Advances in carbon materials for stable lithium metal batteries
2022-02-13JINChengbinSHIPengZHANGXueqiangHUANGJiaqi
JIN Cheng-bin, SHI Peng, ZHANG Xue-qiang, HUANG Jia-qi,
(1.Beijing Key Laboratory of Green Chemical Reaction Engineering and Institution, Department of Chemical Engineering,Tsinghua University, Beijing 100084, China;2.Advanced Research Institute of Multidisciplinary Science, Beijing Institute of Technology, Beijing 100084, China)
Abstract: Lithium (Li) metal is a promising anode material for next-generation high-energy-density batteries.However, the plating/stripping of Li metal is often accompanied by the formation of dendrites, which produce a short lifespan and safety hazards.To date, various approaches have been developed to suppress the dendrite growth and regulate the uniformity of the solid electrolyte interphase.Carbon materials that are lightweight, highly conductive, porous, and chemically and physically stable have been used for stabilizing the Li metal.This review summarizes the advances in carbon materials used as hosts, electrolyte additives, and coating layers in stabilizing Li metal batteries (LMBs).The advantages and limitations of various carbon materials are discussed in terms of their structural and chemical properties.Prospects for the development of carbon materials for improving LMBs are considered.
Key words: Lithium metal batteries;Solid electrolyte interphase;Li dendrites;Inactive Li;Carbon materials
1 Introduction
With the ongoing development of modern society, there has been a great desire for high-quality renewable energy, which directly promotes the boom of interest in energy conversion and storage techniques.Among them, lithium (Li)-ion batteries(LIBs) with long lifespan (thousands of cycles) and high energy content (specific energy of >200 Wh kgand energy density of >600 Wh Lat cell level) have been widely used in daily life and obtained remarkable milestone success.However, the intercalation mechanism that fundamentally enables the remarkably stable cycling of LIBs together decides their upper limit on energy density due to the low theoretical specific capacity of graphite anode (372 mAh g).Such a dilemma can be overcome by replacing the graphite anode with Li metal, which has a high specific capacity of 3 860 mAh gand an ultralow electrochemical potential of -3.04 V (versus the standard hydrogen electrode).The employment of Li metal anode can lead to a ~35% increase in specific energy and ~50% increase in energy density at a cell level.
Consequently, Li metal batteries (LMBs) emerge as promising energy storage devices, which have been on trial before the birth of LIBs by Exxon company.The historic failure of the practical applications of LMBs originates from several issues related to the ultrahigh reactivity of Li metal:(1) Dendritic Li growth.One such typical issue is the uncontrollable growth of mossy Li dendrites, which hold promise to puncture the cell and trigger safety hazards.(2) Inactive Li accumulation.Li dendrites with high tortuosity potentially promotes the incomplete stripping and the subsequent formation of inactive (‘dead’) Li, largely deteriorating the cycling performances and lifespans of LMBs.(3) Instable electrode/electrolyte interface.When in touch with liquid electrolytes, a passive layer that is the well-known solid electrolyte interphase (SEI) forms on the surface of Li metal at the expense of consuming active Li and electrolyte.And SEI produced with state-of-the-art battery chemistry often possesses unsatisfactory mechanical and chemical stability,which experiences repeated breakage/repair processes with the infinitely volumetric changes of a working hostless Li metal.This causes the continuous consumption of Li and electrolytes, further triggeringthe accumulation of inactive Li and accelerating the battery failure.
To resolve the above challenges to propel the advances of LMBs, numerous approaches have been engaged to suppress the formation of Li dendritesand stabilize SEI.Guided by the classical theoretical model of Sand’s equation, reducing the effective local current densities with electronically conductive porous hosts has been validated as an effective strategy to alleviate the growth of Li dendrites.Among various candidate host materials, carbon materials with excellent electronic conductivity, light weight and satisfactory mechanical properties have been considered as the promising hosts.Moreover, carbon materials can be easily produced and functionalized with mature existing techniques(, heteroatom doping, grafting, compositing) into varied morphologies and structures, which potentially exhibit extra multi-functions in addition to reducing the current density.On basis of retrieval on carbon materials for LMBs, it’s found that carbon has been extended as emerging battery materials for LMBs including carbon-based electrolyte additives and carbon coating layers, in addition to as carbon hosts.In detail, the local current density can be effectively reduced to suppress the formation of Li dendrites with the adoption of the porous carbon materials.And the Li plating/stripping can be well confined within the mechanically stable carbon materials to relieve the volumetric effect, which consequently mitigates the SEI fracture and Li dendrite puncture.Additionally, the carbon modified with functional groups or materials can further contribute to the regulation of SEI and Li deposition.Consequently, fruitful successes have been achieved with the adoption of various carbon materials (Fig.1), which largely promote the development of LMBs.
In this contribution, recent advances of various carbon materials for stabilizing Li metal anode are systematically summarized (Fig.1).Carbon materials for LMBs can be classified into interior/exterior/functional hosts, electrolyte additives, and coating layers according to their functions and components in a cell.Then, the advantages and limitations of each carbonbased battery materials are discussed in detail from structure to chemistry.In the final, the prospects on the future development and evaluation of carbon materials in practical LMBs are proposed.
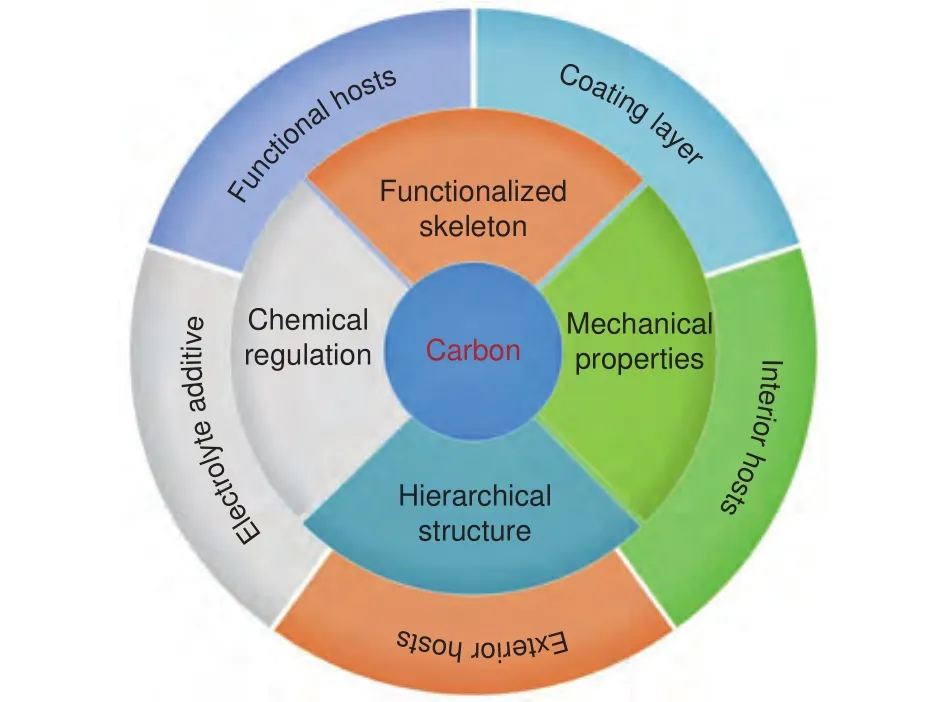
Fig.1 Various carbon products for LMBs with different designs on structure, surface chemistry, functionalization, and mechanical properties.
2 The functions of carbon materials in Li metal batteries
As discussed above, carbon materials with porous structure can hinder the Li dendrite growth by reducing the local current densities.And on basis of the dimensional position relation between carbon materials and Li, the carbon hosts for optimizing Li metal anode can be classified into interior and exterior carbon hosts, which are like the exoskeletons and endoskeletons in the living things.Typically, carbon materials including carbon fibers, layered graphene and carbon powders can generally be defined as the interior carbon hosts.These carbon hosts can be buried within the Li metal to adjust the internal stress and maintain the structure of Li metal anodes, meanwhile, providing excellent electronic network within the whole electrode.In contrast, carbon tubes, cages,spheres, and boxes with abundant internal voids for accommodating Li can be identified as exterior carbon hosts.In general, the exterior carbon hosts physically can accommodate the uniform deposition of Li and relieve the volumetric change of electrodes due to the large surface area and porous struc-ture of carbon.In addition to interior and exterior carbon hosts distinguished by the relative spatial distribution between Li and carbon, carbon hosts that are designed with multi-functions can also be regarded as one of the representative kinds of carbon hosts.These functional carbon hosts are generally assigned with specific surface chemistry (, heteroatom doping,functional group modification) and/or loading substances (, SEI forming agent, redox mediator).Consequently, they can be beneficial to chemically regulate the deposition uniformity of Li by enhancing the interaction between Li ions and functional atoms/groups, promoting the formation of stable SEI, and contributing to the reactivation of inactive Li.
In addition to carbon hosts, carbon can also be processed into other categories of battery materials by tuning the structures and chemistries.A simple solution to meet the above requirement is to migrate carbon materials from electrode to the electrolytes or interlayers between Li and separators, which extends the roles of carbon materials as potential electrolyte additives or coating layers.Carbon materials with porous structure and excellent adsorption ability benefit the loading of electrolyte additives, and facilitate the sustained release and long-lasting effect of additives to regulate the deposition of Li and formation of stable SEI.As to the carbon coating layers with superior mechanical strength, they can be attached either on the surface of Li metal or separators, which can suppress the dendrite puncture and electrode corrosion.With delicate design on the surface chemistry and electronic conductivity of carbon coating layers, they can even be used to regulate the growth direction of Li dendrites.
All in all, carbon materials with light weight,porous structures, high electronic conductivity, excellent chemical and physical stability show high potential to be applied in LMBs in different forms including carbon hosts, electrolyte additives and coating layers.Due to the mature processing techniques towards hierarchical structures and surface chemistry of carbon, more and more carbon-based battery materials can be designed and fabricated to further proceed the practical applications of LMBs.
3 Carbon hosts in Li metal batteries
3.1 Interior carbon hosts
Considering the reality that Li metal is hostless and suffers great volumetric effect, carbon materials with abundant 3D pore structures are favorable for accommodating the Li deposition on the porous surface and within the voids of layers.Moreover, the electronically conductive carbon network guarantees electronic contact throughout the whole electrode.Consequently, the optimized Li metal can deliver improved electrochemical performances during battery evaluation.
Carbon fiber is a widely used material, which can be easily tuned into freestanding fabrics and be directly employed as electrodes.And there have been numerous mature procedures for fabricating fiber materials (, electrospinning, chemical vapor deposition, regeneration of cellulose and protein).Moreover, by conducting treatments towards the precursors during the processing, carbon hosts can be equipped with hierarchically porous surface structures and doped with the electronic conductor, ionic conductor, and lithiophilic materials.Zhang.prepared a coralloid silver-coated carbon fiber-based composite Li anode (CF/Ag-Li) through Ag electroplating and molten Li infusion.Benefiting from the electroplating method, the coated Ag particles on CF exhibited uniform sizes and even distributions, which promoted the formation of a special coralloid morphology of the composite anode (Fig.2a).And during battery operation, this composite anode delivered a dendrite-free morphology and excellent electrochemical performances in both the sulfur and LiFePObased full cells.Considering that carbon itself is not an ideal lithiophilic material, the deposition of Li on pristine carbon is random and uncontrollable.Consequently, it is of great significance to introduce highly lithiophlic materials to continuously regulate the deposition of Li and facilitate the melting-infusion for constructing composite Li/carbon (Li/C) an-ode.Notably, lithiophilicity (materials that can form an alloy or solid solution with Li) is a widely used strategy for confining the deposition behavior of Li metal.The first employment of “lithiophilicity” was used to describe materials that have a low contact angle with liquid Li, alternatively an excellent wettability with liquid Li.With the ongoing researches on Li, the meaning of lithiophilicity has been largely extended (, solid solution, alloying).In another contribution, Hu’s group proposed a different method for harvesting Ag/CF host with high performances.A novel Joule heating method was engaged to fabricate Ag nanoparticles with an ultrafine size of 40 nm homogeneously dispersed on the surface of CF (Fig.2b).By directly visualizing the Li nucleation and growth processes within the Ag/CF(Fig.2c, d), it’s again verified that lithiophilic Ag nanoparticles can effectively reduce the nucleation overpotential and promote the unform deposition of Li within the network of CF.However, with the increasing of Li deposition capacity to a higher value, the function of such a lithiophilic interior carbon host needs to be revealed in future investigations.

Fig.2 (a) Schematic illustration showing the Li deposition within the Ag particle anchored CF[120].(b-d) SEM images of Ag particle anchored CF before and after Li nucleation/growth, respectively[119].(e, f) SEM images of pristine GT and GT after Li deposition, respectively[134].(g) SEM image of Li/rGO composite[124].(Reprinted with permission).
In addition to carbon fiber, various kinds of porous carbon (, activated carbon, MOF-derived carbon, biomass-derived carbon) with 3D hierarchical structures and high specific surface area (SSA) have also successfully been employed to stabilize Li metal anode.However, it should not be neglected that carbon materials with high SSA may contribute to extra exhaustion of active Li and electrolytes due to the formation of larger SEI.How to balance the positive (reduced local current density, relief of volumetric change) and negative effect (extra SEI formation)from high-SSA carbon materials remains to be uncovered in further studies.At present, there are limited researches paying attention to this important parameter.One such report was conducted by Jin.,where they fabricated a scaffold composed of covalently connected graphite nanotubes (GT).This firm and conductive carbon host with a moderate SSA(12 mg) can well accommodate the deposition of Li.As shown in Fig.2e and f, Li can be plated into the tube and on the surface of GT without the formation of Li dendrite.On basis of the analysis of the structural evolution during battery operation, this carbon host enabled the suppression of Li dendrite and reduction of volume change (9%).These enhancements of Li metal anode well indicate the significance of regulation on the microstructures of a host to construct a moderate electrolyte/electrode interface area.The carbon host with an appropriate SSA not only accommodates the Li deposition but also inhibits the extra exhaustion of active Li and electrolyte by SEI-forming reactions.
Besides, layered materials like graphene are other typical interior carbon hosts, since Li can be well stored within the layers of graphene.As Lin.indicated that the almost infinite relative dimensional change of Li during battery operation is one of the major hurdles before the practical application of Li metal anodes.By infusing Li into layered reduced graphene oxide (rGO), a composite anode was prepared (Fig.2g).Since the plating and stripping were well confined within the layers of rGO, the composite anode delivered obviously reduced dimensional variation of 20% and excellent mechanical properties during battery cycling.Of note, due to the limited content of rGO (7 wt%), the composite anode can still ex-hibit an ultrahigh capacity of 3 390 mAh g.With the adoption of such a layered interior carbon host, the optimized Li metal anode can maintain structural integrity during battery operation and deliver much improved electrochemical performance in both half- and full-cell configurations.Obviously, graphene materials show great potential in hosting Li, and the expansion and shrinkage of electrodes are well restrained along with the change of rGO layer spacings.Consequently, this graphene carbon host can well support and maintain the structural integrity of the whole electrode.However, the stress evolution within the composite anode during battery cycling remains unknown,which should be progressively revealed in future work with the assistance of mechanical characterization techniques and simulations.Additionally, there is a great challenge for the practical applications of such graphene hosts, since graphene presently can hardly be produced at a large scale with highly adjustable sizes and low cost.
3.2 Exterior carbon hosts
The network constructed by the exterior carbon host promises excellent electronic conductivity throughout the whole electrode.Meanwhile, exterior carbon hosts serving as armors can directly confine the growth of Li within them and shield the fresh Li away from the corrosion of electrolytes.Obviously, the difficulty lies in how to achieve the controllable growth of Li within the exterior carbon hosts,since both the interior surface and exterior surface of carbon are electronically conductive.
In terms of this consideration, lithiophilic materials are one of the most widely used strategies for enabling the selective deposition of Li within the carbon host.For instance, Cui and his group conducted the pioneering investigation, in which they systematically investigated the nucleation patterns and growth behaviors of Li on various substrates (Pt, Al, Mg, Zn,Ag, Au, Si, Sn, V, Ni, and Cu).And with the aid of binary phase diagrams, it’s found that no nucleation barrier exists on metals exhibiting a definite solubility in Li, while an appreciable nucleation barrier is needed for metals with negligible solubility.Among various metal substrates, Au can alloy with Li to form multiple LiAu phases.And Au also has a solubility zone in Li at a Li atomic ratio near 100% (,0.7 at.% Au in Li at 155C).This reality allows the surface Au to dissolve in Li before the formation of the pure Li phase (βLi), resulting in a solid solution surface layer.With the identical crystal structures of βLi, the solid solution surface layer serves as the buffer layer for the subsequent deposition of Li with the absence of a nucleation barrier.In sharp contrast, carbon materials have no solubility in Li, although they can alloy with Li.And a nucleation overpotential of 14 mV is exhibited.Consequently, there will be a nucleation priority on Au compared to carbon.On basis of these findings, Cui and colleagues designed a nanocapsule comprising hollow carbon host and Au nanoparticles (Fig.3a).And the plating and stripping behaviors of Li were validated to be well confined within the hollow carbon spheres (Fig.3b).Of note, this work provided underlying recognition of Li deposition behaviors, which largely promoted the booming of researches on “lithiophilic” materials for Li metal.And the lithiophlic materials described in this work have been extended and reported by many groups.For instance, Tao and his team used natural wood as a template to harvest an exterior carbon host with aligned channel structures (Fig.3c), which was further modified with lithiophilic nanoseeds of metal oxides (MgO, AlO, and ZnO).The vertical channels inherited from natural wood enabled a homogenous Li ion flux and the nucleation seeds promoted the dendrite-free Li deposition entrapped within the host channel.Due to the superior conductive surface, the Li metal anode/carbonized-wood delivered a high CE of 95% even at an ultra-large current density(15 mA cm).Under laboratory scale, the success of lithiophilic design has been repeatedly verified.However, its significance in real cells tested under a practical setting remains unknown.One of such questions is that whether the lithiliophlic materials still work when they are totally buried in deposited Li or covered by thick dead Li layer.
In addition to lithiophilic design, regulating thestructure and morphology of exterior carbon hosts can also help accommodate Li deposition.Graphite is a representative layered material, which is widely used as anode material in LIBs.Typically, graphite anode experiences repeated Li ions insertion and desertion processes during battery operation.The metallic Li deposition on the surface of graphite is undesired and often happens during the overcharge, fast, or low temperature charging processes, which will accelerate the growth of dendrites and short-circuit of battery.Surprisingly, Cui and his group originally investigated the Li deposition behavior of Li on graphite and revealed the electrochemical dependence of Li deposition on various types of graphite.They started the research to make graphite anodes safe and enabling the application of Li metal anodes.And they successfully found that Li can reversibly plate and strip within the internal space of massive artificial graphite (MAG)particles with the absence of dendrite growth (Fig.3d).In the next, graphite encapsulated Li metal hybrid anode had been produced with both the advantages of graphite (excellent cycling stability) and Li metal(high capacity).However, the composite anode in this work was designed with a relatively low capacity of 744 mAh gdue to the dominant content of graphite.On basis of their findings, the hybrid anode with the twice capacity of graphite can reversibly plate Li after fully lithiating graphite.This plating Li is well confined by the small and isolated space within the graphite particles to avoid the growth of large-sized dendrites.Since there already have been mature graphite producing and processing techniques, this kind of hybrid anode is of great potential to be practically used.And this work initiates a novel solution to optimize Li metal by tuning the Li storage mechanisms (conversion and inserting here).The obtained hybrid anodes hold promise to exhibit the synergetic advantages of both materials at an acceptable expense (, reduc-tion of capacity).

Fig.3 (a) TEM image of hollow carbon with Au nanoparticles inside.(b) Schematic illustration of Li deposition within the hollow carbon with Au nanoparticles[135].(c) SEM image and elemental mappings of MgO modified carbonized-wood[95].(d) SEM images of MAG before and after Li deposition along with a schematic model showing the Li/MAG interaction[139].(e) SEM image of tubular fibers after Li deposition.Inset is the corresponding schematic diagram[138].(f) Schematic process of creep-enabled Li deposition/stripping in a MIEC matrix.(g) SEM image of carbonaceous MIEC tubules.(h) TEM image of the Li plating process in a single carbon tubule with lithiophilic ZnOx[137].(Reprinted with permission).
Tubular carbon hosts are easily considered when seeking appropriate exterior carbon hosts.Considering that both the interior surface or exterior surface is conductive, there should be no rules for the selective deposition of Li within carbon tubes.And it may be puzzled to determine such hosts to be exterior or interior species.Interesting and unexpected findings were observed in several studies to figure out such puzzles.Liu.reported the harvest of a 3D freestanding host composed of hollow carbon fiber with a highly electroactive surface.By gradually plating Li into the host, the ex-situ scanning electron microscopy (SEM) revealed that both the interspaces among fibers and inside the tubular fibers were filled with Li metal (Fig.3e).This special mechanism of deposition behavior enabled the host to deliver high Li accommodation capacity and improved battery performance.Recently, Liu and his colleagues proposed a universal design principle for constructing tubular exterior carbon hosts of Li metal.They claimed that the exterior carbon host should be functionalized with mixed ionic and electronic conductors (MIEC) to facilitate the Li ion and electron transfer (Fig.3f).Additionally, the lithiophilic properties of hosts are also of great significance.In their study, the most impressive finding is that Li metal can reversibly plate in and strip out of the nanotubes by diffusional Coble creep along the MIEC/metal phase boundary (Fig.3g, h).And the proposed Coble creep mechanism can promise the effective relief of stress, desirable electronic and ionic contact, elimination of SEI debris, and controllable deposition/dissolution of Li along with a distance of several micrometers.This work undoubtedly provides a novel solution to the chemical and mechanical instability at the Li metal/electrolyte interphase.Future researches may be devoted to applying this strategy to real cells and evaluating its practical significance, which potentially drives the advances of LMBs.
All in all, interior and exterior carbon hosts with the introduction of hierarchically porous structures and/or lithiopholic materials can help accommodate the Li deposition and avoid the formation of Li dendrites.And numerous kinds of such carbon hosts have been reported in the literature.However, to convert those successes from laboratory scale to practical applications, several concerns must be well understood and solved: (1) The quantitative relation among surface area, pore structure (, shape and size), and electrochemical performance; (2) The strain and stress evolution among the whole anode electrode during battery operation; (3) The low-cost and large-scale production techniques of carbon hosts; (4) The evaluation and failure mechanism analysis of the carbon hosts under practical settings (high cycling capacity and current density); (5) The balance between the electrochemically inert components (, lithiophilic materials) and battery performances (, energy density).With the above concerns being well figured out, universal design principles for high-performance interior and exterior carbon hosts that can accommodate Li deposition, promote cycling performances, and guarantee the energy density of cells can be proposed.
3.3 Functional carbon hosts
In addition to accommodating the Li deposition and relieving the volumetric changes of the electrode,carbon hosts can be designed and processed to exhibit multi-functions.For instance, by regulating the surface chemistry of carbon with heteroatom doping and/or functional groups, the carbon hosts can contribute to the formation of stable SEI or further regulation of Li deposition by enhancing the interaction between Li ions and carbon.Additionally, since carbon is an ideal host material, functional materials that can serve as sustained-release additives can also be loaded.With such designs, a series of interesting and novel carbon hosts have been reported to shape the SEI and even achieve the reclaim of inactive Li.Researches on this aspect are limited at present, and it deserves more attention and effort, which may contribute to competitive candidate solutions towards the application of Li metal anode.
3.3.1 Surface chemistry
Since carbon materials are widely used in the fields like materials science and electrochemistry,there have developed numerous strategies for regulating surface chemistry to functionalize carbon.One of such typical strategies may be heteroatom doping.After that, the properties of carbon including its chemical and physical nature may be largely changed.For instance, the heteroatom dopants can exhibit extra lithiophilicity to enhance the interaction between Li ions and carbon, further controlling the Li deposition.To well understand the lithiophilic chemistry of heteroatom-doped carbon hosts (Fig.4a),a series of first-principles calculations and experiments were conducted by Chen.And three major parameters of electronegativity, local dipole, and charge transfer were proposed to explain the phenomena of lithiophilicity.The Li ion adsorption, Li bond formation (the interaction between O and Li atoms),and charge transfer processes during the deposition of Li were well understood.This work provided an underlying understanding of the lithiophilicity of heteroatom doped carbon and proposed the design criterion for a highly lithiophilic carbon host.
In addition to heteroatom doping, surface modification of carbon can also play a significant role in stabilizing Li metal anode.For instance, Xie.by using atomic layer deposition coated a layer of AlOon the surface of a hollow carbon host(AlO/HCS).This coating layer with low electronic conductivity can make the Li plating happen within the inner carbon walls (Fig.4b).Meanwhile, it can serve as a protecting layer/artificial SEI to prevent the parasitic reactions between active Li sources and electrolytes.Consequently, with the adoption of such a functional carbon host, the LMBs can operate for 500 cycles with high Coulombic efficiency of 99%.Besides, some researches were devoted to modifying the surface chemistry of carbon by the introduction of functional groups (, ―NH, ―COO― ).For instance, Jin.reported the fabrication of a rice husk-derived carbon with abundant fluorine (F)-containing groups on the surface.With the aid of X-ray photoelectron spectroscopy (XPS), it’s found that the F-containing group in the skeleton of carbon resulted in a LiF-rich SEI.LiF is thought to be an ideal SEI component that can promise the Li metal to deliver improved battery performances.The F-containing carbon host in this work initiates a totally new solution for designing SEI with abundant LiF.With a different consideration, Niufunctionalized mesoporous carbon fibers with abundant amine groups (Fig.4c).The strong interaction between Li ions and the ―NHgroups drives the prior nucleation of Li within the surface pores of carbon fiber, further promoting a special self-smoothing deposition of Li(Fig.4d, e).As a consequence, the Li metal full cell with the obtained Li/C composite anode delivered stable cyclability and high energy density of 380 Wh kg(Fig.4f) under realistic parameters (areal capacity ratio of the anode to cathode ≤2, electrolyte amount ≤3 g Ah, cathode capacity ≥4.0 mAh cm).This work was a milestone for the development and application of carbon hosts in real cells, which offered a good reference for the evaluation of LMBs under realistic conditions.And the optimization of carbon with functional groups is easily to be extended to large-scale processing and producing.
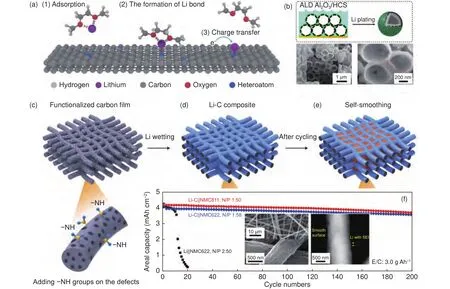
Fig.4 (a) Schematic representation of the Li nucleation on conductive frameworks[113].(b) Schematic illustration and SEM images of Al2O3/HCS electrodes after electrochemical Li plating[143].(c-e) Illustration of self-smoothing behavior in the Li/C anode.(f) Cycling performances of the Li/C || NMC811, Li/C ||NMC622 and Li || NMC622 cells.Inset are SEM images of the flexible carbon film prepared by electrospinning, and the STEM image of an individual Li/C fiber after cycling with a smooth surface and uniform Li layer with SEI[141].(Reprinted with permission).
There may be some overlaps among interior, exterior and functional carbon hosts since all of them can accommodate Li deposition.The difference is that functional carbon hosts are often enabled with specific functions by processing the skeleton of carbon with a heteroatom, modifying the carbon with a protective layer, and grafting the carbon surface with functional groups.These extra functional materials enable the strong interaction between Li ions and carbon, promote the formation of stable SEI, and protect the Li deposits away from corrosion in electrolytes.Compared to the other two kinds of hosts, functional carbon hosts are of greater potential and more advantages for being employed in real cells.However, more studies should be carried out to propose the universal design principles for the functionalization of carbon and develop the facile techniques for large-scale producing carbon hosts.
3.3.2 Carbon capsule
Recently, a new kind of carbon host emerges,which is pre-loaded with soluble functional materials.After the sustained release in the cell electrolyte, such functional materials can deliver multi-functions (,providing lithiophilic seeds for nucleation, contributing to SEI formation, regulation of inactive Li source).Several relevant pioneering works have been revealed in recent publications.One of the representative reports was related to a cubic carbon spansule of NaMg(Mn)F(NMMF@C cube, Fig.5a).When such a functional carbon capsule was employed, functional ions will be sustained release into the electrolyte during battery operation.Among the ions from NaMg(Mn)F, released metal ions will be in-situ reduced to their metallic state, covering the surface of a current collector.This metallic layer will promote the uniform deposition of Li.Meanwhile, the released F ions favor the construction of LiF-rich SEI (Fig.5b,c).Moreover, on basis of the results from SEM and cryo-transmission electron microscopy (cryo-TEM)images, Li preferred to deposit around the carbon cubes (Fig.5b, c).With the advantages mentioned above, the carbon capsule enabled a high Coulombic efficiency of 98% for over 1 000 cycles during the CE measurements.This work well indicated the significance of constructing a functional carbon capsule for ultra-stable Li metal anode.However, the functional materials loaded within carbon capsules are usually electrochemically inert, which might reduce the energy density of batteries.And the effect of functional carbon host is triggered by the side reactions between Li and additives, causing a certain loss of active Li source.There must be a balance between the advantages and disadvantages of carbon capsules, which must be understood and addressed before the application of these carbon hosts.Additionally, the largescale production of such carbon hosts needs to be facile and low-cost.
As is known, the incomplete stripping of Li metal and the corrosion during battery storage contribute to the formation and accumulation of inactive Li,which comprises Li ions in SEI and electrically isol-ated Li.Since inactive Li results from the side reactions between Li and electrolyte, its serious accumulation will lead to exhaustion of active Li and electrolytes, causing sudden battery failure.Numerous researches have been devoted to indirectly reducing the formation of inactive Li by regulating the Li deposition to avoid the growth of dendrites and optimizing the SEI.However, on basis of those inhibitory strategies, the accumulation of inactive Li cannot be totally eliminated.New recognition that reclaiming inactive Li after its formation rather than preventing its formation may also be applicable emerges as a creative solution.To achieve the goal of reclaiming inactive Li, Jinfirstly uncovered the nanostructures and chemistry of inactive Li with cryo-TEM.It’s found that inactive Li formed in typical ester and ether electrolytes comprises abundant LiO in SEI and electrically isolated Li debris.To achieve the conversion and recycle of the inactive Li, a reversible triiodide/iodide redox couple was proposed.In detail,iodine was entrapped within a biomass-derived porous hollow carbon capsule (Fig.5d).During the battery operation, the iodine dissolving in the electrolyte can effectively transform the inactive Li into soluble LiI (Fig.5e), which will shuttle to the cathode side.A delithiated cathode can be lithiated by LiI to collect and restore the Li source.Meanwhile, triiodide will be produced, continuously reclaiming inactive Li and constructing a reversible redox couple.Additionally,the triiodide with high chemical activity can sacrificially reduce the exhaustion of electrolytes by prior reduction at the surface of Li.Consequently, the iodine-based redox couple enabled the LMBs to deliver an ultra-long lifespan of 1 000 cycles in a LiFePOfull cell (Fig.5f).This success can also be translated into pouch cell configurations.The inactive Li reclaiming strategy with functional carbon hosts offers a new opportunity for further understanding the failure mechanism and prolonging the lifespan of LMBs.

Fig.5 (a) STEM and elemental mapping images of the Na, Mg, C, Mn, and F elements of a single NMMF@C cube.(b) Morphology of Li plated on the NMMF@C-modified Cu grid.(c) HRTEM image of the SEI formed on NMMF@C-modified Cu grid[142].(d) SEM images and elemental mappings of iodine/carbon capsule.(e) SEM images of cycled Li foil without and with iodine treatment.(f) Cycling performance of Li || LiFePO4 pouch cell[140].(Reprinted with permission).
After all, due to the high processability of carbon materials, they can be enabled with varied functions by different designs.Inherited from the existing numerous mature techniques for shaping carbon materials, a large variety of functional carbon hosts can be easily produced.With the assistance of these functional carbon hosts, the Li plating/stripping behaviors and nature of SEI can be well controlled.However, in terms of the present strategies, the complexity of the process, high cost, and large-scale producing techniques remain great challenges towards their broad applications.And more investigations should be conducted to resolve the underlying mechanism of optimizing Li metal anode, proposing the universal principles for designing functional carbon hosts.Moreover, the application situations of functional carbon hosts can further be extended to the reclaim of inactive Li by introducing redox couples, which can largely prolong the lifespan of LMBs.And introducing reactive scavengers or highly conductive metals is thought to be a potential methodology to reclaim inactive Li.However, all these imaginations are highly related to the advanced characterization techniques that can perform in-depth, multi-dimension, and lowdamage understanding towards the sensitive Li-metal containing materials.
4 Process of Li/C composite anodes
To adopt the carbon materials in real cells, the large-scale process techniques of Li/C composite anode are of great significance, which at present remains a great challenge.Generally, both electrochemical and mechanical processing can be used to harvest Li/C composite.
Since Li metal owns a low melting point of 180 °C, it can be easily changed into a liquid state.As is known, water can be absorbed into hydrophilic porous structures.A similar phenomenon should be achieved as long as the carbon materials possess superior ‘lithiophilicity’.As is confirmed, the adoption of lithiophilic materials can obviously enhance the lithiophilicity of carbon materials and promote the infusion of molten Li into pore structures of carbon materials.For instance, Cui’s group used a chemical vapor deposition (CVD) method to coat the surface of electrospun carbon fiber with silicon(Si).Benefiting from the lithiophilic coating layer,molten Li can easily flow into the carbon fiber and occupy the voids, obtaining a mechanically and chemically stable composite anode (Fig.6a).In addition, taking advantage of the native surface chemistry of carbon materials can also promote the fabrication of Li/C composite anode.One such typical work was related to layered graphene oxide (GO) film.When the GO film was directly dipped into molten Li metal,an immediate spark reaction happened across the whole GO film to expand the spacing among graphene layers (Fig.6b).This phenomenon can be ascribed to the aggressive reactions between reductive Li metal and residual water or surface functional groups in GO.These reactions caused the sudden pressure release and the combustion of produced hydrogen, which initiated the spark reaction to enable more porous andstable carbon hosts.As a consequence, the desired nanogaps among GO promoted the subsequent intake of molten Li metal, and finally, a flexible and twistable composite Li-rGO was successfully collected.Obviously, such flexibility and twistability enable the composite anodes to deliver high processability during battery manufacturing with folding and rolling processes.The melting and infusing methods for processing Li/C composite anodes have been widely used and developed.However, Li metal needs to be heated and melted, which induces the increase of energy/time (heating and cooling) exhaustion and the possibility of pollution (air and water).
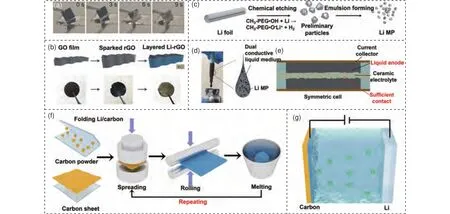
Fig.6 (a) Time-lapse image of Li melt-infusion process for lithiophilic materials[130].(b) Fabrication of a layered Li/rGO composite film[124].(c) Transformation of lithium particle size during a process of chemical ethicizing and emulsification.(d) The digital photo and schematic model showing the Li microparticles dispersed in an electric and ionic dually conductive matrix composed of polymer and carbon.(e) Schematic illustration of a symmetric cell using the semi-liquid Li dispersion with improved interface contact[168].(f) Mechanical processing of Li/C composite anode.(g) Electrochemical deposition for obtaining Li/C composite anode.(Reprinted with permission).
Instead of Li foil, the employment of Li powder as starting materials favors more uniform and facile processing of Li/C composite anodes.In a duration, Li powder has been regarded as an ideal material for constructing anode electrodes of LMBs.In contrast to Li foil, Li powder owns a larger surface area to reduce the local current density, and availability for delicately regulating the areal capacity of the whole electrode.Moreover, the adoption of inorganic/organic materials protecting layers enables the superior safety of Li during processing.However, the complex producing procedures and high producing costs inhibit the large-scale application of Li powder.Another emerging processing strategy with Li powder materials is to transform Li into a semiliquid state by colloidally dispersing Li microparticles into an electronic and ionic dually conductive matrix composed of polymer and carbon (Fig.6c, d).At such a semiliquid state, the composite Li is highly processable.Of note, the dispersion is especially suitable for deployment in solid-state batteries, since it ensures sufficient contact between Li and solid-state electrolyte, largely reducing the interfacial resistance(Fig.6e).However, due to the introduction of large amount of electrochemical inert polymer/carbon matrix, both the volumetric and mass capacity of composite Li metal anode have been obviously reduced to 800 mAh mLand 810 mAh g, respectively.This work undoubtedly initiates a novel strategy for processing Li.And more efforts should be devoted to reducing the loss of capacity and energy density as well as improving the utilization of raw materials away from extra side reactions.Moreover, the procedures for producing the Li metal containing liquid dispersion needs further simplifications.
Ranking the significance for practical applications, fabricating Li/C composite anodes with mechanical methods may be the most promising.In general, carbon materials of different morphologies and structures can be mixed with Li sheets.After repeated spreading, rolling, and melting processes, Li/C composite anodes can be easily harvested (Fig.6f).Of note, more detailed and varied mechanical processes can be extended and developed to facilely obtain Li/C composite anodes.For instance, melting and stirring processes can also contribute to composite anodes.Obviously, mechanical processing can be applied to the large-scale production of Li/C composite anodes with high efficiency and low cost.
Last but not the least, electroplating is a typical and classic strategy for preparing Li/C composite, especially at the laboratory scale (Fig.6g).Both the areal capacity and consuming time can be strictly controlled due to the accuracy of electrochemical processes.However, high efficiency and controllability are achieved at the expense of the high cost of electric power.Additionally, the electroplating process equips the Li with a pretreated SEI, which to some extent reduces the purity of the Li/C composite.And the practical application of this strategy suffers a lot from the large-scale production techniques of large-size carbon electrodes.For freestanding carbon films, it depends heavily on the production and processing techniques.As to carbon electrodes made of a slurry comprising binders and/or conductive additives, the cell energy density is largely reduced.Consequently, this strategy at present may be more applicable on a laboratory scale.
All in all, there have been a variety of processing strategies for harvesting Li/C composites.Among them, mechanical processing is regarded as the most promising method.For instance, by facile roll-to-roll methods, Li/carbon paper composites canbe easily produced at a large scale.Li/carbon paper composites have been employed in Li metal pouch cells, delivering high energy density.With future advances in the production of carbon sources, numerous kinds of carbon materials can be prepared at the industry level, which will be deployed in varied situations and battery systems.
Besides, novel techniques like physical vapor deposition should be further developed and investigated to extend the candidate strategies for harvesting Li/C composites.In addition, it should be noticed that the employment of carbon materials introduces extra volume and mass into composite anodes, which will reduce the energy density of LMBs.Consequently,when designing composite anodes, a vital issue is the relationship between the usage mass of carbon and the energy density of the battery.Generally, to guarantee an energy density over 350 Wh kgin a real cell with lean electrolyte, high loading cathode and limited N/P ratio, the Li to carbon mass ratio should be larger than 1.0.Notably, the selection principles, e.g., structure,morphology, surface chemistry, conductivity, and size, of carbon materials for large-scale production of composite anodes, are also of great significance,which should be further discussed and decided in future researches.
At present, there are limited related reports on processing and adoption of Li/C composite at a large scale and in real cells.Future researches on this aspect depend heavily on the applicability of carbon hosts for Li metal anode.More studies may be conducted to reveal the significance and necessity of Li/C composite in real cells under practical operating conditions.
5 Carbon-based electrolyte additives
In some recent attempts, carbon materials are originally employed as electrolyte additives to regulate the deposition of Li metal anode.The dendritic growth of metals is a widely existing phenomenon.And fruit-full results have been probed to avoid the formation of metal dendrite.For instance, in conventional electroplating industries, nanodiamond particle is one of the frequently used additives to induce the homogenous growth of metal films, which involves the co-deposition of metal ions and nanodiamond.In general, metal ions will absorb the negatively charged nanodiamond particles, which together will be brought to the electrode surface by an electric field and the convection of the electrolyte.In the next, metal ions are reduced to their metallic state.Some of the nanodiamonds will be captured by the plating metals,while others will be released to the electrolyte to continuously guide the Li plating.Inspired by the results from electroplating, Cheng et al.translated this success into LMBs.Nanodiamond nanoparticles were introduced to typical ester electrolytes to control the dendrite-free growth of Li by co-deposition (Fig.7a).On basis of first-principle calculations on adsorption and diffusion process, it’s found that nanodiamond has a much higher binding energy than that of the current collector (Cu foil) and the lowest diffusion barrier of Li among various materials (Fig.7b).All these promise the adsorption of Li ions on nanodiamond during Li plating, which can easily diffuse to result in a uniform distribution of Li.The morphology of Li plating well validated the above results, where Li columns with a small average diameter (0.3 -0.4 μm) were observed (Fig.7c).The design of a carbon-based electrolyte additive was further extended by Jiang et al., where a nitrofullerene (nitro-Cderivative, characterized as C(NO)) was fabricated and applied.Due to the electrostatic interactions between protuberances of Li deposits and nitro-C,nitro-Cwill be absorbed on the surface of Li.And the additive will be reduced to NOions and insoluble C.The former can react with metallic Li to contribute to a stable SEI with high ionic conductivity,while the latter can attach to the surface of Li deposits to homogenize the Li ion flux.The cross-sectional electron probe microanalysis (EPMA) clearly exhibited that many Cparticles anchored on the Li groove, promoting a smooth Li depositing surface(Fig.7d, e).And the high-resolution TEM image revealed a stable SEI resulting from the nitro-Cadditive, which comprised domains of LiF, LiN, LiNOand C(Fig.7f).Consequently, this carbon-based electrolyte additive enabled the full cells with high loading sulfur and LiNiCoMnOcathodes to deliver improved cycling performances.
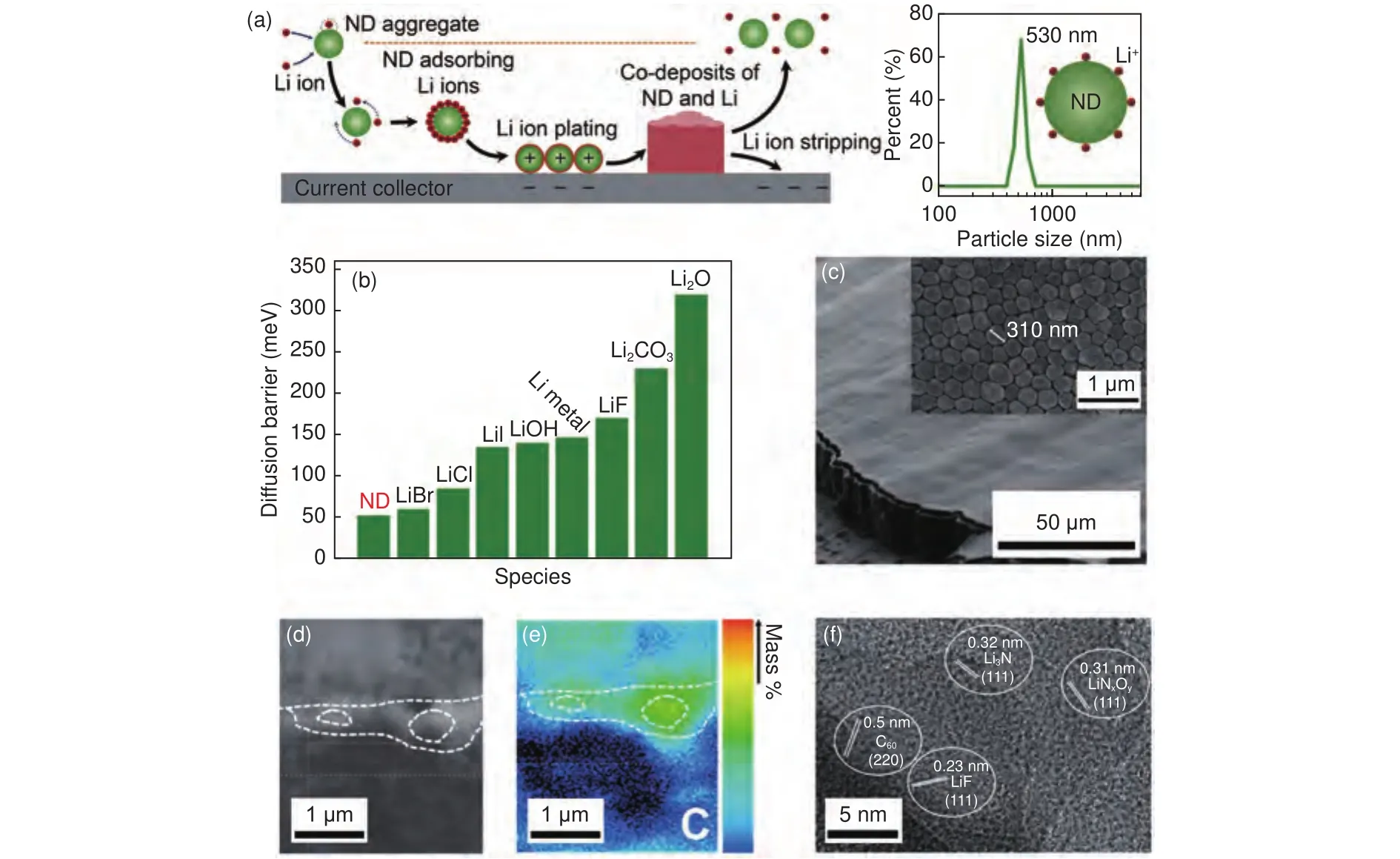
Fig.7 (a) Schematic illustrating the co-deposition of Li ions on nanodiamond, growth of the columnar Li film and the stripping of Li deposits.(b) Diffusion barrier of Li on different surfaces.(c) Li deposits in LiPF6-EC/DEC electrolyte with nanodiamond additives[179].(d, e) EPMA images of cycled Li with nitro-C60 of cross-sectional morphology and C distribution, respectively.(f) HRTEM images of cycled Li with nitro-C60 additive[178].(Reprinted with permission).
In contrast to various carbon hosts, carbon-based electrolyte additives are designed to regulate the deposition of Li mainly in the form of co-deposition.And some surface chemical designs can be adapted to promote the formation of stable SEI.The future developments of such an application of carbon can refer to the fruitful success in the field of electroplating.An obvious advantage of carbon-based electrolyte additives may be that they can function well with a tiny amount of addition, which has no negative effect on the cell energy density.
6 Carbon coating layers
As is widely accepted that a stable interface between Li and electrolyte is the key to the satisfactory cycling performances of LMBs.Numerous strategies have been built by the use of electrolyte additives.However, the produced SEI comprising of Li compounds is brittle of inferior cohesion with Li metal.In addition, such an SEI suffers repeated breakage/repair due to the volumetric expansion of electrodes during battery operation.Consequently, the exhaustion of Li and electrolytes cannot be eliminated.An ideal interface layer should be both chemically and mechanically stable.Additionally, the ability to regulate the Li ion flux and the further controllable deposition of Li metal is essentially important.The requirements on such interfacial layers have largely promoted the researches on artificial SEI or protective coating layers as an alternative solution towards the unsatisfactory native SEI.And carbon materials with their superior chemical and mechanical stability have been regarded as promising coating layers.For example, Zhengdesigned a flexible and interconnected interfacial layer composed of amorphous hollow carbon nanospheres (Fig.8a).Of note, this carbon film can stay stable against the Li metal, and the dendrite growth can be effectively suppressed ascribed to the high mechanical strength(Young’s modulus of 200 GPa) of this carbon.Moreimportantly, this carbon film weakly bonded to the current collector of Cu foil can move up and down along with the deposition and dissolution of Li(Fig.8b, c).As a result, the Li plating is well confined underneath the carbon film.In this contribution,carbon was thought to be static and a stable SEI formed on the top surface carbon spheres, which promoted the uniform Li deposition.Obviously, the structural homogeneity and electronic conductivity of carbon played an important role and should be delicately investigated and controlled.This work has been regarded as a representative reference of using carbon materials for optimizing Li metal anodes, after which the researches on Li metal anodes with protective layer/artificial SEI or host materials have boomed.
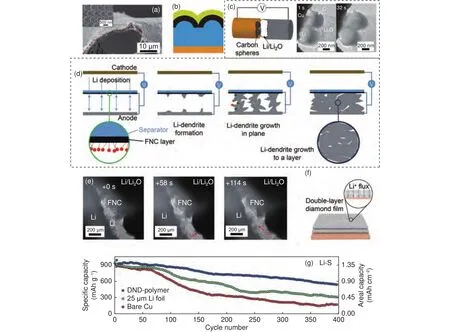
Fig.8 (a) SEM image of the hollow carbon nanosphere thin-film peeled off the Cu substrate.Inset is the SEM image of the carbon-coated polystyrene nanoparticle array.(b) Schematic illustrating the Li deposition under the protection of hollow carbon nanosphere thin-film.(c) Schematic showing the configuration of the in-situ TEM cell, and TEM images of the Li deposition process on Cu wires decorated with hollow carbon nanospheres taken at different times[189].(d)Schematic illustrating the Li deposition in a cell using separator with a carbon-based coating layer.(e) In situ TEM images of Li deposition with the adoption of functionalized carbon[190].(f) Schematic illustrating the double-layer nanodiamond film on a Cu substrate.(g) Cycling performance of the prototypical Li-S cells with Li foil, bare Cu with electrodeposited Li, or double-layer nanodiamond-polymer with electrodeposited Li as the anode[192].(Reprinted with permission).
In another contribution, the growth patterns of Li can be controlled with the adoption of a carbon-based coating layer on the separator to avoid the short-circuit of batteries caused by dendrite puncture.In detail, this carbon was functionalized with p-benzenesulfonic acid (-SOH), in which Hwas further exchanged with Li.And these Li ions can be reduced to Li metal seeds, inducing the deposition of Li from the functionalized carbon-coated separator.Since the Li dendrites grew from both the electrode and separator towards each side, they would be in contact finally.This will lead to the reduction of the potential difference between the tip and the base of Li dendrites to zero (Fig.8d).That’s to say, the driving force for the dendrite growth along the through-plane direction was eliminated.As confirmed by in-situ TEM experiments, the further deposition of Li was to grow in anin-plane direction parallel to the separator and electrode (Fig.8e), making the deposits a uniform Li metal layer.Due to the efficient regulation of Li growth direction, the issues raising from dendrite growth, unstable SEI, and decomposition of electrolyte have been well addressed, promoting the excellent cycling performances of Li/LiFePOfull cells (800 cycles with a capacity retention of 80%).This work provided a creative design for tuning the growth patterns of Li metal with a functionalized carbon coating layer,which can transform the Li dendrites into Li metal layer rather than conventionally preventing the dendritic growth of Li.Because the formation of Li dendrites is thermodynamically favorable, conversion and regulation of Li deposition morphology after the dendrite formation may be an alternative strategy for prolonging the lifespan of LMBs.However, the effectiveness of such carbon coating layers needs to be verified in real cells under practical conditions.
As mentioned above, an ideal coating layer should be mechanically stable against Li metal.To satisfy such considerations, carbon materials like diamonds, diamond-like carbon (DLC), and carbon paper may be a good choice with enough hardness and strength.For instance, Liu et al.used a doublelayered diamond as coating layers to stabilize Li metal (Fig.8f), which had the highest modulus > 200 GPa and electrochemical inertness.Additionally, as the formation of the pinhole is the major reason that accounts for the failure of coating materials, a COMSOL simulation was conducted to validate the effectiveness of such a double-layered design.As illustrated in this work, the doubled layered diamond can stabilize the Li ion flux around the pinhole to a nearly constant value, while the local concentration of Li ion flux increased fiercely in the single-layered diamond.With such a double-layered diamond having electrochemical stability, defect tolerance, low electronic conductivity, and high mechanical strength, the dendrite propagation has been replaced by uniform deposition.And the double-layered diamond enabled the Lisulfur cells to deliver much-improved cycling stability and higher capacity (Fig.8g).In addition to the extrinsic coating layer, a protective carbon coating layer like DLC can be in-situ produced/deposited at the surface of Li metal by techniques like CVD and electrolysis.Such DLC coating layers not only have high mechanical strength, but excellent cohesion with Li metal to prevent corrosion from the electrolyte.
In summary, carbon coating layers have emerged to protect Li metal anodes away from dendrite growth and electrolyte corrosion.Carbon materials can be processed onto a separator or directly on the surface of Li metal at low adoption content.This strategy engaged with carbon coating layers is easy to be extended into large-scale production and application since it can be achieved with simple coating procedures.However, researches on this aspect are bare and limited at present, which appeals for more attention and in-depth investigation.
7 Outlooks
In summary, carbon as a highly processable and designable material has been widely adopted to optimizing Li metal anode by suppressing the volumetric effect, changing the chemistry and structure of SEI, and physically and/or chemically regulating the deposition behaviors of Li.Numerous carbon materials have been designed as interior/exterior/functional hosts,electrolyte additives, and coating layers for LMBs.There is no doubt that the application situations and species of carbon-based battery materials can be further extended and boomed.However, great challenges that hinder the practical applications of carbon materials in real cells remain unsolved: (I) The feasibility evaluation and multi-dimensional recognitions on the function mechanism of carbon materials from structure to chemistry (, porosity, conductivity, lithiophilicity) in cell level under practical operation conditions; (II) Key material parameters and universal design principles towards powerful carbon materials for high-energy-density and long-cycling LMBs(, the balance between performance enhancement and inert electrochemical nature of carbon materials).To achieve the real success of carbon materials in LMBs, there is still a long way to go.
(1) Evaluation of carbon materials in practical cells.In addition to designing various kinds of carbon materials, the evaluation of their effectiveness is also of great significance.To accelerate the practical applications of carbon materials in LMBs, a direct strategy is to evaluate the carbon materials in real cells under practical operating conditions.That’s to say, there should be strict control over the amount of electrolyte (, lean electrolyte of 3 g Ah), the amount of Li source (, limited Li of 50 μm), and the areal capacity ratio between negative and positive electrodes (, N/P of 3).Additionally, the batteries should be tested under high current densities and large cycling capacity (, 3 mA cm, 3 mAh cm).Evaluations with large-size and high-capacity pouch cells are of more significance.With unification in the evaluation standards being constructed, strategies and materials from different research groups can be compared and analyzed, largely promoting the advances and developments of carbon materials in LMBs.
(2) Advanced characterization techniques for understanding the functions of carbon materials.In addition to designing and exploring novel kinds of functional carbon materials, fundamental understanding and recognition of their functions in working batteries are critically important, which can contribute to the establishment of the universal design principles for carbon-based battery materials.However, suffering greatly from the air- and electron beam-sensitive nature of Li-containing materials, it’s hard to atomically reveal the Li deposition behavior and SEI formation with the adoption of carbon during battery operation.The introduction of nondestructive techniques like cryo-TEM promotes the understanding of Li and SEI and sheds light on uncovering the role of carbon materials.However, the fact is that situations become complex with the addition of carbon.How to achieve the high-resolution analysis when carbon is mixed with Li and SEI requires further investigations, and relevant sample preparation techniques are highly required (, cryo-focused ion beam).And more advanced characterizations may be involved and adopted to the researches of carbon materials in LMBs to collect different information (, size, morphology,structure, component, functional groups, and distribution).Besides, employment and design of in-situ/operando characterization techniques can collect dynamic information of carbon materials during battery operation.At present, relevant attempts have been involved in optical microscopy, X-ray diffraction (XRD), nuclear magnetic resonance (NMR), and so on.The advances in characterization techniques largely accelerate the recognition and development of carbon materials in LMBs.With the assistance of cryo-TEM, an underlying recognition towards the evolution of inactive Li in LMBs has recently been uncovered, which promotes emerging researches on the reclaim of inactive Li by functional carbon materials with chemical and physical design,, redox chemistry, pressure, and reconstructing conductive network.
(3) Green and large-scale fabrication of carbon products.With the gradually enhanced attention towards the sustainable developing policies all over the world, green processing and production of materials with limited and reduced environmental concerns(, emission of greenhouse gas) is highly required.However, the reality is that the present production of carbon materials especially from pyrolysis techniques possesses an ultra-low yield, emitting plenty of greenhouse gas.Additionally, employment of sustainable materials does not necessarily equal real sustainability, which should include sustainable production, application, and final disposal.In terms of green production, more efforts are suggested to increase the utilization of raw materials and the yield of products.Alternatively, a hierarchical and stepwise processing technique can be applied to collect and convert the emission into useful products.Another promising approach to guarantee the sustainability of battery systems is to directly use natural large-sized carbonaceous materials, e.g., cellulose and protein films for separators and protective coating layers, without extra carbonizations or chemical treatments.However, the advance of such green battery systems also suffers great challenges and requires much attention from battery community.In addition, the large-scale pro-cessing of carbon with desirable sizes is also a great challenge, especially for freestanding carbon materials.Regulation and optimization of the techniques like CVD, electrospinning, and carbonization may be applicable ideas for figuring out this issue.Conventional slurry coating methods can also be used to harvest carbon products at a large scale at the expense of natural structure loss and increasing contents of electrochemical inert materials (, binder).
All in all, carbon materials with delicate structural and chemical designs have proceeded with the advances of Li metal anodes, which is one of the typical milestones in the development of LMBs.However,both the LMBs and carbon materials are still faced with great challenges, ranging from their practical significance to the potential of large-scale production and application.Due to the ongoing pursuit and desire for renewable energy, the researches on green carbonbased battery materials and LMBs will be driven to experience continuous evolutions and advances, finally achieving their success in practical cells.
This work was supported by National Natural Science Foundation of China (52103342), China Postdoctoral Science Foundation (BX2021136 and 2021M691712), the Seed Fufnd of Shanxi Research Institute for Clean Energy (SXKYJF015).
杂志排行
新型炭材料的其它文章
- 《新型炭材料》新版网站正式开通
- Guide for Authors (1)
- Optimizing the carbon coating to eliminate electrochemical interface polarization in a high performance silicon anode for use in a lithium-ion battery
- Glycine-derived nitrogen-doped ordered mesoporous carbons with a bimodal mesopore size distribution for supercapacitors and oxygen reduction
- 金属有机框架复合酚醛树脂基整体式亲水炭应用于空气水捕集
- 碳基电催化材料选择性合成过氧化氢研究进展
