ARDS患者外周血及肺泡灌洗液中TGF-β/IL-17平衡变化及临床意义分析
2022-03-04李茜屠越兴朱蔚胡秀平江玲芝
李茜 屠越兴 朱蔚 胡秀平 江玲芝

[摘要] 目的 探討急性呼吸窘迫综合征(ARDS)患者外周血及肺泡灌洗液(BALF)中细胞因子及TGF-β/IL-17平衡变化并分析其临床意义。 方法 选择浙江省人民医院重症医学科2015年1月至2019年10月收治的50例ARDS患者(ARDS组)及30例急性心力衰竭患者(对照组)为研究对象,将ARDS组根据住院28 d结局分为生存组和死亡组。采用酶联免疫吸附法(ELISA)测定所有患者外周血及BALF中IL-6、IL-10、IL-17和TGF-β水平,比较ARDS组与对照组及不同结局ARDS患者间外周血及BALF中IL-6、IL-10、IL-17和TGF-β水平及TGF-β/IL-17差异。 结果 ARDS组外周血中IL-6、IL-10、IL-17、TGF-β水平均明显高于对照组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(P<0.001)。ARDS组BALF中IL-6、IL-10、IL-17、TGF-β水平均明显高于对照组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(P<0.001)。死亡组外周血中IL-6、IL-10、IL-17、TGF-β水平均明显高于生存组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(P<0.001)。死亡组BALF中IL-6、IL-10、IL-17、TGF-β水平均明显高于生存组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(P<0.001)。 结论 ARDS患者外周血及肺泡灌洗液中IL-17和TGF-β水平明显升高,TGF-β/IL-17水平明显下降,提示ARDS患者存在免疫失衡,有助于判断病情及调节免疫治疗。
[关键词] 急性呼吸窘迫综合征;IL-17;TGF-β;免疫失衡
[中图分类号] R563.9 [文献标识码] A [文章编号] 1673-9701(2022)02-0001-04
Analysis of the changes and clinical significance of TGF-β/IL-17 balance in peripheral blood and bronchoalveolar lavage fluid of ARDS patients
LI Qian1 TU Yuexing2 ZHU Wei1 HU Xiuping2 JIANG Lingzhi2
1.Department of Emergency Medicine, Zhejiang Provincial People′s Hospital, People′s Hospital of Hangzhou Medical College, Hangzhou 310014, China; 2.Department of Intensive Care Medicine, Zhejiang Provincial People′s Hospital, People′s Hospital of Hangzhou Medical College, Hangzhou 310014, China
[Abstract] Objective To investigate the changes of cytokines and TGF-β/IL-17 balance in peripheral blood and bronchoalveolar lavage fluid (BALF) of patients with acute respiratory distress syndrome (ARDS) and analyze their clinical significance. Methods A total of 50 patients with ARDS (ARDS group) and 30 patients with acute heart failure (control group) admitted to the Department of Intensive Care of Zhejiang Provincial People′s Hospital from January 2015 to October 2019 were selected as the study subjects. The ARDS group was divided into survival group and death group according to the 28-day outcome of hospitalization. Enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of IL-6, IL-10, IL-17 and TGF-β in peripheral blood and BALF of all patients. The levels of IL-6, IL-10, IL-17 and TGF-β and the difference of TGF-β/IL-17 in peripheral blood and BALF between the ARDS group and the control group and ARDS patients with different outcomes were compared. Results The levels of IL-6, IL-10, IL-17 and TGF-β in peripheral blood of the ARDS group were significantly higher than those of the control group, and TGF-β/IL-17 was significantly lower. The differences were statistically significant (P<0.001). The levels of IL-6, IL-10, IL-17, and TGF-β in BALF of the ARDS group were significantly higher than those of the control group, and TGF-β/IL-17 levels were significantly lower, and the differences were statistically significant (P<0.001). The levels of IL-6, IL-10, IL-17, and TGF-β in the peripheral blood of the death group were significantly higher than those of the survival group, and TGF-β/IL-17 levels were significantly lower, and the differences were statistically significant (P<0.001). The levels of IL-6, IL-10, IL-17, and TGF-β in BALF of the death group were significantly higher than those of the survival group, and TGF-β/IL-17 levels were significantly lower, and the differences were statistically significant (P<0.001). Conclusion The levels of IL-17 and TGF-β in peripheral blood and bronchoalveolar lavage fluid of ARDS patients are significantly increased, and the levels of TGF-β/IL-17 is significantly decreased, suggesting that ARDS patients have immune imbalance, which is helpful to determine the condition and regulate immunotherapy.
[Key words] Acute respiratory distress syndrome; IL-17; TGF-β; Immune imbalance
尽管近年来对急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)的研究日益增多,新的药物和呼吸支持技术也不断涌现,但ARDS的发病率和病死率仍居高不下,严重威胁人类健康。ARDS的病理生理基础是弥漫性的肺泡损伤及后继的纤维化,其本质是肺部炎症反应的失控[1]。ARDS过程中牵涉的全身炎症反应(systemic inflammatory response syndrome,SIRS)机制错综复杂,包括细胞因子、炎性介质的释放,中性粒细胞聚集在肺部释放活性氧、肺内皮细胞的凋亡、Toll样受体信号转导和抑制功能及T细胞介导的细胞免疫失衡等[2]。其中,T细胞介导的细胞免疫失衡导致的炎症反应在ARDS的发病中可能起着重要作用。而T细胞在功能上又分为辅助性T细胞(T help cell, Th)和调节性T细胞(regulatory T cell,Treg),这两类T细胞对免疫系统的调节作用相反,其分化过程相互抑制[3]。研究证实在稳态或没有炎症损伤的情况下,初始CD4+Th在转化生长因子-β(transforming growth factor-β,TGF-β)单独诱导下向Treg细胞方向分化,抑制效应细胞的增殖并维持免疫耐受;但在炎症或感染时,免疫体系激活,机体分泌白细胞介素(interleukin,IL)-6和TGF-β共同诱导初始CD4+Th分化为Th17细胞,参与炎症反应和产生自身免疫性疾病,同时抑制Treg细胞的功能,导致Treg/Th17平衡破坏[4]。TGF-β和IL-17分别由Treg和Th17通过不同的信号通路转化而来,并在外周血及肺泡灌洗液(bronchial alveolar lavage fluid,BALF)中可以被检测到[5]。目前国内外对于ARDS患者体内Treg/Th17变化研究較少,本研究通过测定ARDS患者外周血及BALF中TGF-β/IL-17平衡变化来间接反映Treg/Th17平衡变化并进一步分析其临床意义,现报道如下。
1 资料与方法
1.1 一般资料
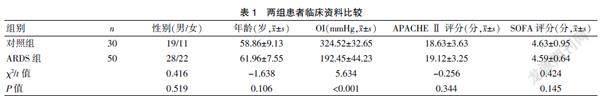
选择浙江省人民医院重症医学科2015年1月至2019年10月收治的50例ARDS患者(ARDS组)及30例急性心力衰竭患者(对照组)为研究对象。ARDS组患者均符合以下纳入标准:(1)ARDS的诊断符合2012柏林标准[6]:①急性起病,1周内新发的气促、呼吸窘迫等;②胸片或胸部CT出现双肺斑片状模糊影且不能用肺不张、肺结节及胸腔积液解释者;③超声心动图检测心脏排除心源性肺水肿;④氧合指数(OI=PaO2/FiO2,即动脉血氧分压/吸入氧浓度)下降,轻度:200 mmHg
1.2 方法
所有患者入院后均接受气管插管或切管切开接呼吸机辅助通气,并根据病情给予补液、抗感染、抑酸护胃、营养心肌、营养支持等治疗。分辨检测ARDS组患者自ARDS诊断成立日及对照组患者入院时的外周血及BALF中IL-6、IL-10、IL-17和TGF-β水平,比较ARDS组与对照组及不同结局的ARDS患者间外周血及BALF中IL-6、IL-10、IL-17和TGF-β水平及TGF-β/IL-17差异,分析其临床意义。其中IL-6、IL-10、IL-17和TGF-β水平的检测均采用酶联免疫吸附法(enzyme linked immunosorbent assay,ELISA)进行测定,试剂盒由北京百奥莱博科技有限公司提供。肺泡灌洗液的提取采用Olympus-P30纤维支气管镜,严格按照操作规范,每次灌洗50 ml,提取20 ml肺泡灌洗液,该操作由经验丰富的副主任医师进行。所有标本留取后及时送检。
1.3 统计学方法
采用SPSS 22.0统计学软件进行数据分析。计量资料以均数±标准差(x±s)表示,组间比较采用t检验;计数资料以[n(%)]表示,组间比较采用χ2检验,P<0.05为差异有统计学意义。
2 结果
2.1 两组外周血中IL-6、IL-10、IL-17、TGF-β水平及TGF-β/IL-17比较
ARDS组外周血中IL-6、IL-10、IL-17、TGF-β水平均明显高于对照组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(t=-3.643、-6.629、-6.683、-4.495、3.637,均P<0.001)。见表2。
2.2两组BALF中IL-6、IL-10、IL-17、TGF-β水平及TGF-β/IL-17比较
ARDS组BALF中IL-6、IL-10、IL-17、TGF-β水平均明显高于对照组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(t=-4.533、-6.324、-6.124、-4.363、3.363,均P<0.001)。见表3。
2.3 ARDS组不同预后结局患者血液中IL-6、IL-10、IL-17、TGF-β水平及TGF-β/IL-17比较
死亡组外周血中IL-6、IL-10、IL-17、TGF-β水平均明显高于生存组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(t=-5.334、-6.543、-7.224、-4.224、3.123,均P<0.001)。见表4。
2.4 ARDS组不同预后结局患者BALF中IL-6、IL-10、IL-17、TGF-β水平及TGF-β/IL-17比较
死亡组BALF中IL-6、IL-10、IL-17、TGF-β水平均明显高于生存组,且TGF-β/IL-17水平明显降低,差异均有统计学意义(t=-3.334、-7.522、-8.568、-4.475、5.223,均P<0.001)。见表5。
3 讨论
ARDS是由各种非心源性的肺内、外致病因素引起的临床急危重症,临床症状主要表现为急性进行性加重的呼吸困难和难治性低氧血症。众多研究已经证实ARDS是全身炎症反应(SIRS)和多脏器功能障碍(multiple organs dysfunction syndrome,MODS)的组成部分和其在肺部的表现,病程早期表现为ALI,而后期则动态演变为ARDS[7-8]。
近年来随着人们对ARDS的研究逐渐深入,越来越多的学者认识到免疫调节在ARDS的发生发展中扮演着重要角色。ARDS导致的SIRS牵涉众多细胞因子和炎症介质,其中T细胞介导的细胞免疫失衡导致的炎症反应在ARDS的发病中可能起着重要作用。机体内初始CD4+T辅助细胞受到外来抗原刺激后通过一系列信号通路增殖分化为各种效應T细胞来发挥生物学效应,如在IL-12诱导下分化为Th1细胞,产生干扰素(IFN)-γ,从而发挥抗感染及抗肿瘤作用;在IL-4诱导下分化为Th2细胞,分泌IL-4、IL-5和IL-13,介导机体的体液免疫和超敏反应并参与自身免疫性疾病的发病;在TGF-β的单独诱导下分化为Treg并分泌TGF-β,在维护机体免疫平衡中发挥重要作用[9-10]。另外,近年来又发现一种以分泌IL-17为主与炎症反应有关的Th17细胞[3]。
信号转导和转录激活因子3(STAT3)和维甲酸相关的孤独核受体γt(RORγt)是调节Th17细胞转录的主要因子,IL-6和TGF-β能够刺激诱导RORγt表达而上调IL-17表达[11-12]。Th17细胞通过IL-17、IL-22、IL-23、IL-6、TNF-α[13-15]等细胞因子发挥其介导炎症反应的功能。介导免疫耐受的Treg细胞和介导炎症反应的Th17细胞间功能和分化过程相互对抗,机体处于正常状态下两者保持平衡,一旦机体发生功能异常时常表现出Treg/Th17失衡,从而引起一系列炎症反应,损伤机体。研究发现在部分脓毒症休克患者的外周血中Treg的比例增加,并且在难以恢复的患者中持续存在,而且Treg相对数量的增加是由于循环中的效应细胞比例下降所造成的,其绝对数量并没有太大变化[16-17]。Li等[18]通过动物实验研究发现,ARDS大鼠体内Treg和Th17细胞都被激活而显著增加,但Th17增加更多,从而导致Treg/Th17比例的失衡。同时,ARDS大鼠体内细胞因子IL-6、TGF-β、IL-10和IL-17水平释放明显增多,推断ARDS大鼠体内存在着免疫失衡,并且这种免疫失衡可以被非选择性磷酸二酯酶(PDE)抑制药己酮可可碱(PTX)所阻断而得以纠正,并推断这种改变可能是由于环磷酸腺苷(cAMP)通路的激活抑制了转录因子Foxp3和RORγt的过度表达来实现的[19]。
脓毒症是导致ARDS的最重要病因,其实质都是全身炎症反应的演变。Mikacenic 等[20]研究发现,ARDS患者中,血清及肺泡分泌的IL-17水平升高与肺泡中性粒细胞百分比上升、肺泡通透性增加及器官功能障碍密切相关。本研究发现,ARDS患者外周血及BALF中IL-6、IL-10、IL-17、TGF-β水平明显升高,同时TGF-β/IL-17水平明显降低,与对照组患者比较,差异均有统计学意义(均P<0.001);进一步对ARDS患者根据不同预后进行比较,结果发现死亡组患者外周血及BALF中IL-6、IL-10、IL-17、TGF-β水平明显升高,同时TGF-β/IL-17水平明显降低,与对照组患者比较,差异均有统计学意义(均P<0.001)。由此可见ARDS时体内的IL-17及TGF-β水平均有明显升高,但是IL-17升高更为明显,因此间接反映体内Treg/Th17比例的TGF-β/IL-17也有明显降低,提示此时机体处于免疫失衡状态,而且这种免疫失衡在病情较重的患者中表现更为明显,对于预后也有一定的提示。此外,笔者还发现这些炎症因子在肺泡灌洗液中的浓度更高,同时检测肺泡灌洗液中的炎症因子对于提早发现和及时诊断ARDS也有一定的提示作用,与笔者前期的研究结果一致[18]。
综上所述,ARDS患者外周血及肺泡灌洗液中IL-17和TGF-β水平明显升高,TGF-β/IL-17平衡明显下降,存在免疫失衡,在蛋白质层面上阐明了ARDS中免疫淋巴细胞Treg和Th17及相关细胞因子的变化,提示可以使用一些干预手段来减轻这种免疫紊乱并恢复机体的免疫自稳态,避免过度炎症反应或免疫麻痹对机体的损害,从而可能发现针对性地治疗ARDS的新靶点,也可为临床新药开发提供新的思路。同时本研究尚存在着以下不足:①本研究样本量偏小,尚需更大规模的动物试验和临床研究来证实;②本研究仅在蛋白质层面上,未进一步在更深层次上揭示具体的信号通路及发生机制。
[參考文献]
[1] Huppert LA,Matthay MA,Ware LB. Pathogenesis of acute respiratory distress syndrome[J]. Semin Respir Crit Care Med,2019,40(1):31-39.
[2] Cardinal-Fernández P,Lorente JA,Ballén-Barragán A,et al. Acute respiratory distress syndrome and diffuse alveolar damage. new insights on a complex relationship[J].Ann Am Thorac Soc,2017,14(6):844-850.
[3] Brinkhoff A,Sieberichs A,Engler H,et al. Pro-infl ammatory Th1 and Th17 cells are suppressed during human experimental endotoxemia whereas anti-infl ammatory IL-10 producing t-cells are unaffected[J]. Front Immunol,2018,9:1133.
[4] Boomer JS,Green JM,Hotehkiss RS.The changing immune system in sepsis:Is individualized immuno-modulatory therapy the answer[J].Virulence,2014,5(1):45-56.
[5] Andreu Ballester JC,Tormo-Calandin C,Gareia-Ballesteros C,et a1.Association of gammadelta T cells with disease severity and mortality in septic patients[J].Clin Vaccine Immunol,2013,20(5):738-746.
[6] ARDS Definition Task Force. Acute respiratory distress syndrome:The berlin definition[J]. JAMA,2012,307(23):2526-2533.
[7] Delano MJ,Ward PA. The immune system′s role in sepsis progression,resoluton,and long-term outcome[J]. Immunol Rev,2016,274(1):330-353.
[8] Kwon MS,Woo SK,Kurland DB,et al.Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage[J]. Int J Mol Sci,2015,16(3):5028-5046.
[9] 马少林,邵蕾,刘畅,等.脓毒症淋巴细胞凋亡和内质网应激状态以及与预后关系的研究[J].中华危重病急救医学,2015,27(2):115-120.
[10] Gupta DL,Bhoi S,Mohan T,et al. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis[J]. Cytokine,2016,88:214-221.
[11] Guo J,Tao W,Tang D,et al. Th17/regulatory T cell imbalance in sepsis patients with multiple organ dysfunction syndrome:Attenuated by high-volume hemofi ltration[J]. Int J Artif Organs,2017,40(11):607-614.
[12] Hotchkiss RS,Monneret G,Payen D. Immunosuppression in sepsis:A novel understanding of the disorder and a new therapeutic approach[J]. Lancet Infect Dis,2013,13(3):260-268.
[13] Kolls JK,Linden A. Interleukin-17 family members and inflammation[J]. Immunity,2004,21(4):467-476.
[14] Zheng Y,Danilenko DM,Valdez P,et al. Interleukin-22,a T(H)17 cytokine mediates IL-23-induced dermal inflammation an d acanthosis[J]. Nature,2006,445(7128):648-651.
[15] Watowich SS,Jetten AM,Nurieva R,et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells[J]. Nature,2007,448(7152):480-483.
[16] 郝艳,郑洪亮.辛伐他汀对急性呼吸窘迫综合征的治疗效果研究[J].中国现代医生,2020,58(35):43-46.
[17] 叶连敏,潘景业.急性呼吸窘迫综合征患者的生物标志物与严重程度的相关性分析[J].中国现代医生,2020, 58(36):92-95.
[18] Li Q,Hu XP,Sun RH,et al. Resolution acute respiratory distress syndrome through reversing the imbalance of Treg/Th17 by targeting cAMP signaling pathway[J].Molecular Medicine Reports,2016,14(1):343-348.
[19] Van der Poll T,Van de Veerdonk FL,Scicluna BP,et al. The immunopathology of sepsis and potential therapeutic targets[J]. Nat Rev Immunol,2017,17(7):407-420.
[20] Mikacenic C,Hansen EE,Radella F,et al. Interleukin-17A is associated with alveolar inflammation and poor outcomes in acute respiratory distress syndrome[J].Crit Care Med,2016,44(3):496-502.
(收稿日期:2021-03-24)
