Current status of liver transplantation for cholangiocarcinoma
2022-02-13PatrickTwohigThoetchaiBeePeeraphatditSandeepMukherjee
Patrick Twohig, Thoetchai Bee Peeraphatdit, Sandeep Mukherjee
Patrick Twohig, Thoetchai Bee Peeraphatdit, Department of Internal Medicine, Division of Gastroenterology and Transplant Hepatology, University of Nebraska Medical Center, Omaha, NE 68198, United States
Sandeep Mukherjee, Department of Internal Medicine, Division of Gastroenterology, Creighton University, Omaha, NE 68124, United States
Abstract Cholangiocarcinoma (CCA) is the second most common liver cancer with a median survival of 12-24 mo without treatment.It is further classified based on its location into intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA.Surgical resection is the mainstay of treatment, but up to 70% of these tumors are inoperable at the time of diagnosis.CCA was previously an absolute contraindication for liver transplantation (LT) due to poor outcomes primary due to early recurrent disease.However, improvement in patient selection criteria and neoadjuvant treatment protocols have improved outcomes for inoperable pCCA patients with recent studies reporting LT may improve survival in iCCA.Future advances in the treatment of CCA should include refining patient selection criteria and organ allocation for all subtypes of CCA, determining effective immunotherapies and the evolving role of personalized medicine in patients ineligible for surgical resection or LT.Our article reviews the current status of LT in CCA, along with future directions in managing patients with CCA.
Key Words: Intrahepatic cholangiocarcinoma; Perihilar cholangiocarcinoma; Liver transplantation; Immunotherapy; Chemotherapy; Transplant
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor that arises from the bile duct epithelium[1].It is further classified based on its location into intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA) with the Whipple procedure the treatment of choice for dCCA[2].In the past 20 years, liver transplantation (LT) has evolved to become the treatment of choice for carefully selected patients with unresectable pCCA[1].Since 2009, a standard model for end-stage liver disease (MELD) exception point is available for patients listed for LT for pCCA[3].In addition, a clinical trial is currently studying if LT is superior to surgical resection for “resectable” pCCA[4].For iCCA, a recent prospective study incorporating neoadjuvant chemotherapyvschemoradiation for selected patients with locally advanced iCCA followed by LT reported 5-year survival of 83%[5].This has increased interest in LT for iCCA and further studies are ongoing.The aim of this article is to review the current role of LT in the management of CCA, specifically pCCA and iCCA.
SURGICAL RESECTION
Surgical resection is the mainstay of CCA treatment.Predictors of poor outcomes are size, positive margins, multiple lesions, and nodal metastasis[1].However, resection is not always possible due to either large size or underlying cirrhosis and recurrence is common leaving LT as a possible option.
CCA is diagnosed with a dominant stricture on cholangiography and one or more of the following criteria positive cytology by endoscopic brushing or biopsy, fluorescence in situ hybridization polysomy, or elevated carbohydrate antigen 19-9 > 100 U/mL in the absence of cholangitis[1,6,7].iCCA is commonly diagnosed with magnetic resonance imaging or computed tomography which demonstrates peripheral rim arterial phase enhancement followed by centripetal hyperenhancement on venous/delayed phase[2,8].However, controversy exists surrounding the diagnosis of CCA given the frequency of incidentally found CCA that was suspected to be hepatocellular carcinoma (HCC) pre-operatively[8].Biopsy may be required to differentiate CCA from HCC, but this carries a risk of tumor seeding.
The treatment and prognosis of CCA is dependent on its location along the biliary tree and likelihood of being completely resected with negative margins[9-11].Surgical resection has been well-established as the standard treatment of CCA.Advances in surgical technique have improved outcomes in CCA patients over the past 20 years due to: (1) Extending the tumor resection to the hepatic parenchyma including caudate lobe, extended R-sided resection; (2) Extending tumor resection to the pancreatic head; (3) Performing vascular resections; (4) Performing lymphadenectomy to remove lymphatic pathways that may disseminate disease; and (5) Preoperative biliary drainage[1].With complete resection and negative margins, 5-year survival rates are approximately 40%[1].However, up to 70% of patients with hilar CCA are inoperable because of the extent of disease at presentation, therefore have a 5-year survival of 0%[2].
LT FOR PCCA
History of LT for pCCA
Historically, pCCA was a contraindication to LT.In the 1980s and early 1990s, LT was performed for pCCA in both Europe and the United States, but 5-year survival was 25%-30% with recurrence occurring in up to 60%[12].The Mayo Protocol for pCCA was subsequently developed in 1993 and is outlined in Figure 1.With a 55% 5-year survival with LT, this has become the standard of care for LT in pCCA[13].Downsides of this protocol were radiation-related injury which could affect surgery and the higher rates of vascular complications resulting in a greater need for vascular grafts[1].Despite these difficulties, refining surgical and neoadjuvant protocol techniques have led to better long-term outcomes with survival increasing to 65% at 5 years and 59% at 10 years[14-16].Since the development of the Mayo protocol in 1993, multicenter studies have validated this protocol and reported 5-year survival of 53%[16].In 2002, Sudanet al[17] reported their experience with a neoadjuvant treatment protocol — using brachytherapy and 5-fluorouracil prior to LT for pCCA, this single center study reported a 45% survival over a median follow-up of 7.5 years[17].Figure 2 illustrates the history of LT for pCCA.Subsequent studies have highlighted the improved overall survival (OS) of patients undergoing LTvssurgical resection, with age and comorbidity-matched patients having better outcomes with LT (3 and 5-year survival 72%vs33% and 64%vs18%, respectively)[18,19].
Despite the significant improvement in survival for pCCA with LT, disagreement exists regarding the need for neoadjuvant therapy.A retrospective study of 28 patients in the European Liver Transplant registry from 1990-2010 reported 5-year survival without neoadjuvant therapy was 59%, highlighting the importance of patient selection pre-transplant as opposed to universal neoadjuvant treatment[20].However, concern was raised about selection bias in this study.Multiple other studies have found poor outcomes in patients who do not receive neoadjuvant treatment[16].A recent multicenter prospective study found that patients with unresectable pCCA treated with neoadjuvant therapy and LT had superior 5-year survival (64%vs18%) than those patients treated with LT alone[18].These results remained significant when controlling for tumor size, nodal status, and presence of primary sclerosing cholangitis (PSC).
Negative surgical margins are critically important as the most common cause of death after LT in CCA patients is abdominal tumor recurrence[1].This is further enhanced by the need for immunosuppression after transplant[21-23].Additional research has identified risk factors for waitlist dropout and disease recurrence, which has helped validate current selection criteria as well as identify patients who would be good candidates for future investigational therapies.
Standard MELD exception point
The standard MELD exception point for pCCA is currently set at Median MELD at transplant (MMaT) minus 3 points[3].To qualify for standard MELD exception points, a patient must have unresectable disease due to either locally advanced tumor with extensive vascular and/or biliary invasion precluding complete resection, or poor hepatic functional reserve from underlying liver disease.It must be a single tumor < 3 cm in diameter with no evidence of intra- or extrahepatic metastasis and patients treated with neoadjuvant therapy at a center with an approved protocol.Further details on the MELD exception for CCA are found in Figure 3.Due to the increased risk of tumor seeding, it is important that transperitoneal aspiration or biopsy (i.e., endoscopic ultrasound-guided biopsy or percutaneous biopsy) of the primary tumor is not performed[24].Due to these limitations together with the long waitlist for LT, living donor liver transplant (LDLT) provides a timely opportunity for access to transplantation, which reduces the risk of waitlist morbidity and mortality[1,2].
The current protocol for pCCA treatment is external beam radiotherapy plus brachytherapy with a continuous infusion of 5-fluorouracil, followed by oral capecitabine until transplant (Figure 1).Other protocols have reported the use of stereotactic beam radiotherapy with gemcitabine plus cisplatin[25,26].However, there are no comparative studies between these different regimens.

Figure 1 Mayo clinic protocol for neoadjuvant chemoradiation and staging laparoscopy prior to liver transplantation.

Figure 2 History of liver transplantation in perihilar cholangiocarcinoma, including the development of the original transplantation protocols, United Network for Organ Sharing approval, and standard exception point for liver transplantation.
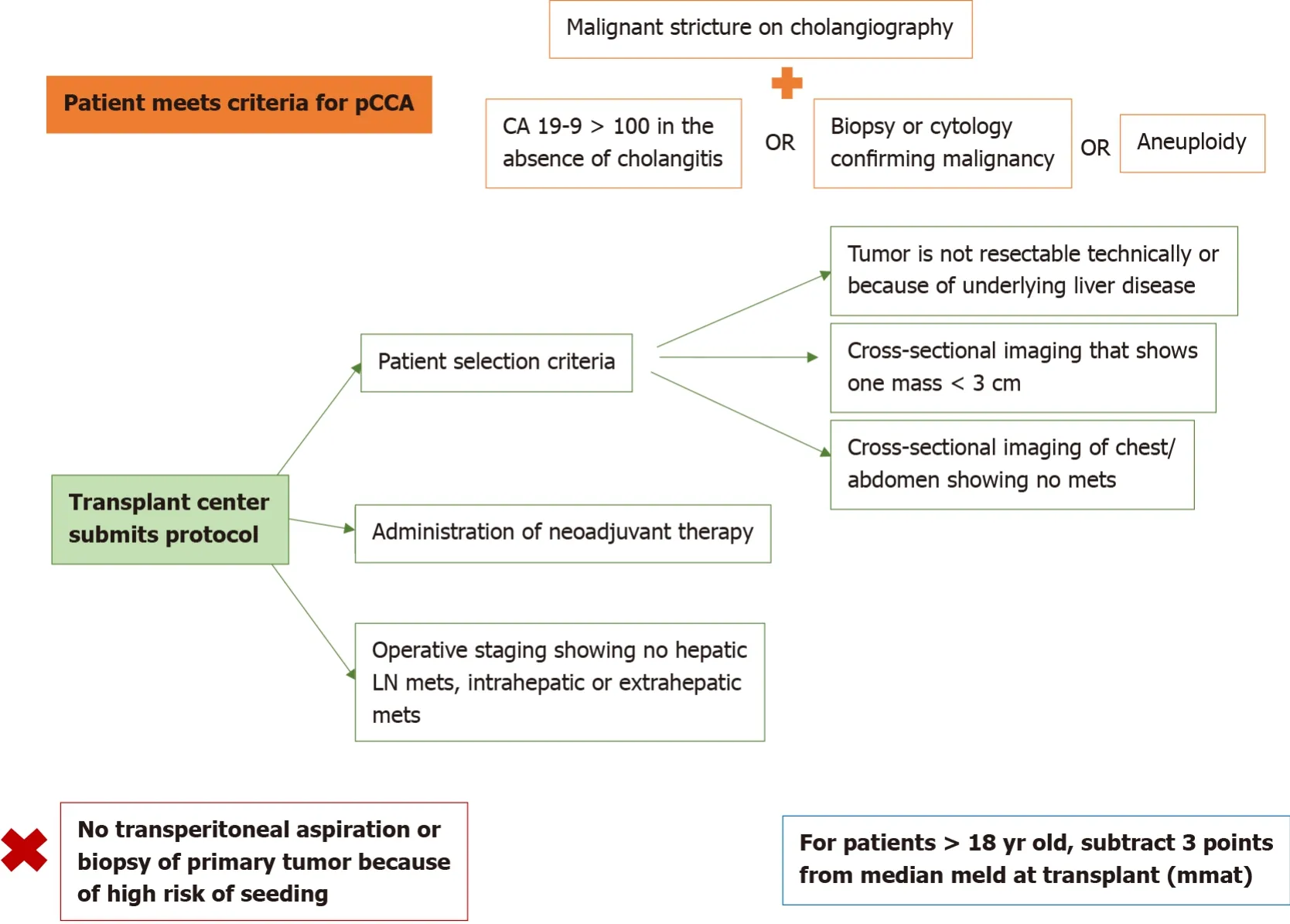
Figure 3 Model for end-stage liver disease exception point for perihilar cholangiocarcinoma, as developed by the United Network for Organ Sharing.
Future directions
A prospective multicenter randomized trial in France is currently comparing neoadjuvant therapy + LTvsliver and extrahepatic bile duct resection for “resectable” pCCA, with 5 year survival as the primary outcome[4].
LT FOR ICCA
Initial experience regarding LT for iCCA occurred in patient’s undergoing LT for suspected HCC which was subsequently diagnosed as iCCA after histologic evaluation of the explant[27].One- and five-year OS in iCCA patients compared to HCC was shown to be 63.6%vs90% and 63.6%vs70.3% in a retrospective study of 44 patients with iCCA on explant LT for HCC[27].A review of studies completed on LT in iCCA is reviewed in Table 1.
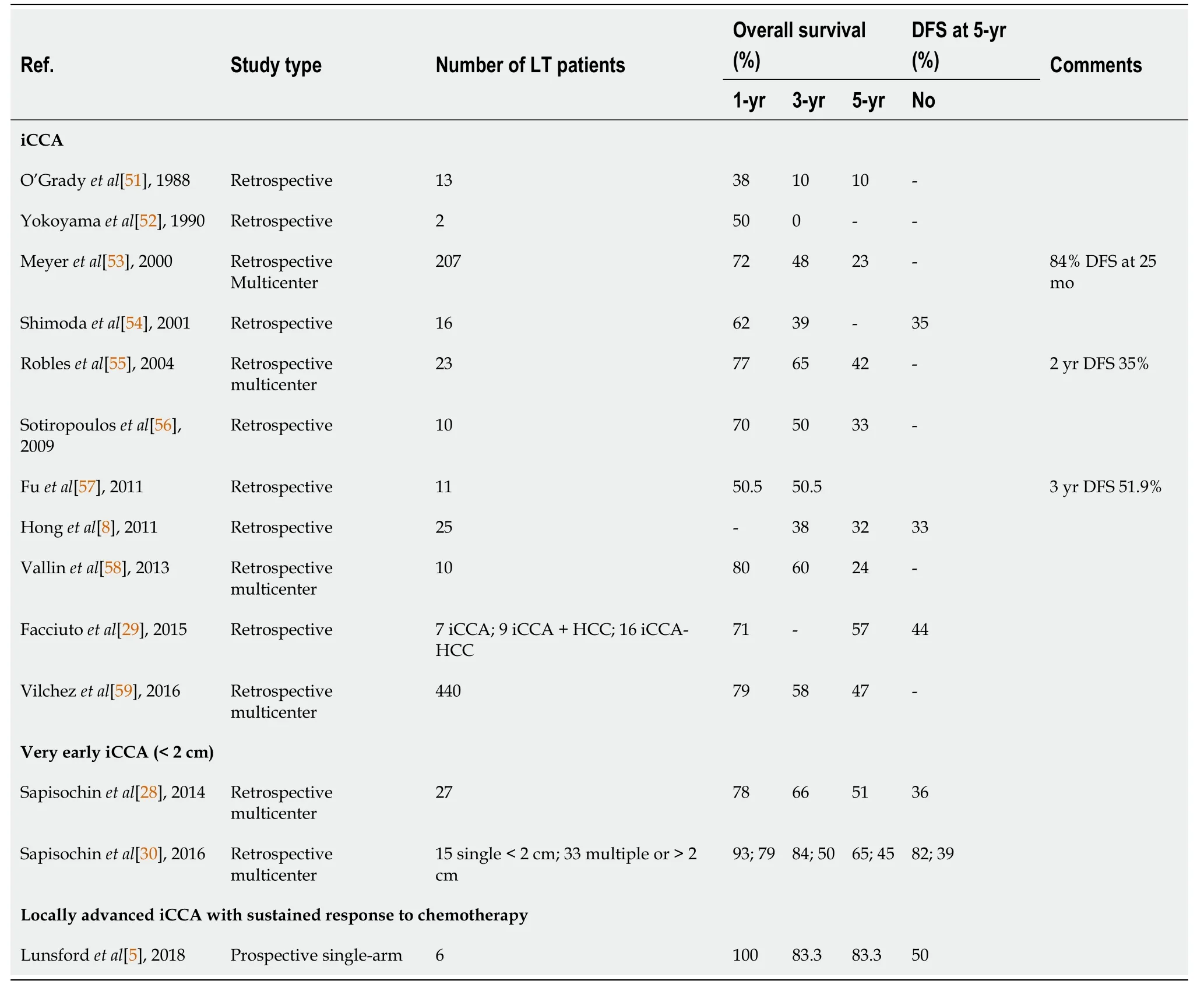
Table 1 Studies assessing patient survival and disease-free survival after receiving a liver transplant for intrahepatic cholangiocarcinoma
Very-early iCCA in cirrhosis
Although surgical resection is the ideal treatment for iCCA, up to 70% of iCCA is unresectable at diagnosis with a median survival of 12 mo even with chemoradiation[1,8].Historically, LT for iCCA carries a high risk of recurrence and thus has not been considered an indication for LT.
In 2014, a Spanish multi-center retrospective trial of 2301 patients undergoing LT for HCC found 8 patients had iCCA in the explant.These patients had a 73% 5-year survival[28].A single-center retrospective study of LT for HCC from New York of 32 patients found 7 patients had iCCA in the explant.OS of these patients was 57%[29].An international multi-center retrospective trial of 48 iCCA patients which included 15 patients with tumors < 2 cm and 32 patients with > 2 cm tumors reported that patients with < 2 cm tumors had a 65% 5-year survival, and the > 2 cm tumor group had a 45% 5-year survival[30].A multi-center retrospective French study of patients examined outcomes of LTvslocal resection for iCCA or iCCA-HCC for tumors < 2 cm and 2-5 cm.Better outcomes were found for LT in terms of OS and recurrence free survival[31].These studies have laid the foundation for a multi-center prospective trial in France which is assessing outcomes for LT in iCCA < 2 cm and 2-5 cm[32].
Locally advanced iCCA
A single center prospective case series analysis at Methodist Houston of 6 patients with large locally advanced unresectable iCCA were treated with neoadjuvant chemotherapy followed by LT[5].The average total tumor burden was 10 cm in size with 4 lesions.Outcomes were positive with 80% 3-year survival and 50% recurrence free survival[5].However, as this was only a small single center study, the investigators are developing a multi-center trial to determine if this may be a feasible treatment option for the future.
Similar to neoadjuvant and adjuvant protocols for pCCA, centers that have performed LT for iCCA have used regimens including fluorouracil or capecitabine combined with oxcaliplatin, leucovorin, and gemcitabine[8].
Risk factors for recurrent iCCA after LT
Patients with multifocal tumors, perineural invasion, infiltrative tumor subtypes, and a lack of neoadjuvant and adjuvant therapies have been associated with high risk of recurrence and poor outcomes after LT for iCCA[8].Interestingly, tumor size did not predict the risk of recurrence.
Risks for recurrent iCCA after surgical resection
Recurrence of iCCA has been shown to occur in approximately 66% of patients who undergo curative resection[33].Risk factors that increase the likelihood for recurrence include surgical margin < 10 mm, female sex, and presence of liver cirrhosis[33].
Currently, iCCA has no standard MELD exception.The options are to transplant based on calculated MELD score, or to use a LDLT.Although it is possible for a clinician to appeal to the National Liver Review Board (NLRB), there is no current policy or guidance regarding iCCA (unlike what exists for HCC or hCCA), which makes it challenging for NLRB to make decisions on allocation.
Future direction
Until iCCA has an established, suitable indication for MELD exception, surgical resection will remain the standard of care.However, retrospective data suggests patients with small iCCA (< 2 cm) may have good outcomes with LT.The role of neoadjuvant chemoradiotherapy and LT for iCCA > 2 cm in non-cirrhotic patients remains to be defined.
ALTERNATIVE TREATMENT STRATEGIES
Downsizing
Rayaret al[34] treated 45 patients with Yttrium-90 + chemotherapy and were able to downgrade 8 (18%) patients for resection.Given organ scarcity, using chemotherapy to downgrade to resection may be another option to LT[35].
Immunotherapy and personalized medicine
Historically, advanced, unresectable CCA has been treated with gemcitabine-based chemotherapy[1,26].Recent advances in oncology have focused on the identification of biomarkers and molecular profiles that may be used as novel targets for chemotherapy[36-38].In vitroandin vivostudies have suggested significant heterogeneity exists in biomarkers and molecular targets for CCA, especially iCCA[39].This is further influenced by genetic variation, as well as the etiology for iCCA (e.g., PSC, liver-fluke, viral hepatitis)[38].Treatments currently under evaluation include T-cells, antibodies, oncolytic viruses, cancer vaccines, and combinations of traditional chemotherapy with immunotherapy.These treatments are designed to target unique pathobiological pathways involved in CCA[40].For example, patients with fibroblast growth factor receptor (FGFR) mutations (seen in 30% of patients with iCCA) are diagnosed at a younger age but typically have a more indolent coursevsthose with Kirsten rat sarcoma (KRAS) and p53 mutations which are more aggressive with poorer prognosis[41-46].These genes are being evaluated as targets for future treatment to inhibit tumor growth[40,41,47,48].Chemotherapy and immune checkpoint inhibitors have synergistic effects, which may increase tumor cell destruction while also decreasing the dosage of chemotherapy needed which may improve side effect profiles[41].Radiotherapy is known to increase the sensitivity of the immune system to tumors, which in combination with immunotherapy has been efficacious for CCA.There are ongoing trials assessing the efficacy of immunotherapy, alone or in combination with chemotherapy to treat CCA.Additional promising tumor markers currently being evaluated for CCA include isocitrate dehydrogenase, programmed cell death protein 1, epidermal growth factor receptor, mechanistic target of rapamycin, mitogenactivated protein kinase and breast cancer pathways[41,49].The identification of novel therapeutic pathways for CCA would provide a promising paradigm shift in the treatment of patients who are not candidates for resection or LT[50].
CONCLUSION
CCA is becoming increasingly prevalent worldwide.Typically presenting at advanced stages that are inoperable, there has been a rapid evolution of treatments for unresectable CCA, including LT and new immunotherapies.Future research will evaluate the efficacy of novel pharmacotherapies in treating advanced CCA.Continuing to refine patient selection criteria for LT in CCA as well as optimizing neoadjuvant treatment regimens will be helpful.If LT is established as an acceptable therapy for iCCA, determining universal criteria for referral as well as organ allocation such as MELD exceptions will be crucial.Additionally, given the presence of iCCA in explanted livers suspected to be HCC, refining pre-transplant tumor staging and radiologic identification of iCCA will be helpful.
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Gastric per-oral endoscopic myotomy: Indications, technique, results and comparison with surgical approach
- Survival after curative pancreaticoduodenectomy for ampullary adenocarcinoma in a South American population: A retrospective cohort study
- Application value of mixed reality in hepatectomy for hepatocellular carcinoma
- Association of anastomotic leakage with long-term oncologic outcomes of patients with esophagogastric junction cancer
- Laparoscopic Kasai portoenterostomy can be a standard surgical procedure for treatment of biliary atresia
- Routine laboratory parameters in patients with necrotizing pancreatitis by the time of operative pancreatic debridement: Food for thought
