Synthesis and nematicidal activity of 4,5,5-trifluoro-N-(heteroaryl methyl) pent-4-enamide
2022-02-21LIUChengYANGHaipingZHANGRuifengLIZhongPeterMAIENFISCHXUXiaoyong
LIU Cheng, YANG Haiping, ZHANG Ruifeng, LI Zhong,Peter MAIENFISCH*,,2, XU Xiaoyong*,
(1. Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology,Shanghai 200237, China; 2. CreInSol MCB, CH-4118 Rodersdorf, Switzerland)
Abstract: Plant parasitic nematodes may cause severe damages to crops globally. In this study, fifteen novel 4,5,5-trifluoropent-4-enamide derivatives were designed and synthesized, and their nematicidal activities both in vitro and in vivo (in sand) were determined. Compounds with high activity in sand were further investigated for their in vivo activities in matrix. Results of the in vitro test showed that some of compounds exhibited better nematicidal activity. Among the synthesized molecules,compounds B8 containing a furan ring exhibited excellent nematicidal activity against Meloidogyne incognita and Bursaphelenchus xylophilus, with LC50/72 h values of 1.22 mg/L and 0.53 mg/L,respectively. Furthermore, most of the compounds showed 100% inhibition rate against M. incognita at 40 mg/L in sand in the in vivo test. Among which compound B10 containing a benzothiazole ring showed the best nematicidal activity. It exhibited 66.0% inhibition rate at 2.5 mg/L. Results of the in vivo test in matrix showed that compound B6 containing a thiophene ring was the most active compound. It showed 31.0% inhibition rate at 5 mg/L. Preliminary analysis on structure-activity relationship showed that the compounds containing non-substituted five-membered ring such as thiophene, furan and thiazole demonstrated better bioactivity than those compounds containing bulky six-membered ring or fused ring in the molecule.
Keywords: 4,5,5-trifluoropent-4-enamide; Meloidogyne incognita; Bursaphelenchus xylophilus;nematicidal activity
0 Introduction
Nematode infestation is one of the major stresses affecting crop production worldwide. Plant parasitic nematodes could survive with a wide range of hosts and may damage more than 3 000 crops[1]. The crops causing major economic losses include citrus, sugar beet, soybean, tomato, strawberry, cotton, and sugar cane, etc.[2]. The global economic losses reach approximately 173 billion US dollars each year[3].Among plant parasitic nematodes, root-knot nematodes and pine wood nematodes are more destructive than others. Root-knot nematodes feed on the roots of plants and cause the loss of nutrients in the plant roots, which may reduce the ability of plants to resist drought and adversity[4-5]. Pine wood nematodes could cause severe pine wood wilt, which has brought destructive harm to pine forests in North America,East Asia, and European countries[6]. Currently,nematode control is mainly achieved by synthetic fumigant and non-fumigant nematicides. Fumigant nematicides such as methyl bromide has been withdrawn from the market in many countries for causing the degradation of the ozone layer[7-9]. Nonfumigant organophosphates and carbamates nematicides, including fenamiphos[10], fosthiazate[11],carbofuran[12]and aldicarb, also quit the market gradually after the awakening of food safety and environmental protection awareness. More products with high efficacy, low toxicity, low-residual and good environmental compatibility are urgently needed.
Up to now, only six new nematicides products come out, including fluensulfone[13-15], tioxazafen[16-17],fluropyram[18-20], fluazaindolizine[21-22], cyclobutrifluram[23], and sanfushaxianzhi[24](Scheme 1). Although molecular design based on targets becomes a trend in the finding of pesticide lead compound, the target information of some new nematicides such as fluensulfone, tioxazafen, fluazaindolizine is unverified.Crystal structure of protein complex is unavailable,which cannot provide the template for homologous modeling, and makes it difficult to carry out molecular docking and virtual screen based on target. Thus,bioisosterism, fragment splicing, and scaffold hopping will still play an important role in the finding of nematicidal lead compound.
In our previous research, a series of amide derivatives containing the trifluorobutene moiety were designed and synthesized based on the structure of fluensulfone, and some compounds showed good nematicidal activitiy againstMeloidogyne incognita[25](Scheme 2).

Scheme 1 Six new nematicides products

Scheme 2 Some high nematicidal activity compounds found in our previous report
In some pesticides, methylene was often introduced into amide bonds to adjust the flexibility of chain and change the binding mode with the target,such as fluopimomide, beflubutamid-M, and pyribencarb.In nematicides, it was found that ethylene and cyclobutyl were connected the amide bond of fluopyram and cyclobutrifluram, respectively (Scheme 3).

Scheme 3 Some pesticides containing methylene, ethylene, cyclobutyl to connect amide bond
In this study, methylene was introduced into amide bond of the molecules which were prepared in our previous work to explore the change of nematicidal activity, and thus compounds B1-B15(Scheme 4) were designed and synthesized. Their nematicidal activities againstM. incognitaandBursaphelenchus xylophiluswere evaluated. The synthetic routes of intermediate a15 and compounds B1-B15 were shown in Scheme 5.

Scheme 4 Design of target compounds

Scheme 5 General synthetic route of intermediate a15 and target compounds B1-B15
1 Materials and methods
1.1 Chemicals and instruments
Melting point was determined by Büchi Melting Point B-540 instrument (Büchi Labortechnik AG, Flawil,Switzerland), uncorrected; NMR data were recorded by Brucker AM-400 nuclear magnetic resonance instrument (400 MHz), TMS was used as internal standard for1H and13C NMR, CFCl3was used as internal standard for19F NMR, CDCl3or DMSO-d6as solvent; chemical shifts were reported inδ, coupling constant (J) was presented in Hz. High resolution mass spectrometry (HRMS) was performed on a waters micromass liquid chromatography-time of flight (LC-TOF) spectrometer under electrospray ionization conditions. The reagents and solvents used in the experiment were analytical or chemical purity,and without further purification. All reagents and solvents were commercially available.
1.2 General synthetic procedure for compounds
1.2.1 Synthesis of 4,5,5-trifluoropent-4-enoyl chloride (2) To a 100 mL single-necked bottle was added 4,5,5-trifluoropent-4-enoic acid 1 (2 mmol) and dichloromethane (15 mL), and then oxalyl chloride(2.5 mmol) dissolve in dichloromethane (10 mL) was added dropwise into the reaction solution at 0 ℃.After addition of oxalyl chloride, dimethyl formamide(0.05 mL) was added and the mixture was stirred at room temperature for 3 h. The solvent was removed under reduced pressure to afford 4,5,5-trifluoropent-4-enoyl chloride (2) (Scheme 5).
1.2.2 Synthesis of (4-(p-tolyloxy) phenyl)methanamine (a15) To a 250 mL single-necked bottle was added 4-fluorobenzonitrile (20 mmol),pcresol (22 mmol), dimethyl formamide (80 mL) and cesium carbonate (40 mmol). The mixture was heated to 100 ℃ for 4 h. Flask was allowed to cool to room temperature, and then the reaction mixture was removed under reduced pressure. Saturated aqueous sodium bicarbonate (30 mL) was added to the residue and the resulting mixture was extracted with dichloromethane (20 mL × 3). The organic layers were combined and dried over anhydrous sodium sulfate.The solvent was removed under reduced pressure and the residue was purified by column chromatography to afford the 4-(p-tolyloxy) benzonitrile with the yield of 72%.
To a 100 mL single-necked bottle was added 4-(p-tolyloxy) benzonitrile (3 mmol) and dry tetrahydrofuran (15 mL). Then, LiAlH4(6 mmol) was added in batches (2 batches) under argon atmosphere at 0 ºC. After addition of LiAlH4, the solution was stirred at room temperature for 4 h. Then, ice water(0.3 g), NaOH solution at 1 mol/L (0.3 mL) and water(0.9 g) were slowly added to the reaction solution at 0 ºC. The solution was stirred for 10 minutes, and then the reaction mixture was filtered through celite.The celite was washed three times with ethyl acetate and all organic solvents were collected. Saturated sodium chloride solution (30 mL) was added to the organic layer, and extracted with ethyl acetate (30 mL ×3). The organic layers were combined and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and the residue was purified by column chromatography to afford (4-(p-tolyloxy)phenyl) methanamine (a15), which was directly used in the next step (Scheme 5).
1.2.3 General synthetic procedure for target compounds (B1-B15) To a 100 mL single-necked bottle was added amine a1-a15 (3 mmol), triethylamine (4.5 mmol) and dichloromethane (20 mL), and then 4,5,5-trifluoropent-4-enoyl chloride (2) (3 mmol)was dissolved in 10 mL dichloromethane and added dropwise into the reaction solution at room temperature.The solvent was removed under reduced pressure and saturated aqueous sodium bicarbonate (30 mL) was added to the residue, the mixture was extracted with dichloromethane (20 mL × 3). The organic layers were collected and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and the residue was purified by column chromatography to afford compounds B1-B15. All the compounds were synthesized according to this procedure (Scheme 5).
1.3 Compounds data
4,5,5-Trifluoro-N-(pyridin-3-ylmethyl)pent-4-enamide (B1):Yellow liquid, yield 51%.1H NMR (400 MHz, CDCl3),δ: 7.68(d,J= 3.2 Hz, 1H), 7.29 (d,J= 3.2 Hz, 1H), 6.91 (s, 1H), 4.74(d,J= 6.0 Hz, 2H), 2.78 − 2.56 (m, 2H), 2.48 (t,J= 7.4 Hz,2H).19F NMR (376 MHz, CDCl3, full coupled),δ: −104.00 −−104.65 (m, 1F), −122.62 − −123.51 (m, 1F), −175.31 −−176.13 (m, 1F).13C NMR (101 MHz, CDCl3),δ: 170.8,167.0, 153.0 (ddd,1JCF= 286.2, 274.0,2JCF= 46.7 Hz), 142.1,127.8 (ddd,1JCF= 233.8,2JCF= 53.2, 16.5 Hz), 119.9, 40.7,31.7, 21.7 (dd,2JCF= 21.8 Hz,3JCF= 2.3 Hz). HRMS (ESI)calcd. for C9H10F3N2OS (M + H)+, 251.0467, found,251.0468.
4,5,5-Trifluoro-N-(pyridin-3-ylmethyl)pent-4-enamide(B2): Yellow liquid, yield 72%.1H NMR (400 MHz,DMSO−d6),δ: 8.53 (t,J= 5.0 Hz, 1H), 8.50 – 8.39 (m, 2H),7.71 – 7.59 (m, 1H), 7.34 (dd,J= 7.6, 4.8 Hz, 1H), 4.30 (d,J=6.0 Hz, 2H), 2.66 – 2.52 (m, 2H), 2.42 (t,J= 7.2 Hz, 2H).19F NMR (376 MHz, DMSO−d6, full coupled),δ: −105.46 –−106.13 (m, 1F), −123.33 – −124.12 (m, 1F), −173.33 –−174.11 (m, 1F).13C NMR (101 MHz, DMSO−d6),δ: 170.3,152.5 (ddd,1JCF= 283.2, 272.6 Hz,2JCF= 47.8 Hz), 148.7,148.0, 135.0, 134.9, 128.8 (ddd,1JCF= 233.5 Hz,2JCF= 52.9,15.4 Hz), 123.4, 39.8, 30.6, 21.3 (dd,2JCF= 21.5 Hz,3JCF= 2.3 Hz). HRMS (ESI) calcd. for C11H12F3N2O (M + H)+, 245.0902,found, 245.0903.
4,5,5-Trifluoro-N-(pyrimidin-2-ylmethyl)pent-4-enamide(B3): Yellow liquid, yield 72%.1H NMR (400 MHz, DMSO−d6),δ: 8.75 (d,J= 4.9 Hz, 2H), 8.53 (d,J= 4.6 Hz, 1H), 7.38(t,J= 4.9 Hz, 1H), 4.47 (d,J= 6.0 Hz, 2H), 2.64 – 2.52 (m,2H), 2.45 (t,J= 7.0 Hz, 2H).19F NMR (376 MHz, DMSO−d6,full coupled),δ: −105.58 – −106.21 (m, 1F), −123.28 –−124.14 (m, 1F), −173.38 – −174.08 (m, 1F).13C NMR (101 MHz, DMSO−d6),δ: 170.3, 166.9, 157.3, 152.6 (ddd,1JCF=282.9, 272.4 Hz,2JCF= 47.8 Hz), 128.8 (ddd,1JCF= 233.3 Hz,2JCF= 52.9, 15.4 Hz), 119.7, 44.9, 30.6, 21.3 (dd,2JCF= 21.6 Hz,3JCF= 2.3 Hz). HRMS (ESI) calcd. for C10H10F3N3NaO(M + Na)+, 268.0673, found, 268.0676.
4,5,5-Trifluoro-N-(pyrazin-2-ylmethyl)pent-4-enamide(B4): Yellow liquid, yield 30%.1H NMR (400 MHz, CDCl3),δ: 8.59 (d,J= 6.8 Hz, 1H), 8.55 – 8.45 (m, 2H), 6.75 (s, 1H),4.60 (d,J= 5.2 Hz, 2H), 2.73 – 2.58 (m, 2H), 2.51 (t,J= 7.4 Hz, 2H).19F NMR (376 MHz, CDCl3, full coupled),δ: −104.03 –−104.62 (m, 1F), −122.73 – −123.52 (m, 1F), −175.26 –−176.10 (m, 1F).13C NMR (101 MHz, CDCl3),δ: 170.9, 153.0(ddd,1JCF= 286.2, 273.9 Hz,2JCF= 46.7 Hz), 152.34, 144.0,143.7, 143.5, 127.8 (ddd,1JCF= 233.8 Hz,2JCF= 53.2, 16.3 Hz),42.4, 31.8, 21.7 (dd,2JCF= 21.9 Hz,3JCF= 2.3 Hz). HRMS(ESI) calcd. for C10H10F3N3NaO (M + Na)+, 268.0673, found,268.0676.
4,5,5-Trifluoro-N-(2-(trifluoromethyl)benzyl)pent-4-enamide (B5): Colorless liquid, yield 29%,1H NMR (400 MHz, CDCl3),δ: 7.64 (d,J= 8.0 Hz, 1H), 7.56 – 7.48 (m, 2H),7.42 – 7.34 (m, 1H), 6.01 (s, 1H), 4.61 (d,J= 6.0 Hz, 2H),2.72 – 2.57 (m, 2H), 2.43 (t,J= 7.4 Hz, 2H).19F NMR (376 MHz, CDCl3, full coupled)δ:−59.43 (s, 3F), −104.11 –−104.76 (m, 1F), −122.70 – −123.55 (m, 1F), −175.35 –−176.18 (m, 1F).13C NMR (101 MHz, CDCl3),δ: 170.6, 153.0(ddd,1JCF= 284.4 and 272.3,2JCF= 46.4 Hz), 136.4 (q,3JCF=2.8 Hz), 130.6, 132.3, 128.1 (q,2JCF= 30.2 Hz), 127.8 (dd,1JCF=234.3,2JCF= 53.0, 16.4 Hz), 127.7, 126.0 (q,3JCF= 5.5 Hz),124.4 (q,1JCF= 272.0 Hz), 40.2, 31.9, 21.7 (dd,2JCF= 21.7,3JCF= 2.4 Hz). HRMS (ESI) calcd. for C13H12F6NO (M + H)+,312.0824, found, 312.0824.
4,5,5-Trifluoro-N-(thiophen-2-ylmethyl)pent-4-enamide(B6): Yellow liquid, yield 49%.1H NMR (400 MHz, DMSO−d6),δ: 8.57 (J= 5.2 Hz, 1H), 7.38 (dd,J= 4.4, 2.0 Hz, 1H),6.97 – 6.91 (m, 2H), 4.43 (d,J= 6.0 Hz, 2H), 2.65 – 2.52 (m,2H), 2.38 (t,J= 7.2 Hz, 2H).19F NMR (565 MHz, CDCl3,decoupled),δ: −104.2 (dd,J= 85.0, 32.2 Hz), −122.98 (dd,J=114.2, 86.1 Hz), −175.70 (dd,J= 113.0, 32.2 Hz).13C NMR(101 MHz, CDCl3)δ:170.5, 153.0 (ddd,1JCF= 286.1, 273.8 Hz,2JCF= 46.7 Hz), 140.7, 127.9 (ddd,1JCF= 235.3 Hz,2JCF=52.5, 16.7 Hz), 126.9, 126.0, 125.2, 38.3, 31.8, 21.7 (dd,2JCF=21.8 Hz,3JCF= 2.3 Hz). HRMS (ESI) calcd. for C10H10F3NNaOS(M + Na)+, 272.0332, found, 272.0334.
N-((1H-indol-3-yl)methyl)-4,5,5-trifluoropent-4-enamide(B7): Reddish brown liquid, yield 30%.1H NMR (400 MHz,CDCl3),δ: 8.36 (s, 1H), 7.59 (d,J= 8.0 Hz, 1H), 7.37 (d,J=8.4 Hz, 1H), 7.27 – 7.19 (m, 1H), 7.18 – 7.08 (m, 2H), 5.77 (s,1H), 4.60 (d,J= 5.2 Hz, 2H), 2.80 – 2.55 (m, 2H), 2.37 (t,J=7.6 Hz, 2H).19F NMR (376 MHz, CDCl3, full coupled),δ:−104.10 – −104.69 (m, 1F), −122.67 – −123.53 (m, 1F),−175.15 – −175.98 (m, 1F).13C NMR (101 MHz, CDCl3),δ:170.6, 153.0 (ddd,1JCF= 286.1, 273.8 Hz,2JCF= 46.8 Hz),136.5, 128.0 (ddd,1JCF= 234.1 Hz,2JCF= 53.3, 16.3 Hz),126.4, 123.4, 122.5, 119.9, 118.7, 112.2, 111.5, 35.3, 32.0,21.8 (dd,2JCF= 21.9 Hz,3JCF= 2.3 Hz). HRMS (ESI) calcd.for C14H13F3N2NaO (M + Na)+, 305.0877, found, 305.0879.
4,5,5-Trifluoro-N-(furan-2-ylmethyl)pent-4-enamide(B8): White solid, yield 44%, m.p. 56.0−57.3 ºC.1H NMR(400 MHz, CDCl3),δ: 7.35 – 7.33 (m, 1H), 6.31 (dd,J= 3.2, 2.0 Hz,1H), 6.25 – 6.18 (m, 1H), 5.99 (s, 1H), 4.43 (d,J= 5.6 Hz,2H), 2.74 – 2.57 (m, 2H), 2.43 (t,J= 7.4 Hz, 2H).19F NMR(565 MHz, CDCl3, decoupled)δ: −104.32 (dd,J= 85.0,32.2 Hz), −123.07 (dd,J= 114.1, 86.0 Hz), −175.72 (dd,J=114.3, 32.1 Hz).13C NMR (101 MHz, CDCl3),δ: 170.5, 153.0(ddd,1JCF= 286.1, 273.9 Hz,2JCF= 46.7 Hz), 151.0, 142.3,127.9 (ddd,1JCF= 233.9 Hz,2JCF= 53.3, 16.3 Hz), 110.5,107.5, 36.5, 31.8, 21.7 (dd,2JCF= 21.9 Hz,3JCF= 2.3 Hz).HRMS (ESI) calcd. for C10H10F3NNaO2(M + Na)+, 256.0561,found, 256.0563.
N-([1,1'-biphenyl]-4-ylmethyl)-4,5,5-trifluoropent-4-enamide (B9): White solid, yield 47%, m.p. 154.0−154.4 ºC.1H NMR (400 MHz, CDCl3),δ: 7.65 – 7.51 (m, 4H), 7.48 –7.41 (m, 2H), 7.40 – 7.30 (m, 3H), 5.83 (s, 1H), 4.49 (d,J=5.6 Hz, 2H), 2.79 – 2.61 (m, 2H), 2.47 (t,J= 7.4 Hz, 2H).19F NMR (376 MHz, CDCl3, full coupled),δ: −104.01 – −104.61(m, 1F), −122.55 – −123.42 (m, 1F), −175.25 – −176.08 (m,1F).13C NMR (101 MHz, DMSO−d6),δ: 170.1, 152.6 (ddd,1JCF= 283.3, 272.6 Hz,2JCF= 47.9 Hz), 139.9, 138.7, 138.6,128.9, 128.8 (ddd,1JCF= 233.5 Hz,2JCF= 52.9, 15.4 Hz),127.8, 127.3, 126.6, 126.5, 41.8, 30.7, 21.3 (dd,2JCF= 21.5 Hz,3JCF= 2.2 Hz). HRMS (ESI) calcd. for C18H16F3NNaO (M +Na)+, 342.1081, found, 342.1081.
N-(benzo[d]thiazol-2-ylmethyl)-4,5,5-trifluoropent-4-enamide (B10): Yellow solid, yield 85%, m.p. 73.0−74.2 ºC.1H NMR (400 MHz, CDCl3),δ: 7.96 (d,J= 8.0 Hz, 1H),7.90 – 7.80 (m, 1H), 7.54 – 7.43 (m, 1H), 7.43 – 7.34 (m, 1H),6.76 (s, 1H), 4.85 (d,J= 5.6 Hz, 2H), 2.81 – 2.62 (m, 2H), 2.53 (t,J=7.4 Hz, 2H).19F NMR (376 MHz, CDCl3, full coupled),δ:−103.76 – −104.45 (m, 1F), −122.49 – −123.35 (m, 1F),−175.30 – −176.08 (m, 1F).13C NMR (101 MHz, CDCl3),δ:171.0, 168.3, 153.0 (ddd,1JCF= 286.3, 274.0 Hz,2JCF= 46.6 Hz), 152.3, 135.0, 127.8 (ddd,1JCF= 233.7 Hz,2JCF= 53.1, 16.3 Hz),126.3, 125.4, 122.6, 121.8, 41.6, 31.7, 21.6 (dd,2JCF= 21.9 Hz,3JCF= 2.3 Hz). HRMS (ESI) calcd. for C13H12F3N2OS (M +H)+, 301.0623, found, 301.0623.
N-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)methyl)-4,5,5-trifluoropent-4-enamide (B11): Yellow liquid, yield 37%.1H NMR (400 MHz, CDCl3),δ: 8.72 (s, 1H), 7.95 (s, 1H), 7.05(s, 1H), 4.73 (d,J= 4.4 Hz, 2H), 2.80 – 2.64 (m, 2H), 2.59 (t,J= 7.2 Hz, 2H).19F NMR (376 MHz, CDCl3, full coupled),δ:−62.26 (s, 3F), −103.98 – −104.66 (m, 1F), −122.60 – −123.47(m, 1F), −175.30 – −176.12 (m, 1F).13C NMR (101 MHz,CDCl3),δ: 170.3, 152.5 (dd,1JCF= 281.4 and 271.0,2JCF=47.7 Hz), 143.7 (q,3JCF= 2.8 Hz), 134.4 (q,3JCF= 3.6 Hz),130.0, 128.7 (dd,1JCF= 232.0,2JCF= 52.4, 15.4 Hz), 125.1 (q,2JCF= 32.9 Hz), 123.9 (q,1JCF= 271.2 Hz), 41.9, 30.5, 21.3(dd,2JCF= 21.5,3JCF= 2.4 Hz). HRMS (ESI) calcd. for C12H10ClF6N2O (M + H)+, 347.0387, found, 347.0387.
N-(2-(1H-pyrazol-1-yl)benzyl)-4,5,5-trifluoropent-4-enamide (B12): Yellow solid, yield 88%, m.p. 53.2−55.2 ºC.1H NMR (400 MHz, CDCl3),δ: 7.74 (dd,J= 14.0, 2.0 Hz,2H), 7.64 – 7.55 (m, 1H), 7.45 – 7.35 (m, 2H), 7.34 – 7.27 (m,1H), 7.11 (s, 1H), 6.50 (t,J= 2.2 Hz, 1H), 4.30 (d,J= 6.0 Hz,2H), 2.76 – 2.51 (m, 2H), 2.41 (t,J= 7.4 Hz, 2H).19F NMR(376 MHz, CDCl3, full coupled),δ: −104.37 – −104.99 (m,1F), −122.93 – −123.76 (m, 1F), − 175.09 – −175.87 (m, 1F).13C NMR (101 MHz, DMSO−d6),δ: 170.2, 152.5 (ddd,1JCF=283.3, 272.6 Hz,2JCF= 47.9 Hz), 140.3, 138.6, 133.9, 131.4,128.80 (ddd,1JCF= 233.5 Hz,2JCF= 52.9, 15.4 Hz), 128.2,128.1, 127.6, 125.5, 106.6, 38.4, 30.6, 21.3 (dd,2JCF= 21.5 Hz,3JCF= 2.2 Hz). HRMS (ESI) calcd. for C15H14F3N3NaO (M +Na)+, 332.0986, found, 332.0988.
4,5,5-Trifluoro-N-((1-methyl-1H-imidazol-2-yl)methyl)pent-4-enamide (B13): Light yellow solid, yield 65%, m.p.78.8−79.3 ºC.1H NMR (400 MHz, CDCl3),δ: 7.89 (s, 1H),6.90 (d,J= 1.2 Hz, 1H), 6.83 (d,J= 1.2 Hz, 1H), 4.46 (d,J=5.6 Hz, 2H), 3.69 (s, 3H), 2.75 – 2.57 (m, 2H), 2.48 (t,J= 7.4 Hz, 2H).19F NMR (376 MHz, CDCl3, full coupled),δ: −104.15 –−104.91 (m, 1F), −122.87 – −123.70 (m, 1F), −175.10 –−175.85 (m, 1F).13C NMR (101 MHz, DMSO−d6),δ: 169.8,152.5 (ddd,1JCF= 283.2, 272.5 Hz,2JCF= 47.8 Hz), 144.5,128.8 (ddd,1JCF= 233.5 Hz,2JCF= 52.9, 15.4 Hz), 126.3,121.8, 34.7, 32.2, 30.4, 21.2 (dd,2JCF= 21.5 Hz,3JCF= 2.2 Hz).HRMS (ESI) calcd. for C10H13F3N3O (M + H)+, 248.1011,found, 248.1010.
4,5,5-Trifluoro-N-((1-methyl-1H-pyrazol-4-yl)methyl)pent-4-enamide (B14): White solid, yield 40%, m.p. 62.9−63.6 ºC.1H NMR (400 MHz, CDCl3),δ: 7.36 (s, 1H), 7.31 (s,1H), 5.96 (s, 1H), 4.25 (d,J= 5.6 Hz, 2H), 3.83 (s, 3H), 2.73 –2.54 (m, 2H), 2.38 (t,J= 7.4 Hz, 2H).19F NMR (376 MHz,CDCl3, full coupled),δ: −104.20 – −104.82 (m, 1F), −122.75 –−123.63 (m, 1F), −175.28 – −176.08 (m, 1F).13C NMR (101 MHz, CDCl3),δ: 170.4, 153.0 (ddd,1JCF= 286.1, 273.8 Hz,2JCF= 46.8 Hz), 138.5, 129.4, 127.9 (ddd,1JCF= 233.8 Hz,2JCF= 53.2, 16.3 Hz), 118.5, 38.8, 34.0, 31.9, 21.8 (dd,2JCF=21.8 Hz,3JCF= 2.3 Hz). HRMS (ESI) calcd. for C10H13F3N3O(M + H)+, 248.1011, found, 248.1012.
4,5,5-Trifluoro-N-(4-(p-tolyloxy)benzyl)pent-4-enamide(B15): Light yellow solid, yield 48%, m.p. 102.0−103.7 ºC.1H NMR (400 MHz, CDCl3),δ: 7.21 (d,J= 8.0 Hz, 2H), 7.14(d,J= 8.0 Hz, 2H), 7.00 – 6.87 (m, 4H), 5.74 (s, 1H), 4.41 (d,J= 5.6 Hz, 2H), 2.81 – 2.58 (m, 2H), 2.44 (t,J= 7.4 Hz, 2H),2.34 (s, 3H).19F NMR (376 MHz, CDCl3, full coupled),δ:−104.02 – −104.71 (m, 1F), −122.59 – −123.50 (m, 1F),−175.28 – −176.06 (m, 1F).13C NMR (101 MHz, DMSO−d6),δ: 175.2, 161.2, 159.6, 157.8 (ddd,1JCF= 283.1, 272.6 Hz,2JCF= 47.9 Hz), 139.4, 137.7, 135.6, 134.1, 134.0 (ddd,1JCF=234.3 Hz,2JCF= 53.0, 15.7 Hz), 123.8, 123.3, 46.8, 35.9, 26.6(dd,2JCF= 21.5 Hz,3JCF= 2.2 Hz), 25.4. HRMS (ESI) calcd.for C19H19F3NO2(M + H)+, 350.1369, found, 350.1367.
1.4 Nematicidal activity
The second-stage juveniles (J2) ofM. incognitaused in all tests were cultured by Huzhou Modern Agricultural Biotechnology Innovation Center,Chinese Academy of Sciences, China.
1.4.1 Thein vitronematicidal activity The compound was initially dissolved in acetone to obtain solution with the concentration of 10000 mg/L, a certain amount of the above acetone solution was diluted to the determined concentration using distilled water containing surfactant. An equal volume of the suspensions of second-stage juveniles (J2) ofM.incognitaorB. xylophiluswas added to a 96-well plate to form the final concentration to be measured(all compounds have good solubility and the solution is clear and transparent). Each well contained approximately 50 nematodes; each treatment was repeated twice. The 96-well plate was placed in an incubator and incubated at room temperature. The survival of nematodes was observed and recorded after 24 h, 48 h, and 72 h, respectively. Nematodes were considered dead if their bodies were straight or they did not move when strongly prodded their bodies with a needle. The LC50values of tested compounds were calculated using the probit method. Fluensulfone was served as a positive control and the negative control was the solution above without the tested compound. The nematicidal corrected mortality was calculated according to formula (1)[25].

In this formula,Mis corrected mortality (%),M1is mortality of treatment (%),M2is mortality of negative control (%).
1.4.2 Thein vivonematicidal activity The test was carried out by the tube method, which was divided into two types of sand and matrix. The compound was initially dissolved in acetone to obtain 10000 mg/L solution, the solution was diluted to the tested concentration using distilled water containing surfactant (all compounds have good solubility and the solution is clear and transparent). The one-weekaged cucumber seedlings were replanted in sterilized sand (or matrix) in test tubes (one seedling per test tube, tube size: 20 mm × 250 mm), and the roots of each seedling were treated with 3 mL of test solution.Then approximately 2000 living J2 nematodes were inoculated into the rhizosphere sand of each host plant, fluensulfone was served as positive control, and the negative control group was prepared in the same way without compound to be tested. Distilled water without nematodes was served as blank control. Each treatment was repeated three times. All the test tubes were incubated at 25 ºC for 20 d, with 10 h in the daylight and 14 h in the dark per day. The number of root knots in each test tube were counted and recorded as a score. The inhibition on J2 ofM. incognitawas calculated according to the following formula (2).
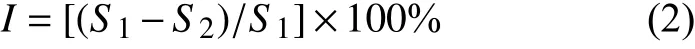
In this formula,Iis inhibition rate (%),S1is score of negative control andS2is score of treatment.
Scoring criteria: 0: 0–5 knots; 5: 6–10 knots; 10:11–20 knots; 20: more than 20 knots[25].
2 Result and discussion
2.1 In vitro nematicidal activity against M.incognita
According to the bioassay data of thein vitrotest in Table 1, the mortality of compound B1 againstM.incognitawas 87.0% after 72 h of treatment at the concentration of 40 mg/L. Then, the thiazole ring of compound B1 was replaced by different heterocycles to synthesize compounds B2-B15. Among the synthesized compounds, the mortalities of B6 and B8 againstM. incognitaat the concentration of 40 mg/L were 89.0% and 93.0%, respectively. In order to further explore the nematicidal activities of these three compounds, their LC50/72 hvalues were calculated.Among which compound B8 containing a furan ring exhibited the best nematicidal activity with LC50/72 hvalue of 1.22 mg/L (Table 1), but it was still inferior to that of compound A23[25].
2.2 In vivo nematicidal activity against M.incognita (sand)
First, the inhibitory activities of synthesized compounds againstM. incognitain sand at the concentration of 40 mg/L were tested (Table 2).Results showed that compounds B1-B4, B6-B8 and B11 had 100% inhibition rate againstM. incognitaat40 mg/L, while the inhibition rates of B10 and B14 were 97.3% and 92.5%, respectively. When the test concentration was reduced to 10 mg/L, compounds B1, B2, B4, B6, B8 and B10 could still maintain more than 50% root-knot inhibition rate. The inhibitory activities of these six compounds were further investigated by reducing the concentration, and it was found that compound B10 containing a benzothiazole ring exhibited 66.0% inhibition rate at 2.5 mg/L.
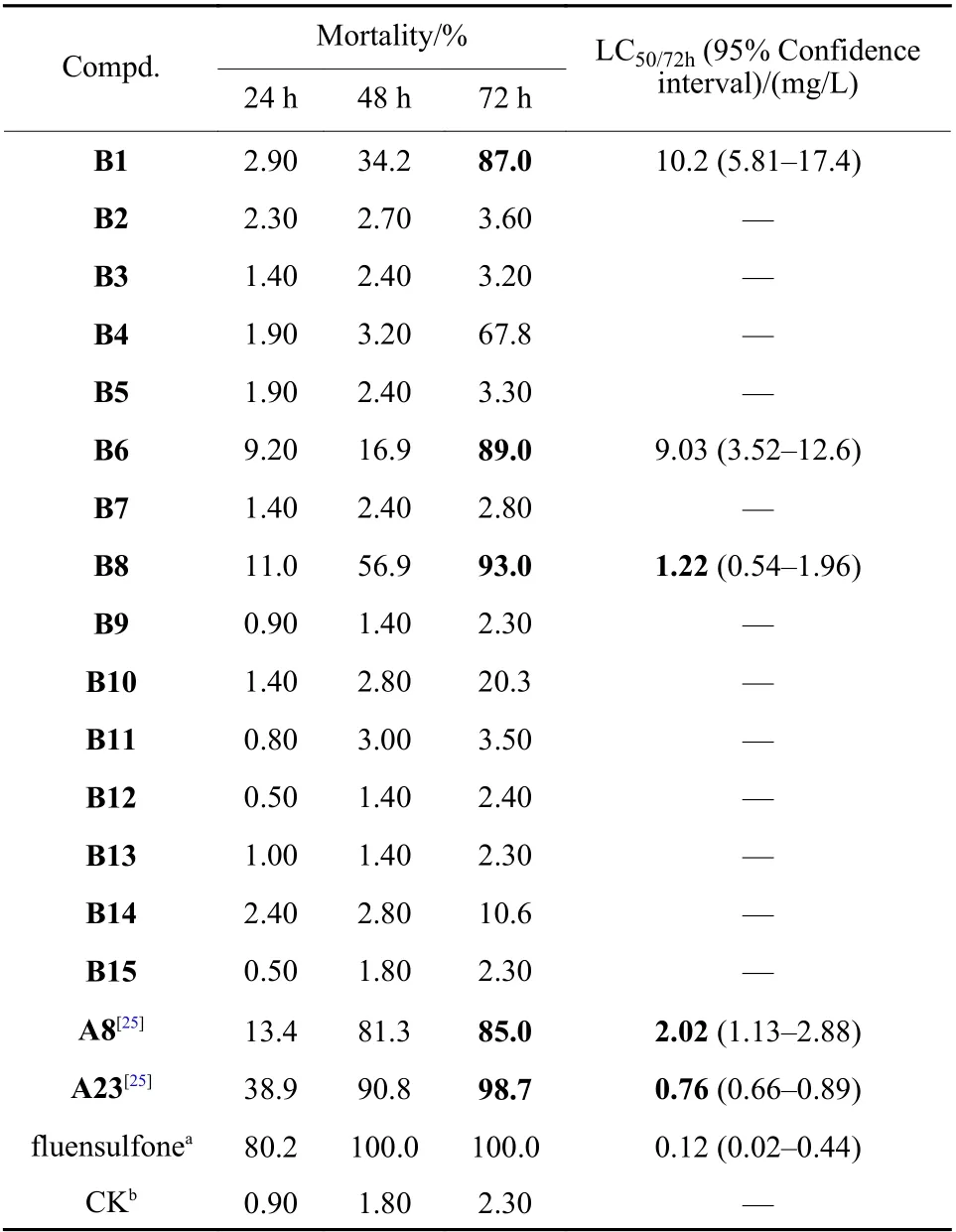
Table 1 In vitro nematicidal activity of the target compounds B1-B15, A8 and A23 against M. incognita (40 mg/L)
2.3 In vivo nematicidal activity against M.incognita (matrix)
Thein vivotest in matrix can simulate the real field environment of nematodes survival, which is important for the evaluation of the bioactivity and further modification of a nematicidal lead compound.Considering that compounds B1, B2, B4, B6, B8 and B10 showed good inhibitory activity at low concentration (10 mg/L) in sand, the activities of these six compounds againstM. incognitain matrix were further tested. It was found that compounds B1,B4, B6, B8 and B10 showed more than 50%inhibition rate againstM. incognitaat 40 mg/L (Table 3).When the concentration was reduced to 20 mg/L,compound B6 containing a thiophene ring and compound B8 bearing a furan ring still exhibited rather high bioactivities with more than 50%inhibition rate, while compound B10 bearing a benzothiazole ring had only 21.6% inhibition rate,which exhibited excellent activity in sand. It was speculated that this compound might easily be decomposed by the composition in the matrix. After further reducing the concentration, it was found that compound B6 containing a thiophene ring exhibited the best activity with 31.0% inhibition rate at 5 mg/L.But it was inferior to compound A8[25], which showed the inhibition rate of 56.2% at 5 mg/L. When the concentration was reduced to 2.5 mg/L, compound B6 showed 21.1% inhibition rate againstM. incognita,but the bioactivity of compound A8 totally disappeared. Preliminary analysis on structure-activity relationship showed that the non-substituted fivemembered ring such as thiophene, furan and thiazole was significant toin vivoactivity in matrix of compounds we prepared. The work of chain extension or cyclobutyl replacement is in progress.

Table 2 In vivo nematicidal activity of the target compounds B1-B15 against M. incognita in sand
2.4 In vitro nematicidal activity against B.xylophilus
Thein vitronematicidal activities of these fifteen compounds againstB. xylophiluswere determined again, and the results were shown in Table 4.Compounds B1, B6 and B8 showed better activityagainstB. xylophilusand the inhibition rates were 87.9%, 94.3% and 100.0% after 72 h of treatment at the concentration of 40 mg/L, respectively, which is similar to that againstM. incognita(Table 1). It was also found that fluensulfone exhibited no bioactivity againstB. xylophilus. It indicated that the mechanism of these three compounds was different from that of fluensulfone. In order to further analyze and compare the activities of B1, B6 and B8 againstB. xylophilus, LC50/72 hvalues of these three compounds were tested, and shown in Table 4. Of these threecompounds, compound B8 containing a furan ring exhibited the best activity againstB. xylophilus, with LC50/72 hof 0.53 mg/L, followed by compound B6 containing a thiophene ring (2.33 mg/L). Compound B1 containing a thiazole ring showed the worst nematicidal activity, with LC50/72 hof 14.7 mg/L.Since the bioactivity of compounds A8 and A23 againstB. xylophilushad not been tested[25], the comparison could not be carried out continuously.

Table 3 In vivo nematicidal activity of highly active compounds against M. incognita in matrix

Table 4 In vitro nematicidal activity of the target compounds B1-B15 against B. xylophilus (40 mg/L)
3 Conclusion
In this paper, based on the structural characteristics of commercial nematicide fluensulfone, 15 novel 4,5,5-trifluoropent-4-enamide derivatives B1-B15 were designed and synthesized by replacing the sulfone with methylene amide bond.
The biological activities of these compounds againstM. incognitaandB. xylophiluswere tested.The results showed that compounds B1, B6 and B8 exhibited excitingin vitroactivity againstM.incognitaandB. xylophilus, LC50/72 hvalues of compound B8 containing a furan ring were 1.22 mg/L and 0.53 mg/L, respectively. But the activity of compound B8 againstM. incognitawas still inferior to compound A23 (LC50/72 h, 0.76 mg/L) which was prepared in our previous work. In thein vivotest againstM. incognita, most of the compounds were showed more than 90% inhibition rate at 40 mg/L in sand, among which compound B10 containing a benzothiazole ring exhibited the best activity with 66.0% inhibition rate at 2.5 mg/L. However, in matrix, compound B6 containing a thiophene ring was the most active compound, and showed 50.4% and 31.0% inhibition rate at 10 mg/L and 5 mg/L, respectively. When the concentration was reduced to 2.5 mg/L, compound B6 still showed 21.1% inhibition rate againstM. incognita, which was better than that of compound A8. Preliminary analysis on structureactivity relationship showed that the compounds containing non-substituted five-membered ring such as thiophene, furan and thiazole demonstrated better bioactivity than those compounds containing bulky six-membered ring or fused ring in the molecule. This result will be benefit for further structural modification of novel nematicidal lead compound.
