 2006, and December 31, 2008, showed that the greatest relative change for the study period was observed for the DPP-4 inhibitors, increasing from 0.4% to 7.3% or 0.15% per month[56]. Of note,during the period that the study was conducted GLP-1 RA and SGLT2-i were not available and, therefore, were not included in the analysis. The same pattern was observed in a study in Germany in elderly patients with an initial diagnosis of DM2 between January 2011 and December 2015, where the use of DPP-4 inhibitors raised from 13.4% to 19.8% during the study period[57]. The results of the study showed that DPP-4 inhibitors might be preferred over other drugs due to the good safety profile in elderly patients with DM2. At this point we should mention that there is lack of evidence regarding the trends of prescription of DPP-4 inhibitors. Another rather important issue is that there are large differences in prescription patterns, suggesting that the screening and management of DM2 varies among different countries.
THE PLACE OF DPP-4 INHIBITORS IN THE THERAPEUTIC ALGORITHM OF HYPERGLYCEMIA
In general, DPP-4 inhibitors cause a clinically meaningful reduction in blood glucose,have a low risk of hypoglycemia and a neutral effect on body weight, while their safety profile is overall favorable. They are also easy to use, requiring no dose titration and can be taken at any time of day regardless of meal times. Furthermore, DPP-4 inhibitors exhibit non-glycemic favorable effects including reductions in systolic blood pressure, total cholesterol and triglycerides, as well as improvement in β-cell function[35]. For the above reasons, until recently, they were a safe choice for the up titration of antidiabetic therapy after metformin. However, the large CVOTs with the newest agents, namely GLP-1 RA and SGLT2-i, have changed the treatment algorithm as well as the selection of DPP-4 inhibitors as a second-line add-on therapy to metformin[5].
DPP-4 inhibitors still have a place in the treatment of certain patients, such as those who take many drugs due to longstanding DM2 and have multiple co-morbidities, as well as in those with renal impairment, where other anti-diabetic medications might be contraindicated. The frail elderly population may also benefit due to the low risk of hypoglycemia with DPP-4 inhibitors. Post-hoc analysis of the SAVOR-TIMI 53 data established the safety and efficacy of saxagliptin in the elderly[58], an observation that has been confirmed by other studies of DPP-4 inhibitors in this patient population[59,60]. We should stress that saxagliptin is contraindicated in patients with HF due to the increased risk of hospitalizations for HF associated with its use[47].
Patients with advanced renal failure have fewer options of glucose lowering agents and often resort to treatment with complicated insulin regimens facing their accompanying increased hypoglycemia risk. Linagliptin might be a good choice as initial therapy in a patient with CKD at risk for hypoglycemia, while other DPP-4 inhibitors might be used with proper dose adjustment in these patients[38,39]. More recently, renoprotection was suggested as another beneficial property of DPP-4 inhibitors[36], which may be of clinical importance as diabetic nephropathy is a major complication of DM. Experimental data suggest that the modulation of innate immunity and inflammation are probably involved in these kidney-protective effects.The degradation of DPP-4, which is known to be expressed on the cell membrane of many types of cells including immune cells, as well as of several chemokines and cytokines[36], the attenuation of oxidative stress, fibrosis and cellular apoptosis in the kidney[37] are plausible underlying mechanisms.
According to recent guidelines, in patients with DM2 and established atherosclerotic CVD a GLP-1 RA or an SGLT2-i with proven CV safety (
, it has label indication of reducing CVD events) should be preferably used. In patients with HF or CKD an SGLT2-i should be used due to the beneficial effects of these drugs in CVOTs, unless they are contraindicated (according to GFR levels); then a GLP-1 RA should be used[61].
And then one day, just like that, the nightmare is over. The war has ended. Those of us who are still alive are freed. I have lost everything that was precious to me, including my family. But I still have the memory of this girl, a memory I carry in my heart and gives me the will to go on as I move to America to start a new life.
When the therapeutic goals are not achieved with the previous antidiabetic agents, a combination with a DPP-4 inhibitor is recommended as a possible third-line therapy.The triple therapy of metformin with a DPP-4 and an SGLT2-i has a very low risk of hypoglycemia, leads to a further reduction in HbA1c, followed by weight loss and a reduction of blood pressure secondary to SGLT2-i administration[62-64]. Moreover,the dual effects of DPP4-i on α-cells and β-cells of the pancreas may combine well with the pancreatic islet-independent action of SGLT2-i.
DPP-4 inhibitors still remain a reasonable second-line add-on therapy to metformin,especially in individuals at high risk for hypoglycemia (
, elderly) or when an oral regimen is preferred. DPP-4 inhibitors can also be combined with insulin therapy. The combination of basal insulin with a DPP-4 inhibitor is a practical treatment option without the need for multiple injections and glucose self-measurements for the adjustments of insulin[61].
CONCLUSION
Despite the establishment of SGLT2-i and GLP-1 RA as a second-line therapy in current diabetes treatment algorithms, DPP-4 inhibitors still remain a useful tool for the management of patients with diabetes. Furthermore, the lack of evidence with SGLT2-i and GLP-1 RA in elderly patients with diabetes as well as the contraindication of SGLT2-i in patients with CKD grade 3A and lower, make DPP-4 inhibitors a safe choice in such populations. Concluding, DPP-4 inhibitors still appear to have a place in the management of patients with DM2 as a safe class of oral glucose lowering agents with great experience in their use.
1 Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L,Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9
edition.
2019; 157:107843 [PMID: 31518657 DOI: 10.1016/j.diabres.2019.107843]
2 World Health Organization. Diabetes. [cited 25 April 2021]. In: World Health Organization[Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
3 Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G,Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD.
2020; 41: 255-323 [PMID: 31497854 DOI:10.1093/eurheartj/ehz486]
4 Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials.
2011; 343: d6898 [PMID: 22115901 DOI:10.1136/bmj.d6898]
5 Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A,Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes(EASD).
2018; 41: 2669-2701 [PMID: 30291106 DOI: 10.2337/dci18-0033]
6 Caveney E, Turner JR. White paper. A review of FDA guidance: understanding the FDA guidance on assessing cardiovascular risks for new antidiabetic therapies. [cited 25 April 2021]. In: IQVIA[Internet]. Available from: http://www.quintiles.com/Library/white-papers/newfda-guidance-onantidiabetic-therapies
7 Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes.
2007; 356: 2457-2471 [PMID: 17517853 DOI:10.1056/NEJMoa072761]
8 U.S. Food and Drug Administration. Guidance for industry: diabetes mellitus-evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. [cited 25 April 2021]. In:U.S. Food and Drug Administration [Internet]. Available from: www.fda.gov/downloads/Drugs/Guida nceComplianceRegulatoryInformation/Guidances/ucm071627.pdf
9 European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. [cited 25 April 2021]. In: European Medicines Agency[Internet]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guide line/2012/06/WC500129256.pdf
10 Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin,Cardiovascular Outcomes, and Mortality in Type 2 Diabetes.
2015; 373: 2117-2128[PMID: 26378978 DOI: 10.1056/NEJMoa1504720]
11 Butler J, Usman MS, Khan MS, Greene SJ, Friede T, Vaduganathan M, Filippatos G, Coats AJS,Anker SD. Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and metaanalysis.
2020; 7: 3298-3309 [PMID: 33586910 DOI: 10.1002/ehf2.13169]
12 Butler J, Zannad F, Fitchett D, Zinman B, Koitka-Weber A, von Eynatten M, Zwiener I, George J,Brueckmann M, Cheung AK, Wanner C. Empagliflozin Improves Kidney Outcomes in Patients With or Without Heart Failure.
2019; 12: e005875 [PMID: 31163986 DOI:10.1161/CIRCHEARTFAILURE.118.005875]
13 Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE,McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC;DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease.
2020; 383: 1436-1446 [PMID: 32970396 DOI: 10.1056/NEJMoa2024816]
14 Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R,Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C,Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy.
2019; 380: 2295-2306 [PMID: 30990260 DOI: 10.1056/NEJMoa1811744]
15 Bellastella G, Maiorino MI, Longo M, Scappaticcio L, Chiodini P, Esposito K, Giugliano D.Glucagon-Like Peptide-1 Receptor Agonists and Prevention of Stroke Systematic Review of Cardiovascular Outcome Trials With Meta-Analysis.
2020; 51: 666-669 [PMID: 31813360 DOI: 10.1161/STROKEAHA.119.027557]
16 Nathan DM. Finding new treatments for diabetes--how many, how fast... how good?
2007; 356: 437-440 [PMID: 17267901 DOI: 10.1056/NEJMp068294]
17 Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.
2008; 31: 173-175 [PMID: 18165348 DOI: 10.2337/dc08-9016]
18 Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes.
2003; 26:2929-2940 [PMID: 14514604 DOI: 10.2337/diacare.26.10.2929]
19 Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent)diabetic patients.
1993; 36: 741-744 [PMID: 8405741 DOI: 10.1007/BF00401145]
20 Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line.
1987; 84: 3434-3438 [PMID: 3033647 DOI: 10.1073/pnas.84.10.3434]
21 Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7-36)-amide.
1989; 38: 902-905 [PMID:2661287 DOI: 10.2337/diab.38.7.902]
22 Hare KJ, Vilsbøll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action.
2010; 59: 1765-1770 [PMID: 20150286 DOI: 10.2337/db09-1414]
23 Gutzwiller JP, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2.
1999; 276: R1541-R1544 [PMID: 10233049 DOI: 10.1152/ajpregu.1999.276.5.R1541]
24 Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D,Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans.
1999; 44: 81-86 [PMID: 9862830 DOI: 10.1136/gut.44.1.81]
25 Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes.
2003; 88: 2719-2725 [PMID: 12788879 DOI: 10.1210/jc.2003-030049]
26 Vilsbøll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus.
2004; 47:357-366 [PMID: 14968296 DOI: 10.1007/s00125-004-1342-6]
27 Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulindependent) diabetes.
1986; 29: 46-52 [PMID: 3514343 DOI: 10.1007/BF02427280]
28 Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects.
1967; 46: 1954-1962 [PMID: 6074000 DOI: 10.1172/JCI105685]
29 Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects.
1995; 44: 1126-1131 [PMID: 7657039 DOI:10.2337/diab.44.9.1126]
30 Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications.
2011; 124: S3-18 [PMID: 21194578 DOI: 10.1016/j.amjmed.2010.11.002]
31 Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes.
2004; 89: 2078-2084 [PMID: 15126524 DOI:10.1210/jc.2003-031907]
32 Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A,Keymeulen B, Lasseter KC, Kipnes MS, Snyder K, Hilliard D, Tanen M, Cilissen C, De Smet M, de Lepeleire I, Van Dyck K, Wang AQ, Zeng W, Davies MJ, Tanaka W, Holst JJ, Deacon CF,Gottesdiener KM, Wagner JA. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes.
2006; 91: 4612-4619 [PMID: 16912128 DOI:10.1210/jc.2006-1009]
33 Craddy P, Palin HJ, Johnson KI. Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison.
2014; 5: 1-41[PMID: 24664619 DOI: 10.1007/s13300-014-0061-3]
34 Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies: viewpoints on the way to consensus.
2009; 32 Suppl 2: S223-S231 [PMID: 19875556 DOI:10.2337/dc09-S315]
35 Kim NH, Yu T, Lee DH. The nonglycemic actions of dipeptidyl peptidase-4 inhibitors.
2014; 2014: 368703 [PMID: 25140306 DOI: 10.1155/2014/368703]
36 Kodera R, Shikata K. Renoprotective effects of incretin-based drugs: A novel pleiotropic effect of dipeptidyl peptidase-4 inhibitor.
2016; 7: 29-31 [PMID: 26816598 DOI:10.1111/jdi.12380]
37 Yang CT, Lin WH, Li LJ, Ou HT, Kuo S. Association of Renal and Cardiovascular Safety With DPP-4 Inhibitors vs. Sulfonylureas in Patients With Type 2 Diabetes and Advanced Chronic Kidney Disease.
2021; 110: 464-472 [PMID: 33866549 DOI: 10.1002/cpt.2262]
38 Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review.
2011; 13: 7-18 [PMID: 21114598 DOI:10.1111/j.1463-1326.2010.01306.x]
39 Forst T, Pfützner A. Linagliptin, a dipeptidyl peptidase-4 inhibitor with a unique pharmacological profile, and efficacy in a broad range of patients with type 2 diabetes.
2012; 13: 101-110 [PMID: 22149370 DOI: 10.1517/14656566.2012.642863]
40 Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors.
2010; 12:648-658 [PMID: 20590741 DOI: 10.1111/j.1463-1326.2010.01212.x]
41 Deacon CF, Knudsen LB, Madsen K, Wiberg FC, Jacobsen O, Holst JJ. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity.
1998; 41: 271-278 [PMID: 9541166 DOI: 10.1007/s001250050903]
42 Kalyani RR. Glucose-Lowering Drugs to Reduce Cardiovascular Risk in Type 2 Diabetes.
2021; 384: 1248-1260 [PMID: 33789013 DOI: 10.1056/NEJMcp2000280]
43 Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J,Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F,Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes.
2015; 373: 232-242 [PMID: 26052984 DOI:10.1056/NEJMoa1501352]
44 Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M,Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT,von Eynatten M, McGuire DK; CARMELINA Investigators. Effect of Linagliptin
Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial.
2019; 321: 69-79 [PMID: 30418475 DOI: 10.1001/jama.2018.18269]
45 Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, Keller A,Mattheus M, Baanstra D, Meinicke T, George JT, von Eynatten M, McGuire DK, Marx N;CAROLINA Investigators. Effect of Linagliptin
Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial.
2019; 322: 1155-1166 [PMID: 31536101 DOI: 10.1001/jama.2019.13772]
46 White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR,Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes.
2013; 369: 1327-1335[PMID: 23992602 DOI: 10.1056/NEJMoa1305889]
47 Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R,Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK,Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus.
2013; 369: 1317-1326 [PMID: 23992601 DOI: 10.1056/NEJMoa1307684]
48 McMurray JJV, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, Kothny W, Lewsey JD,Krum H; VIVIDD Trial Committees and Investigators. Effects of Vildagliptin on Ventricular Function in Patients With Type 2 Diabetes Mellitus and Heart Failure: A Randomized Placebo-Controlled Trial.
2018; 6: 8-17 [PMID: 29032139 DOI: 10.1016/j.jchf.2017.08.004]
49 Phrommintikul A, Wongcharoen W, Kumfu S, Jaiwongkam T, Gunaparn S, Chattipakorn S,Chattipakorn N. Effects of dapagliflozin
vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: a randomised study.
2019; 85: 1337-1347[PMID: 30767253 DOI: 10.1111/bcp.13903]
50 Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis.
2018; 319: 1580-1591 [PMID: 29677303 DOI:10.1001/jama.2018.3024]
51 DeVries JH, Rosenstock J. DPP-4 Inhibitor-Related Pancreatitis: Rare but Real!
2017;40: 161-163 [PMID: 28108536 DOI: 10.2337/dci16-0035]
52 Dicembrini I, Montereggi C, Nreu B, Mannucci E, Monami M. Pancreatitis and pancreatic cancer in patientes treated with Dipeptidyl Peptidase-4 inhibitors: An extensive and updated meta-analysis of randomized controlled trials.
2020; 159: 107981 [PMID: 31870827 DOI:10.1016/j.diabres.2019.107981]
53 U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin.[cited 25 April 2021]. In: U.S. Food and Drug Administration [Internet]. Available from:https://www.fda.gov/
54 Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A,Wender R, Matthews DR; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD).
2012; 35: 1364-1379 [PMID:22517736 DOI: 10.2337/dc12-0413]
55 Athanasakis K, Prodromiadou E, Papazafiropoulou A, Koutsovasilis A, Driva S, Ziori M,Georgopoulos E, Gougourelas D, Sotiropoulos A, Bousboulas S, Melidonis A, Liatis S. Twenty-year trends in the prescription costs of Type 2 diabetes: Real world data and empirical analysis in Greece.
2020; 162: 108095 [PMID: 32112790 DOI: 10.1016/j.diabres.2020.108095]
56 Liao EP. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications.
2012; 125: S1-S2 [PMID: 22998891 DOI: 10.1016/j.amjmed.2012.05.001]
57 Kostev K, Rockel T, Jacob L. Prescription Patterns and Disease Control in Type 2 Diabetes Mellitus Patients in Nursing Home and Home Care Settings: A Retrospective Analysis in Germany.
2018; 12: 136-139 [PMID: 28539088 DOI: 10.1177/1932296817710477]
58 Tatosian DA, Guo Y, Schaeffer AK, Gaibu N, Popa S, Stoch A, Langdon RB, Kauh EA. Dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes treated with saxagliptin, sitagliptin, or vildagliptin.
2013; 4: 431-442 [PMID: 24163113 DOI: 10.1007/s13300-013-0045-8]
59 Baranov O, Kahle M, Deacon CF, Holst JJ, Nauck MA. Feedback suppression of meal-induced glucagon-like peptide-1 (GLP-1) secretion mediated through elevations in intact GLP-1 caused by dipeptidyl peptidase-4 inhibition: a randomized, prospective comparison of sitagliptin and vildagliptin treatment.
2016; 18: 1100-1109 [PMID: 27300579 DOI: 10.1111/dom.12706]
60 Alsalim W, Göransson O, Tura A, Pacini G, Mari A, Ahrén B. Persistent whole day meal effects of three dipeptidyl peptidase-4 inhibitors on glycaemia and hormonal responses in metformin-treated type 2 diabetes.
2020; 22: 590-598 [PMID: 31789451 DOI:10.1111/dom.13934]
61 American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment:
.
2019; 42: S90-S102 [PMID: 30559235 DOI:10.2337/dc19-S009]
62 Del Prato S, Rosenstock J, Garcia-Sanchez R, Iqbal N, Hansen L, Johnsson E, Chen H, Mathieu C.Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: Post-hoc analysis of concomitant add-on
sequential add-on to metformin and of triple
dual therapy with metformin.
2018; 20: 1542-1546 [PMID: 29446523 DOI: 10.1111/dom.13258]
63 Gallwitz B. A safety evaluation of empagliflozin plus linagliptin for treating type 2 diabetes.
2017; 16: 1399-1405 [PMID: 28934557 DOI: 10.1080/14740338.2017.1382471]
64 Matthaei S, Catrinoiu D, Celiński A, Ekholm E, Cook W, Hirshberg B, Chen H, Iqbal N, Hansen L.Randomized, Double-Blind Trial of Triple Therapy With Saxagliptin Add-on to Dapagliflozin Plus Metformin in Patients With Type 2 Diabetes.
2015; 38: 2018-2024 [PMID: 26324329 DOI: 10.2337/dc15-0811]
