Historical evolution, overview, and therapeutic manipulation of co-stimulatory molecules
2022-02-12HenryVelazquezSotoFernandaRealMariaJimnezMartnez
Henry Velazquez-Soto, Fernanda Real, Maria C Jiménez-Martínez
Henry Velazquez-Soto, Fernanda Real, Maria C Jiménez-Martínez, Department of Immunology and Research Unit, Institute of Ophthalmology “Conde de Valenciana”, Mexico City 06800, Mexico
Maria C Jiménez-Martínez, Department of Biochemistry, Faculty of Medicine, National Autonomous University of Mexico, Mexico City 04510, Mexico
Abstract Co-stimulatory molecules are key mediators in the regulation of immune responses and knowledge of its different families, structure, and functions has improved in recent decades.Understanding the role of co-stimulatory molecules in pathological processes has allowed the development of strategies to modulate cellular functions.Currently, modulation of co-stimulatory and co-inhibitory molecules has been applied in clinical applications as therapeutic targets in diseases and promising results have been achieved.
Key Words: Co-stimulatory molecules; Immune modulation; Monoclonal antibodies; Biological therapy; Autoimmune diseases; Oncological diseases
INTRODUCTION
Regulation of the immune response is a crucial process in the initiation and control of inflammatory phenomena.Various mechanisms capable of regulating T cell activation have been described.Co-stimulatory molecules were initially described as accessory signals present in antigen-presenting cells (APCs) that interacted with T cells during the immunological synapse[1].They comprise a diversity of glycoproteins expressed in the membrane of APCs, and they interact with other glycoproteins that function as their receptors on T cells, modulating in a positive or negative way the activation, proliferation, differentiation, and function of T cells[2].In recent decades, advances in the knowledge of co-stimulatory molecules and the development of biological drugs allowed a therapeutical targeting of co-stimulatory molecules in distinct diseases[3].
A BRIEF HISTORY OF CO-STIMULATORY MOLECULES
A two-signal model of T cell activation was first proposed in the second half of the 1960s.The two signals were antigen recognition by an antigen receptor and the interaction with co-stimulatory molecules.Although the mechanisms were not known, the model proposed that in the absence of a second signal or "co-stimulation," the T cell would enter a state of paralysis or inactivation[4,5].By the second half of the 1980s, a series of investigations had experimentally demonstrated the existence of costimulatory molecules and their participation in T cell activation[6-9].The findings resulted in the description of a diversity of molecules and the investigation of their function in different disease models, which led to therapeutic applications.One example is the 2018 Nobel Prize in Physiology and Medicine, awarded to Tsuku Honjo and James P Alisson, for their contributions to the discovery of cytotoxic T lymphocyte–associated antigen (CTLA)-4 and programmed death (PD)-1 protein and the development of methods of molecular blockade for the treatment of oncological diseases[10-12].
OVERVIEW OF ANTIGEN PRESENTATION AND INVOLVEMENT OF COSTIMULATORY MOLECULES
APCs are part of the innate immune system and act as an interface between antigen recognition and the adaptive response of T cells during antigen presentation[3].Activation of T cells requires the appropriate activation and integration of three signals.The first signal is the antigen, which is presented in the context of the major histocompatibility complex, and its recognition by the T cell receptor (TCR).The first signal is not sufficient to activate T cells.Activation continues with a second signal that involves the participation of surface molecules expressed on dendritic cells that interact with their respective receptors on the T cell.The third signal involves the production of cytokines, which not only favor the activation state but also promote the polarization of T cells into their various helper/cytotoxic subpopulations[3,13] (Figure 1).In that dynamic microenvironment, the spatiotemporal expression of various co-stimulatory molecules on dendritic cells and T cells, as part of the second signal, is the key to regulating T cell activation, inhibition, survival, and polarization.
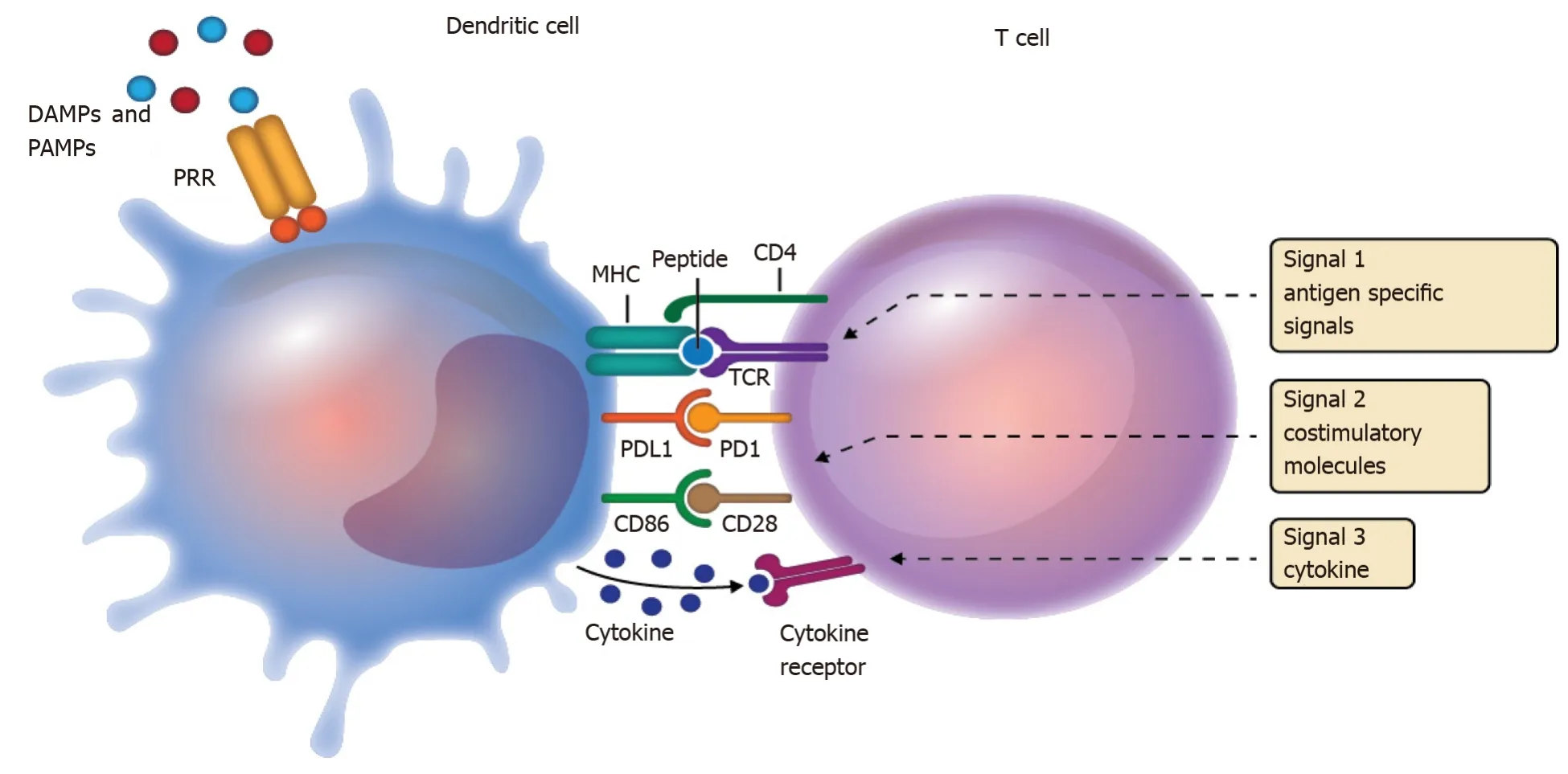
Figure 1 Overview of antigen presentation and the three-signal model.
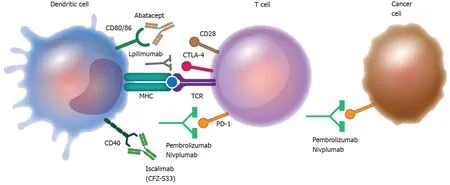
Figure 2 Therapeutical manipulation of co-stimulatory molecules.
Activating and inhibitory signals
Co-stimulatory molecules are transmembrane glycoproteins that induce activation or inhibition cascades that enhance or diminish TCR signaling[14,15].Stimulatory, or activating signals (co-stimulation by CD28 or CD40), lead to the production of growth factors, cell expansion, and survival.Inhibitory signals (co-inhibition by PD1 or CTL-4) attenuate TCR-induced signals, resulting in decreased cell activation, inhibition of growth factor production, inhibition of cell cycle progression, and in some cases, promotion of cell death[14].
Families of co-stimulatory molecules
Co-stimulatory molecules are divided into two main families by their molecular structure.The first (Table 1) is the immunoglobulin superfamily which includes CD226, the CD2/signaling lymphocytic activation molecule family, T cell immunoglobulin and mucin (TIM) family, butyrophilin (BTN) family, and leukocyte-associated immunoglobulin-like receptor (LAIR) family.Because of its historical relevance, the most studied is the B7 family, which includes CD80, CD86, and its receptor CD28.The second (Table 2) is the tumor necrosis factor superfamily (TNFR SF), which includes three subfamilies, the divergent type (OX-40, CD27, glucocorticoid-induced TNFRrelated protein), the S-type (CD267), and the conventional type [FAS, herpes virus entry mediator, receptor activator of nuclear factor kappa-B (RANK), and CD40].CD40 and its ligand CD40L are the most investigated co-stimulation molecules of the TNFR SF[3].

Table 1 Immunoglobulin super family co-stimulatory molecules
Co-stimulatory molecules and their study in human disease
The involvement of co-stimulatory molecules in clinical conditions has been explored.Mutations in ICOSL, CD40, or C267 have been associated with immunodeficiencies; increased expression of CD86, CD28, CD27, and CD70 has been reported in autoimmune diseases and allergies[16-23].Some of the most interesting findings are summarized in Tables 3 and 4.
Therapeutic application of co-stimulatory or co-inhibitory molecules
Numerous scientific studies have shown the involvement of co-stimulatory molecules in the regulation of the inflammatory process[3].Subsequently, both experimental trials in various disease models and preclinical trials have demonstrated promising results achieved by the therapeutic manipulation of these molecules[24,25].The preclinical results support their application at the clinical level either by inhibiting the function of activating co-stimulatory molecules to promote tolerogenic functions or by inhibiting inhibitory co-stimulatory molecules to promote pro-inflammatory functions.Blockade of the co-inhibitory molecules PD1 and CTLA-4 by the monoclonal antibodies pembrolizumab, ipilimumab, and nivolumab is a therapeutic indication in cancer treatment, particularly melanoma.On the other hand, therapeutic approaches for autoimmune diseases have exploited the blockade of the co-stimulatory molecules CD80/CD86 by abatacept or CD40 by iscalimab.In both cases, co-stimulatory molecule-targeted therapies have shown promising results[26-32] (Figure 2 and Table 5).
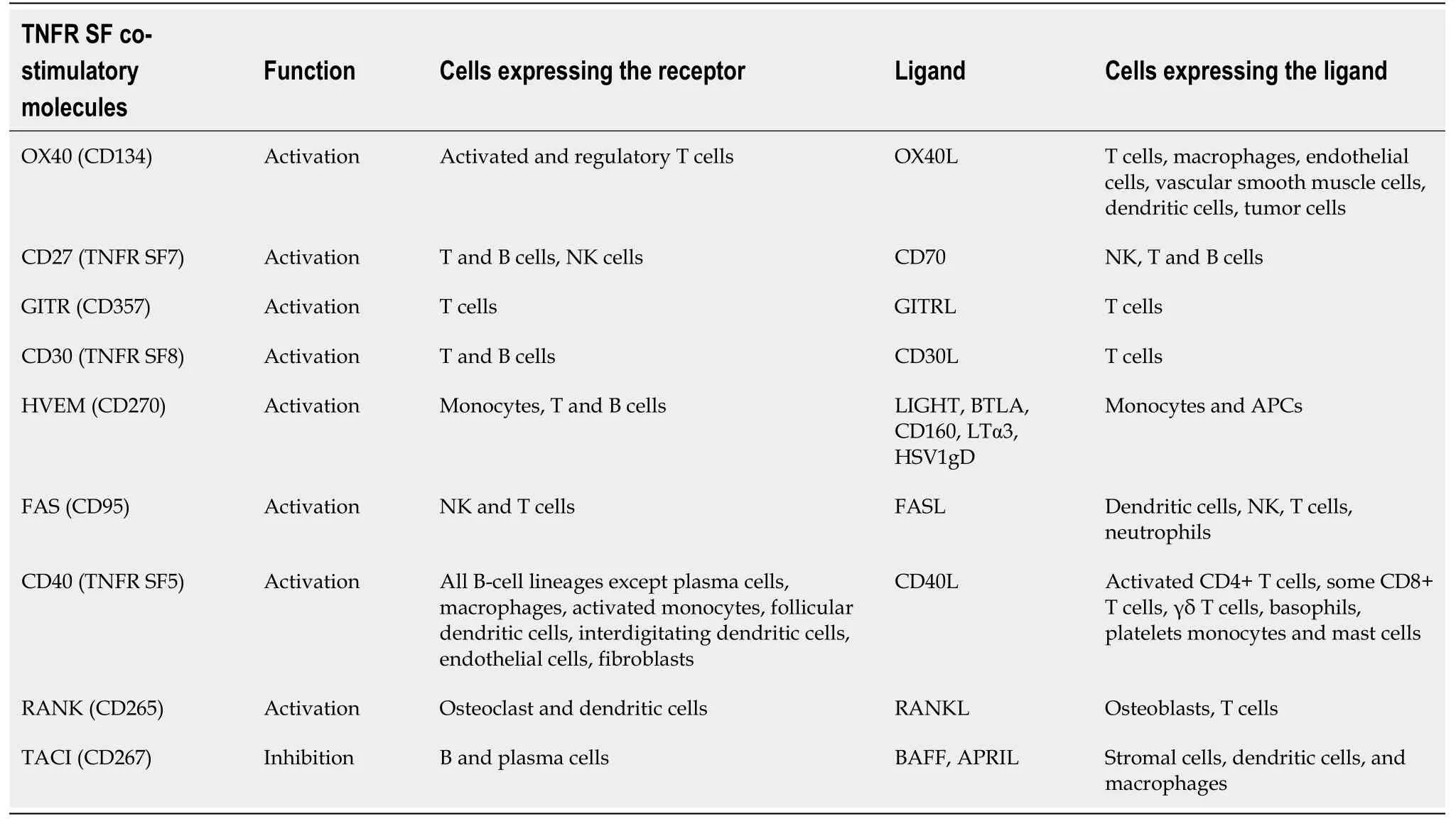
Table 2 Tumor necrosis factor receptor super family co-stimulatory molecules

Table 3 Immunoglobulin super family co-stimulatory molecules studied in various diseases
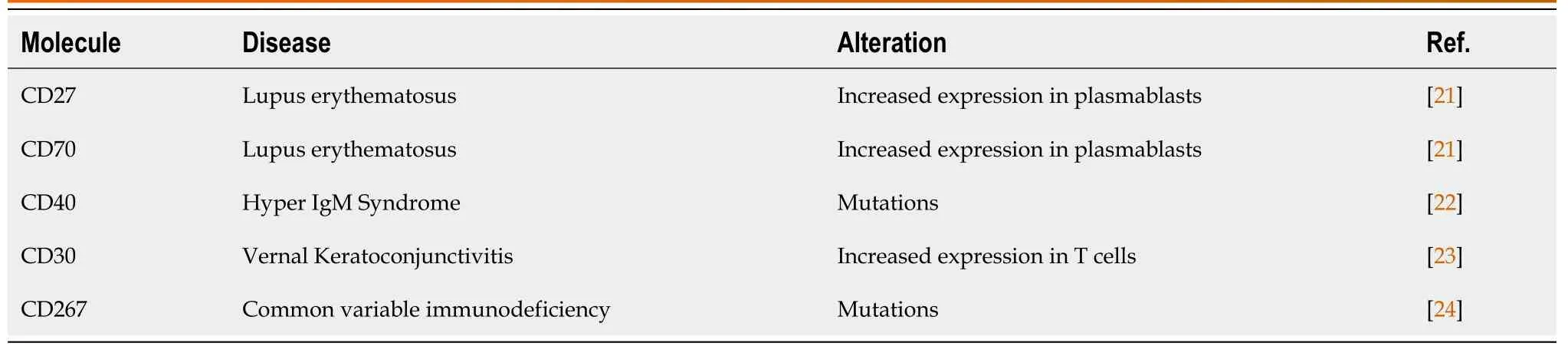
Table 4 Tumor necrosis factor superfamily co-stimulatory molecules studied in various diseases

Table 5 Co-stimulatory molecule manipulation in various diseases
CONCLUSION
Challenging limitations need to be overcome before these therapeutical tools are approved for clinical use[33].Nevertheless, understanding the function and the possibility of therapeutic manipulation of co-stimulatory molecules represents a milestone for immunology and pharmacology.The knowledge gained from the study of co-stimulatory molecules has allowed a deeper understanding of the pathophysiology of many diseases.The therapeutic use of these molecules has been well exploited in autoimmune diseases and oncology, where they serve as effective adjuvants to conventional therapy.However, we should not exclude the potential that these molecules have in many other contexts.They will undoubtedly continue to be an area of great interest for research and drug development.
