Quercitrin:a New Immunosuppressant Inhibits Calcineurin*
2022-02-10WANGMengQiLIUJiQiongTONGLiXIEYuFeiCHENHuiZHAOChangYunWANGLeiLIUYuanYuanGAOYaDanZHANGMuXIANGBenQiongWEIQun
WANG Meng‑Qi,LIU Ji‑Qiong,TONG Li,XIE Yu‑Fei,CHEN Hui,ZHAO Chang‑Yun,WANG Lei,LIU Yuan‑Yuan,GAO Ya‑Dan,ZHANG Mu,XIANG Ben‑Qiong*,WEI Qun*
(1)Department of Biochemistry and Molecular Biology,Beijing Normal University,Gene Engineering and Biotechnology Beijing Key Laboratory,Beijing 100875,China;2)Beijing Normal University Publishing Group,Beijing 100088,China;3)School of Chemistry and Bioengineering,Changsha University of Science and Technology,Changsha 410114,China)
Abstract Objective This study aimed to search for immunosuppressants with high‑efficiency and low‑toxicity from Chinese herbal medicine using calcineurin (CN) as the target enzyme.Methods Natural compound which could inhibit the activity of the target enzyme,CN,was screened and one effective compound was identified and isolated.The immunosuppressive effect and toxic side effects of the compound were evaluated at the cellular and animal levels.The underlying immunosuppressive mechanism was explored using fluorescence spectroscopy,molecular docking,Western blot,dual‑luciferase reporter gene,real‑time PCR, etc.Results Quercitrin inhibited CN activity both in vitro and in vivo.In addtion,it reduced the proliferation of splenocyte and ameliorated DTH symptoms in mice.Toxicological study showed that quercitrin had no or minimal toxic side effects on experimental animals.Fluorescence spectroscopy and molecular docking analysis showed that quercitrin may bind to two sites on the CN and regulate immune responses through the CN‑NFAT pathway.Conclusion We identified a new CN inhibitor,quercitrin,which could be a useful novel immunosuppressant with low toxicity.
Key words quercitrin,calcineurin,immunity,CN‑NFAT signal pathway,interaction
Immunosuppressive drugs are widely used in organ transplantation and autoimmune diseases.The discovery of cyclosporin A (CsA) and tacrolimus(FK506) in the 1980s opened up new avenues for organ transplantation and the treatment of autoimmune diseases[1].These agents reduced transplant rejection rates and improved patient survival[2‑3].In 1991,Liuet al.[4]reported that calcineurin was the target of both CsA and FK506.
Calcineurin (CN),is a unique calcium/calmodulin‑dependent serine/threonine protein phosphatase;it is a heterodimer composed of a~60 ku catalytic A subunit (CNA) and a~19 ku regulatory B subunit (CNB)[5].CNA has one N‑terminal catalytic domain and three regulatory domains,namely a CNB‑binding domain (BBH),a CaM‑binding domain(CBD) and an autoinhibitory domain (AID)[6].It is involved in a host of critical physiological and pathological processes including muscle growth,T lymphocyte activation,apoptosis and cardiac hypertrophy[5,7].CN controls T cell activationviathe CN‑nuclear factor of activated T cells (NFAT)pathway.In response to various triggers,calcium enters the T cell and binds to calmodulin,which then activates CN.The activated CN dephosphorylates NFAT,leading to its nuclear translocation and the transcription of target genes,including cytokine genes such as IL‑2 in human T cells,and the corresponding cytokines activate the immune response[8‑9].CsA and FK506 combine with their specific cytoplasmic receptors (cyclophilin and FK506‑binding protein(FKBP),respectively) to form complexes that bind to CN and inhibit the immune response[1,10].
Until now,CsA and FK506 remain the drugs of choice in the clinic[11‑12],but their dramatic side effects,including nephrotoxicity,neurotoxicity and hypertension[12‑14],limit their utility.These side effects of CsA and FK506 are particularly important since patients generally need to take the drugs for a long time.Therefore,effective CN inhibitors of low toxicity would be extremely desirable.In our laboratory we have established a mature system for screening inhibitors of CN,and have already identified several compounds[15‑17].
Chinese traditional herbal medicine is an important source of modern drugs.Many Chinese herbals have been evaluated as potential therapeutic agents.Albizzia julibrissinDurazz,also known as the Persian silk tree or pink siris,is a small domed or flat‑topped,spreading tree with smooth,gray‑brown bark and doubly pinnate leaves[18].The flowers of this plant are used to treat depression,insomnia,amnesia,sterility and obesity in oriental traditional medicine[19‑20],and immunity‑related studies have also been reported[21‑22].Using our screening system we identified a low toxicity flavonoid that inhibited calcineurin activity inAlbizzia julibrissin.In initial work we showed that this compound was quercitrin,which has been reported to be an inhibitor of sortase A[23],to have anti‑inflammatory and anti‑tumor activityviathe NF‑κB pathway[24‑25],and an excellent therapeutic effect in systemic lupus erythematosus(SLE)[26]and imiquimod‑induced psoriasis‑like skin lesions murine model[27].
To explore its immunomodulatory activity,we investigated its effect on the proliferation of ConA‑stimulated murine spleen lymphocytes,LPS‑stimulated murine spleen lymphocytes,thein vitromixed lymphocyte reaction (MLR),and delayed type hypersensitivity (DTH).We also used fluorescence spectroscopy and molecular docking to examine the effects of quercitrin on CN and obtained preliminary evidence that quercitrin acts on the CN‑NFAT signaling pathwayin vivo.
1 Materials and methods
1.1 Ethics statement
All animal experimental procedures were authorized by the Ethics and Animal Welfare Committee,School of Life Science,Beijing Normal University (NO.CLS‑EAW‑2013‑015) and were executed in strict accordance with United Kingdom Scientific Procedures Act (1986).All efforts were made to minimize the number of animals used and their suffering.
1.2 Materials
Flowers ofAlbizzia julibrissinDurazz were purchased from Beijing Tongrentang head store(Beijing,China).BL21 (DE3) strains harboring CNA cDNA and the cDNA for a deletion of CNA encoding the catalytic domain only were from our laboratory.BALB/c and C57BL/6 mice (6-8 weeks) were purchased from Vital River Laboratories (Beijing,China).Jurkat cells were obtained from the American Type Culture Collection.(FL)‑NFAT plasmid and the p‑renilla luciferase(RL)‑SV40 plasmid were from our laboratory.The other reagents were all of standard laboratory grade.
1.3 Expression and purification of proteins
CNA and CNAa (catalytic domain only) were expressed and purified as described previously[28].Protein purity was assessed by SDS‑PAGE.Protein concentrations were measured by the procedure of Bradford.
1.4 Extraction and isolation of compounds for screening
Air‑dried flowers ofAlbizzia julibrissinDurazz was ground into a powder,then extracted with 70%ethanol.The concentrated extract was dispersed in water and partitioned successively with petroleum ether,ethyl acetate (EtOAc) and n‑butanol.The EtOAc fraction was subjected to silica gel chromatography using trichloromethane and methanol(80∶1-0∶1,v/v)as solvent,to yield 7 fractions.The third fraction was subjected to polyamide column chromatography and high‑speed countercurrent chromatography (HSCCC) and then recrystallized with methanol to yield the compound.
1.5 Compound identification
The compound was identified as quercitrin by1H NMR and13C NMR analysis and comparison with the literature.
1.6 CN activity assays in vitro
Enzyme activity was measured using the method described in detail previously[16].Phosphatase activities are presented as percentages of control phosphate activity.Quercitrin stock solution was dissolved in DMSO.Assays using p‑nitrophenyl phosphate (p‑NPP) (Sigma‑Aldrich,America) as substrate were monitored by spectrophotometer at 410 nm.When the substrate was32P‑RIIpeptide (BioMoL Research Labs,America),the released32P was quantified by liquid scintillation spectrometry,and vehicle served as the matched control.
1.7 Cell viability assay
Cell viability was measured using the CCK‑8 assay as described previously[15].Final concentrations of quercitrin were 2,5,10,20,50,100,500,1 000 and 2 000 μmol/L,those of CsA(TCI,America) were 2,5,10,20 and 50 μmol/L.The highest content of DMSO was 0.4%.Plates were incubated for 20,44 and 68 h at 37℃in 5% CO2,then 20 μl of CCK‑8(Dojindo,Japan) reagent was added to the wells and incubated for 4 h.The experiment was repeated five times.Cell viability was calculated from the following equation:Viability/%=(A450of quercitrin group/A450of control group)×100.
1.8 Con A/LPS-induced mouse splenocyte proliferation
Splenocyte proliferation was measured as described[15].The final concentrations of quercitrin were 2,10,50,100,500 and 1 000 μmol/L.Proliferation was measured using the CCK‑8 assay as described above.Cell growth inhibition was calculated from the following equation:Inhibition/%=[(A450of control group-A450of quercitrin group)/A450of control group]×100.
1.9 Mixed lymphocyte reaction(MLR)
A unidirectional MLR was performed with five replicates as previously described[15].The final concentrations of quercitrin were 0.1,0.5,2,10,50 and 100 μmol/L.The highest content of DMSO was 0.1%.After 48,72 and 96 h of incubation,proliferation was measured using the CCK‑8 assay.
1.10 Toxicity test
The acute toxicity test was performed in mice using the maximum amount of drug method together with the fixed‑dose procedure,which is a sequential testing scheme proposed by the British Toxicology Society in 1984[29].Briefly,in the test the estimated median lethal dose(LD50)is used to assess the toxicity of the substance being investigated.If theLD50is larger than 2 000 mg/kg of body mass,the substances will be termed“unclassified”.Sixteen mice,eight of each sex,were divided into two groups.Quercitrin was administered orally at a dose of 5 000 mg/kg to one group and intraperitoneally at a dose of 2 000 mg/kg to the other.Quercitrin dissolved in DMSO was diluted with a 0.5% aqueous solution of sodium carboxymethyl cellulose.The highest content of DMSO was 10%.The mice were observed for two weeks to assess any changes in reactivity,gait,motor activity,respiration rate,etc.,and especially survival.
To further investigate the effect of quercitrin in animals,BALB/c mice were randomly divided into the following three groups (six per group):control group,CsA group and quercitrin group.Mice in the control group received an intraperitoneal injection of 0.2 ml 0.9% NaCl daily for 4 d,mice in the CsA group received the same volume containing 200 mg/kg CsA on the first day and 40 mg/kg on the following three days,and mice in the quercitrin group received the same volume containing 200 mg/kg quercitrin on the first day and 40 mg/kg on the following three days.CsA and quercitrin dissolved in 0.9% NaCl.Blood was taken and assays were performed to determine levels of glutamic‑pyruvic transaminase,glutamic‑oxalacetic transaminase,alkaline phosphatase,total bilirubin,urea nitrogen and serum creatinine.
1.11 Delayed type hypersensitivity(DTH)assay
DTH assay was carried out as previously described[30].Male BALB/c mice were randomly divided into the following five groups (eight per group):control group,CsA group,and three experimental quercitrin groups.Drugs were administered intraperitoneally to the appropriate groups for 5 d:the control group (0.9% NaCl),CsA group (40 mg/kg) and quercitrin groups (10,20,40 mg/kg).
1.12 Fluorescence quenching of proteins in the presence of quercitrin
Fluorescence measurements were made with a Fluoro Max‑2 Fluorimeter (Jobin Yvon‑Spex,France).Fluorescence emission spectra were recorded as described before[31].Molar ratios of drug to protein were 0,1,1.5,2,3,4,6,8 and 12.Fluorescence quenching was analyzed using a Stern‑Volmer quenching plot[32](Equation(1)),as follows:

whereF0andFare the fluorescence intensities in the absence and presence of quencher,respectively,[Q]is the concentration of quencher,andKDis the Stern‑Volmer quenching constant,defined asKD=KQτ0,whereKQis the bimolecular quenching constant andτ0 (assumed to be 10‑8s)[33]is the lifetime of the fluorophore in the absence of quencher.
1.13 Docking
Docking experiments were carried out with Autodock 4.0.The macromolecule CN was treated as rigid,and quercitrin was treated as flexible.The first 35 docking results were compared with the potential binding areas from the fluorescence analysis.All figures were rendered using PyMOL.
1.14 CN activity assays of Jurkat cells
CN activityin vivowas determined using the assay described previously[16].Phosphatase activities are shown as percentages of control activity.
1.15 Western blot analysis of CN in Jurkat cells
Jurkat cells plated in 6‑well plates were treated with 100 μg/L phorbol 12‑myristate 13‑acetate(PMA)(Sigma‑Aldrich,America) and 1 μmol/L ionomycin(Ion) (Sigma‑Aldrich,America).Cells received PMA and Ion or a combination of CsA or different doses of quercitrin and PMA and Ion.The cells were harvested by centrifugation after 24 h and washed twice with PBS.Then,they were lysed on ice for 15 min.Cell‑free supernatants were collected for Western blot analysis.Protein quantitation was performed with a bicinchoninic acid (BCA) protein quantitation kit(Thermo Fisher Scientific,America).The samples were separated by discontinuous SDS‑PAGE and transferred to PVDF membranes (Merck Millipore,America),which were blotted with primary antibodies(Cell Signaling Technology,America) and secondary antibodies,and specific bands were visualized using an electrochemiluminescence (ECL) kit (Engreen,China).
1.16 Dual-luciferase reporter gene assay
NFAT transcription activity was investigated using the two‑reporter gene assay system.Jurkat cells plated in 24‑well plates were cotransfected with the p‑firefly luciferase (FL)‑NFAT plasmid and the p‑renilla luciferase‑SV40 endogenous control plasmid.Twenty‑four h after transfection,cells were incubated with PMA+Ion or a combination of different doses of quercitrin and PMA+Ion for 24 h.NFAT activity was measured using a Dual‑Luciferase Reporter Kit (Promega,America) according to the manufacturer’s protocol.
1.17 Relative quantitative real-time PCR for IL-2
RNA was extracted from Jurkat cells using TRIzol reagent (Invitrogen,America).Relative quantitative real‑time PCR was performed to detect the transcriptional level of IL‑2.The primer sequences for IL‑2 were TGTCACAAACAGTGCACCTACTTC(forward)and TGTGGCCTTCTTGGGCATGT(reverse).For β‑actin they were GTGACAGCAG‑TCGGTTGGAG (forward),and AGTGGGGTGGC‑TTTTAGGAT (reverse).β‑actin was used as an endogenous control gene.Real‑time quantitative PCR was performed on an Applied Biosystems 7500 Real‑Time PCR System using SYBR green dye.The reactions were performed in triplicate and the results are shown as.
1.18 Statistical analysis
The results are expressed as the mean±standard error of the mean.The GraphPad Prism 5.0 software was employed to calculate data and draw plots.Studentttest was used to analyze the differences between groups,and significant were deemed at three levels:P<0.05(*),P<0.01(**),P<0.001(***/###).
2 Results
2.1 Expression and purification of CNA and its deletion mutant
CNA and CNAa (Figure 1a) were expressed,purified in a series of steps and analyzed by SDS‑PAGE(Figure 1b).Both were electrophoretically pure.
2.2 Identification of the screened flavonoid as quercitrin
We tracked the inhibitory activity of different fractions fromAlbizzia julibrissinby CN activity assays.After continuous isolation and activity detection,we finally obtained a compound giving the strongest inhibition of CN.
The compound was a yellow amorphous powder,and the NMR data were as Table 1.

Table 1 1H(500 MHz)and 13C(125 MHz)NMR assignments of quercitrin in DMSO-d6
Comparison of the compound’s1H NMR and13C NMR spectra with those reported in the literature[34],allowed us to identify it as quercitrin,purity>95%(Figure 2).

Fig.1 Characterization of proteins

Fig.2 Compound identification
2.3 Quercitrin inhibits CN activity in vitro
Quercitrin inhibits CN activityin vitrowith either p‑NPP or32P‑RII peptide as substrate.IC50values were 1.59 mmol/L (p‑NPP,Figure 3a) and 22.59 μmol/L (32P‑RII peptide,Figure 3b),respectively.The effects of quercitrin were strikingly different from those of CsA and FK506,which inhibit CN activity with RIIas substrate but stimulate CN activity with p‑NPP as substrate[35].
2.4 Inhibition of Con A or LPS-induced proliferation and the MLR by quercitrin

Fig.3 Inhibition of CN activity in vitro by quercitrin
The cytotoxic effect of quercitrin on mice spleen cells measured over 24,48 and 72 h by CCK‑8 assay,was lower than that of CsA.TheIC50values of CsA were 18.85 μmol/L (24 h),8.82 μmol/L (48 h) and 4.86 μmol/L (72 h).Quercitrin only had a weak inhibitory effect on growth of the cells at concentrations equal to or above 500 μmol/L.
To evaluate the immunosuppressive effects of quercitrin,we using Con A or LPS‑stimulated proliferation assays and the MLR assay.Quercitrin inhibited the growth of T lymphocytes,B lymphocytes and one‑way mixed lymphocytes(Figure 4a-c),and inhibition was time‑and dose‑dependent.

Fig.4 Inhibition of Con A or LPS-stimulated proliferation and MLR by quercitrin
2.5 Quercitrin is non-toxic and has no significant hepatotoxicity and nephrotoxicity in mice
Mice treated with quercitrin (2 000 mg/kg,i.p.and 5 000 mg/kg,i.g.) were fully observed for 14 d(twice daily).The mice exhibited some degree of malaise in the initial half hour,and then returned to normal.There were no toxic reactions,deaths or obvious weight changes over 14 d.On day 14,the mice were killed and dissected to observe their organs;there were no visible lesions.Substances estimated with an indicating that quercitrin can be identified as“unclassified”.
In order to further study the effect of quercitrin on the activities of the mouse kidney and liver,three groups of mice (control,CsA and quercitrin),after treatment with the respective drugs for four d,were used to provide sera for blood biochemistry analysis.From Figure 5,it can be seen that quercitrin was less toxic than CsA at equivalent doses.
2.6 Quercitrin alleviates paw edema in DTH mice
The DTH assay was employed to evaluate the impact of quercitrin on immunoregulation.The results are shown in Table 2.Low doses of quercitrin had no significant effect on the paw edema.However,high doses of quercitrin and CsA alleviated the paw edemas in the DTH mice.Quercitrin had a similar effect on spleen cell numbers in the DTH mice(Table 2).

Fig.5 Toxicity of quercitrin in mice

Table 2 Percentage inhibition of paw swelling by quercitrin and CsA in DTH(n=8)
2.7 Quercitrin reduces CN activity,not expression,in cells
We investigated quercitrin inhibition of CN activityin vitrousing Jurkat cells,human T cell leukaemia cells.The Jurkat cells were cultured in 6‑well plates and treated with quercitrin for 24 h.Inhibition of CN activity by quercitrin was dose‑dependent,and theIC50value was 107.54 μmol/L(Figure 6a).
We also assessed the effect of quercitrin on CN expression in Jurkat cells.Figure 6b shows that quercitrin had no effect on the expression of CN.
2.8 Quercitrin inhibits of NFAT activity and IL-2 transcription
CsA,a classical CN inhibitor,exerts its immunosuppressive effect by inhibiting the CN‑NFAT pathway,and IL‑2 is one of the NFAT target genes.To test whether quercitrin also acted on this signaling pathway,we measured the effect of quercitrin on NFAT activity and IL‑2 transcript levels.As shown in Figure 6c and 6d,quercitrin inhibited both NFAT andIL‑2 transcription in a dose‑dependent manner.

Fig.6 Effect of quercitrin in Jurkat cells
2.9 Fluorescence quenching of CNA and its deletion mutant by quercitrin
First,we confirmed that 2% DMSO did not alter the basic shape of the emission spectrum of CNA,then examined the effect of quercitrin.Figure 7a shows that the fluorescence of CNA was gradually quenched as the concentration of quercitrin increased,and there was a slight blue shift of the maximum emission wavelength in the presence of quercitrin.We plotted regression curves according to Equation (1),(Figure 7b) and calculated aKQof (1.32 ± 0.37) ×1013L·mol-1·s-1(n=3,relative coefficientR>0.99),This was greater than 2×1010L·mol-1·s-1implying that a static quenching mechanism was operative;in other words,quercitrin and CNA form a complex that results in quenching of the fluorescence.We then plotted a regression line based on Equation(2)(Figure 7c),and calculated aKAof (1.60±0.40) × 106L·mol-1with a number of binding sitesnof (1.17 ±0.07) (R>0.99).These data show that quercitrin binds to CN with a stoichiometry of 1∶1,and the binding intensity is quite strong.

In order to explore more accurately the binding region between quercitrin and CNA,we used fluorescence spectroscopy to study the interaction between quercitrin and CNAa.As Figure 7d shows,the fluorescence of CNAa also gradually declined as the concentration of quercitrin increased.We plotted regression curves according to Equation (1)(Figure 7e) and calculatedKQto be (1.71 ± 0.24) ×1013L·mol-1·s-1(n=3,relative coefficientR>0.99),indicating that quercitrin and CNAa form a stable complex.We then plotted a regression line based on Equation (2) (Figure 7f),and calculatedKA=(2.30±0.43)×107L·mol-1and the number of binding sites to ben=(1.45±0.08)(R>0.99).These data indicate that the binding stoichiometry of quercitrin:CNAa was also about 1∶1,and the binding of quercitrin to CNAa was stronger than to CNA.
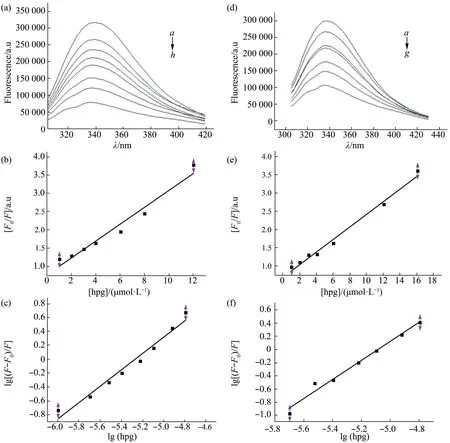
Fig.7 Fluorescence spectra of CNA and CNAa in presence of quercitrin
2.10 Possible sites of binding of quercitrin to CN in catalytic domain identified by docking analysis
We calculated 35 results,which could be ranked in 7 groups,generated by Autodock 4.There were two high‑ranked docking positions of quercitrin on CN,which were coherent with the fluorescence results.Their free energy of binding were-6.35 kcal/mol(site1) and -5.50 kcal/mol (site2) (Figure 8a).The actual amino acid residues in simulations of site1were Pro340,Trp342,Tyr124 (Figure 8b);and of site2Lys52,Ala48,Pro338(Figure 8c).

Fig.8 Docking of quercitrin to CN
3 Discussion
CsA and FK506 are widely used as immunosuppressants in organ transplantation as well as in the treatment of autoimmune disorders[36]in spite of side effects on liver,kidney,nervous system and other problems[14].In our search for alternative immunosuppressants we extracted flavonoids,the main component of the silk tree flower,and obtained quercitrin,which is the major flavonoid in these flowers.Quercitrin has been reported to be an anti‑oxidant[37],anti‑inflammatory[24‑25],to regulate the autoimmune response[26‑27]and to induce apoptosis[38].It is also an inhibitor of Sortase A[23].It is first reported to inhibit CN activity with both p‑NPP and32P‑RIIpeptide as substrate,unlike CsA and FK506[39].
Because of the important role of CN in immune regulation,we investigated the effect of quercitrin on immune reactions.Quercitrin was active in the mouse spleen lymphocytes proliferation assay,in responses to the T lymphocyte mitogen Con A,and the B lymphocyte mitogen LPS.It also inhibited the MLR in mice.Inhibition of the MLR was stronger than of T cell and B cell proliferation.Given the critical role that CN plays in T lymphocyte activation,we presume that quercitrin exerts its immunosuppressive effect by inhibiting CN activityin vivo.
DTH is a reaction in which a specific antigen activates T lymphocytes to release cytokines[40].In our experiments,quercitrin alleviated paw edema in a dose‑dependent manner in mice,which pointed to a suppressive effect on T cell‑dependent immune responsese.Quercitrin had the same effect as CsA at 40 mg/kg.In the DTH test described above,we observed that the mice of the CsA group displayed fur ruffling and emaciation,which were not seen in the quercitrin‑treated group,suggesting that quercitrin has lower toxicity than CsA.The toxic side effects of conventional medicine have received much attention in the last few years,with the result that more medicinal plants and their derivatives are being used as therapeutic alternatives[41].At present,CsA and FK506 are still widely used as immunosuppressants,but their long term side effects on liver,kidney and nerve[42‑44]are a serious disadvantage.Therefore,novel immunosuppressants with low toxicity and few side effects are greatly needed.In this study,we performed an acute toxicity test in mice with quercitrin,and used two different routes of administration,intraperitoneal injection and oral gavage.No mice died or exhibited abnormal reactions at doses of 2 000 mg/kg (i.p.) and 5 000 mg/kg (i.g)during the experiment,which indicates that theLD50is above 2 000 mg/kg with intraperitoneal injection and above 5 000 mg/kg with oral gavage.So we can consider quercitrin to have no or minimal toxicity.CsA has severe hepatotoxicity and nephrotoxicity,and can cause transaminase and total bilirubin levels to increase[45].Therefore we measured some specific biochemical indexes of liver and kidney function.The results of blood biochemistry showed that levels of serum creatinine,urea nitrogen,total bilirubin,alkaline phosphatase,glutamic‑oxaloacetic transaminase,glutamic‑pyruvic transaminase increased in mice receiving large intraperitoneal injections of CsA,but not in mice treated with quercitrin.Our previous study of quercitrin treatment of systemic lupus erythematosus in mice also showed that quercitrin had lower toxicity than CsA as long‑term medication[26].Overall,quercitrin had less toxic side effects on experimental animals than CsA,and this advantage may become more obvious in long‑term treatment.
Because CN controls T cell activity and other immune‑related activitiesviathe CN‑NFAT signaling pathway,we examined the relationship of quercitrin to the CN‑NFAT pathway in Jurkat cells and confirmed that quercitrin regulates T cells and immune activities by modulating CN‑NFAT signaling pathway as CsA does.
In our previous study,quercitrin inhibited CN activity with both substrates,unlike CsA and FK506 did.This suggested that quercitrin bound directly to CN.To explore the interaction between quercitrin and CN we used fluorescence spectroscopy and docking.Quercitrin caused fluorescence quenching of CNA,the stoichiometry of quercitrin and CNA was 1∶1 and the binding constantKAwas (1.60 ± 0.40) ×106L·mol-1,indicating strong binding.Quercitrin bound even more strongly to CNAa.It thus seems likely from all our results that quercitrin binds directly to the catalytic domain of CNA.To further explore the possible binding area of quercitrin with CN,we calculated each Trp‑quercitrin distances by energy transfer experiments,and generated possible concrete binding sites for quercitrin in quercitrin‑CN complex by Autodock 4 (Figure 8b,c),which all located in the catalytic domain of CNA but not the catalytic center[5].Additionally,Figure 7a shows that there was a slight blue shift of the maximum emission wavelength in the presence of quercitrin,which indicates protein secondary structure may change.Our study on CD spectra of CNA with quercitrin,which was inSupplementary data,showed a much tighter structure of the enzyme in presence of quercitrin.
In conclusion,our data indicate that quercitrin is a CN inhibitor of low toxicity that acts as an immunosuppressant by inhibiting the CN‑NFAT signaling pathway.Unlike CsA and FK506,it binds directly to the catalytic domain of CN.It may be a useful new immunosuppressive agent.
4 Conclusion
We used CN as the target enzyme to explore the inhibitory effect of natural compound quercitrin and its possible mechanism of action.Quercitrin inhibited CN activity bothin vitroand in intact cells,and also had good performance in reducing splenocyte proliferation and improving DTH symptoms in mice.The results of toxicological study showed that quercitrin had no or minimal toxic side effects on experimental animals.Fluorescence spectroscopy and molecular docking analysis showed that quercitrin may bind to two sites on the CN and participate in the regulation of immune response through the CN‑NFAT pathway.In summary,we identified a new CN inhibitor,quercitrin,which could be a useful novel immunosuppressant with low toxicity.
Supplementary materialPIBB_20210381_Doc_S1.pdf is available online(http://www.pibb.ac.cn or http://www.cnki.net).
杂志排行
生物化学与生物物理进展的其它文章
- Protein Aggregation and Phase Separation in TDP-43 Associated Neurodegenerative Diseases*
- 细菌的信号转导系统及其在耐药中的作用*
- CRBGP Inhibited The Activity of Glioma U251 Cells Through Suppressing FAK-AKT Pathway and The Secretion of Interleukin-6*
- Bradykinin Upregulated The Expression of Cyclooxygenase-2 in The Submucosal Plexus of Enteric Nervous System of Guinea Pig*
- 阿尔茨海默病体外诊断纳米技术*
- α-Synuclein as a Diagnostic Marker and Therapeutic Target for Parkinson Disease*
