Study on metabonomics in patients with acute coronary syndrome based on LC-MS technology
2022-02-05YUPengYANGZhejunSHIRuijieSUNWeixinCHENXiaohuSHENLe
YU Peng, YANG Zhe-jun, SHI Rui-jie, SUN Wei-xin,3, CHEN Xiao-hu, SHEN Le✉
1. Department of Cardiology, Jiangsu Province Hospital of Chinese Medicine, Nanjing 210029, China
2. First Clinical Medical College, Nanjing University of Chinese Medicine, Nanjing 210023, China
3. Department of Cardiology, Yancheng TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Yancheng 224001, China
Keywords:Acute coronary syndrome Unstable angina Acute myocardial infarction Metabolomics Differential metabolites
ABSTRACT Objective: To investigate the plasma metabolic components of patients with unstable angina(UA) and acute myocardial infarction(AMI), and to screen potential biomarkers and explore possible biological mechanisms in order to provide reference for early evaluation of acute coronary syndrome. Methods: Plasma samples from patients with UA and AMI were collected to obtain their general information, and the metabolites were detected by LCMS technology. Combined with univariate statistical analysis, the significantly different metabolites and their pathways were further determined by partial least squares discriminant analysis and orthogonalized partial least squares discriminant analysis models. Results: A total of 33 samples from UA group and 47 samples from AMI group were included for testing.Then 54 metabolites and 20 pathways were found to be significantly different between them.Metabolites such as pantoprazole, acetylcarnitine, palmitoyl ethanolamide, betaine, caprylic acid, bilirubin, histidine, oleic acid, citrate, vanillin can be used as potential biomarkers to distinguish them. The pathways include ABC transporters, aminoacyl-tRNA biosynthesis,central carbon metabolism in cancer and so on. Conclusion: There are significant differences in the plasma metabolic components of UA and AMI. Studies related to metabolomics may guide significance for exploring the biological nature of ACS.
1. Introduction
Acute coronary syndrome (ACS) is a type of syndrome characterized by acute myocardial ischemia and hypoxia leading to myocardial damage and even necrosis. It has epidemiological characteristics of high morbidity and high mortality. The clinical manifestations can be divided into three categories: unstable angina pectoris (UA), acute non-ST-segment elevation myocardial infarction (NSTEMI) and acute ST-segment elevation myocardial infarction (STEMI). The latter two can be collectively referred to as acute myocardial infarction (AMI), which is more severe [1-2].The occurrence of ACS is closely related to the formation and rupture of atherosclerotic plaques, with insidious progression and rapid onset, causing a heavy burden to society. In recent years, with the development of enzymology, myocardial necrosis markers,especially troponin I have gradually become an indispensable part in the diagnosis and risk classification of ACS. However, the early evaluation value is limited, because it often changes after 3-4 h of onset, which affects the prognosis and outcome to a certain extent.Therefore, it is urgent to find new specific biomarkers for the early identification and intervention of ACS.
Metabolomics originated in the 1990s and is mostly used to quantify and characterize small molecule metabolites, which has been widely used in medicine, chemistry, pharmacology,toxicology, botany and other fields today [3]. The technical methods of metabolomics mainly include nuclear magnetic resonance(NMR) and mass spectrometry (MS). Compared with NMR,MS technology is more sensitive and has a wider coverage [4].Untargeted metabolomics, as a kind of metabolomics, is well known for its advantages of simultaneous quantitative analysis of large-scale data, and has achieved remarkable results in the pathophysiological mechanisms of the cardiovascular system over the years [5]. Studies have shown that although the endophenotype of ACS is similar, the large individual biological differences and unknown mechanisms provide an opportunity for the application of non-targeted metabolomics [6]. Past metabolomic studies on ACS have found a large number of potential biomarkers, such as alanine,valine, homocysteine, etc., but most of them focus on comparison with healthy subjects, and ACS has not been disassembled. In this study, patients with UA and AMI were used as the research subjects, and LC-MS technology was used to analyze metabolites and improve the KEGG enrichment pathway, in order to better understand the metabolic differences and nature between the two.
2. Materials and methods
2.1 Case source
A total of 80 ACS patients who were admitted to the Cardiovascular Department of Jiangsu Province Hospital of Chinese Medicine from November 2019 to October 2021 and met the inclusion criteria were retrospectively selected, including 33 UA patients and 47 AMI patients. This study was approved by the Ethics Committee of Jiangsu Province Hospital of Chinese Medicine. Approval Number:2018NL-105-03.
2.2 Diagnostic criteria
It is based on the "2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation" [7], "Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes(2016)" [8] and "Guidelines for the diagnosis and treatment of acute ST-segment elevation myocardial infarction (2019)" [9].
(1) UA: ① resting angina pectoris: lasting more than 20 minutes;② new-onset angina pectoris: spontaneous or exertional attack;③ exacerbating angina pectoris: past history of related medical conditions, symptoms worsening within the past 30 d; or appearing within 30 d after myocardial infarction.
(2) AMI: including NSTEMI and STEMI.NSTEMI: The symptoms are more severe than UA and may present as retrosternal crushing pain, which may radiate to left upper arm,neck, or jaw. The 12-lead ECG may show ST-segment depression in 2 or more adjacent leads and the serum troponin (cTn) is increased,with at least 1 time higher than the upper limit of normal.STEMI:There is severe squeezing pain in the retrosternal or precordial area,which can radiate to the left upper arm, jaw, neck, shoulder and back. It often accompanies with nausea, vomiting, sweating, and dyspnea, which cannot be completely relieved by nitroglycerin. The 12-lead ECG show that the ST-segment was arched upward. The serum troponin (cTn) is increased, with at least 1 time higher than the upper limit of normal.
2.3 Inclusion criteria
(1) They are all between the ages of 30-70, regardless of gender;
(2) All of them met the diagnostic criteria of Western medicine and the above-mentioned TCM syndrome differentiation criteria;
(3) They all voluntarily participated in the research and signed the informed consent.
2.4 Exclusion criteria
(1) Those with functional impairment of important organs;
(2) Those with a history of severe gastrointestinal bleeding and/or intracranial hemorrhage;
(3) Pregnant or breastfeeding women;
(4) Those currently participating in other clinical trials.
2.5 Research Methods
2.5.1 Clinical data collection
(1) General information of the enrolled patients was collected,including gender, age, and past history.
(2) Blood was collected after 8 hours of fasting for:
a. Blood routine, including white blood cell count (WBC), red blood cell count (RBC), platelet count (PLT), hemoglobin (Hb), and percentage of neutrophils (N%);
b. Biochemical, including alanine aminotransferase (ALT),aspartate aminotransferase (AST), glucose (Glu), total cholesterol(TC), triglycerides (TG), low density lipoprotein cholesterol(LDL-C), high density lipoprotein cholesterol (HDL-C);
c. Serum Troponin I (cTnI);
d. Metabolomics related tests.
(3) Echocardiography was performed and left ventricular enddiastolic diameter (LVIDd), left ventricular end-systolic diameter(LVIDs), and ejection fraction (EF) were recorded.
2.6 Metabolomics analysis
2.6.1 Instruments and Reagents
Triple TOF 5600/6600 mass spectrometer; Agilent 1290 Infinity LC ultra-high liquid chromatograph; Cryogenic high-speed centrifuge;Column: Waters, ACQUITY UPLC BEH Amide 1.7 µm, 2.1 mm×100 mm column; Waters, ACQUITY UPLC HSS T3 1.8 µm,2.1 mm×100 mm column; acetonitrile; ammonium acetate.
2.6.2 Sample collection and preparation
The cubital venous blood samples were collected, centrifuged at 1 000 rpm for 10 min at 4 ℃, and the supernatant plasma was collected and stored at -80 ℃. After excluding the partially hemolyzed samples, the frozen samples were taken out and lysed slowly at 4 ℃. Then, 100 µL samples were respectively taken, 400µL of pre-cooled methanol and acetonitrile solution was added, and the vortex time was 60 s. Then they were placed at -20 ℃ for 1 h,centrifuged at 14 000 RCF for 20 min at 4 ℃, and the supernatant was lyophilized and stored at -80 ℃.
2.6.3 Chromatography and mass spectrometry
Chromatographic conditions: The samples were separated using an Agilent 1290 Infinity LC Ultrahigh Liquid Chromatography System HILIC column. Mobile phase: A is an aqueous solution containing 25 mmol/L ammonium acetate and 25 mmol/L ammonia, and B is acetonitrile. The flow rate of the mobile phase was 0.3 mL per minute, and the column temperature was 25 ℃. The gradient elution program is: 0~0.5 min, 95% B; 0.5-7 min, 65% B; 7-8 min, 40% B;8-9 min, 40% B; 9-9.1 min, 95% B; 9.1-12 min, 95% B.
Mass spectrometry conditions: Electrospray ionization (ESI) was used for detection in positive and negative ion modes, respectively.After sample detection, primary and secondary spectra were collected using an AB Triple TOF 6600 mass spectrometer. The ESI conditions after HILIC chromatographic separation are: Gas 1: 60,Gas2: 60, Curtain gas: 30, source temperature: 600℃, IonSapary Voltage: ±5 500 V; TOF MS scan m/z range: 60-1 000 Da, production Scan m/z range: 25-1 000 Da, TOF MS scan accumulation time:0.20 s/spectra, production scan accumulation time: 0.05 s/spectra;secondary mass spectrometry was acquired using information dependent acquisition and high sensitivity mode. Declustering potential: ±60 V, Collision Energy: 35 ±15 eV, IDA settings are as follows: Exclude isotopes within 4 Da, Candidate ions to monitor per cycle: 6.
2.7 Statistical processing
After the data were preprocessed by Pareto-scaling, multivariate and univariate statistical analysis was performed.
3. Results
This study finally included 80 patients, including 33 UA patients and 47 AMI patients.
3.1 General situation
Gender, history of hypertension, history of type 2 diabetes, and history of atrial fibrillation were compared between the UA and AMI groups: Gender (P=0.438), history of hypertension (P=0.238),history of diabetes (P=0.408), history of atrial fibrillation (P=0.362),with no significant difference.
Age, WBC, RBC, PLT, Hb, N%, ALT, AST, Glu, TC, TG, LDL-C,HDL-C, cTnI, LVIDd, LVIDs, EF were compared between UA and AMI groups: Age (P=0.571), RBC (P=0.642), PLT (P=0.691),Hb (P=0.642), Glu (P=0.509), TG (P=0.694), HDL-C (P=0.794),LVIDd (P=0.447), with no significant difference. And WBC(P=0.032), N% (P=0.037), ALT (P=0.001), AST (P=0.002), TC(P=0.007), LDL-C (P=0.004), cTnI (P=0.016), LVIDs (P=0.020),and EF (P=0.025), with significant differences.
3.2 PLS-DA
The PLS-DA model was established, and the parameters (R2Y, Q2)were shown in Table 2. It can be seen from the PLS-DA score charts in Figure 1A, B that the UA group and the AMI group have a certain degree of discrimination in both positive and negative ion modes,suggesting that there are metabolic differences between the two groups of plasma samples.
3.3 OPLS-DA
Subsequently, the OPLS-DA model was established, and the parameters (R2Y, Q2) were shown in Table 3. It can be seen from the OPLS-DA score maps in Figure 1C, D that the UA group and the AMI group have a certain degree of discrimination in the positive and negative ion modes, suggesting that there are metabolic differences between the two groups of plasma samples.
3.4 Univariate statistical analysis
Among the commonly used univariate statistical analysis methods,in addition to fold-of-variance analysis and t-test, there is also a Volcano Plot that integrates the first two methods. In the volcano plot in Figure 2, the red and blue dots are the differential metabolites screened by univariate statistical analysis, red is up-regulated, and blue is down-regulated.
3.5 Significantly different metabolites
In general, metabolites with similar expression patterns clustered within the same cluster. From the heat map of differential metabolite clustering in Figure 3, it can be seen that there are differences in positive and negative ion patterns between the UA group andthe AMI group, suggesting that the metabolic patterns of the two groups are significantly different, and there may be many significant differential metabolites.
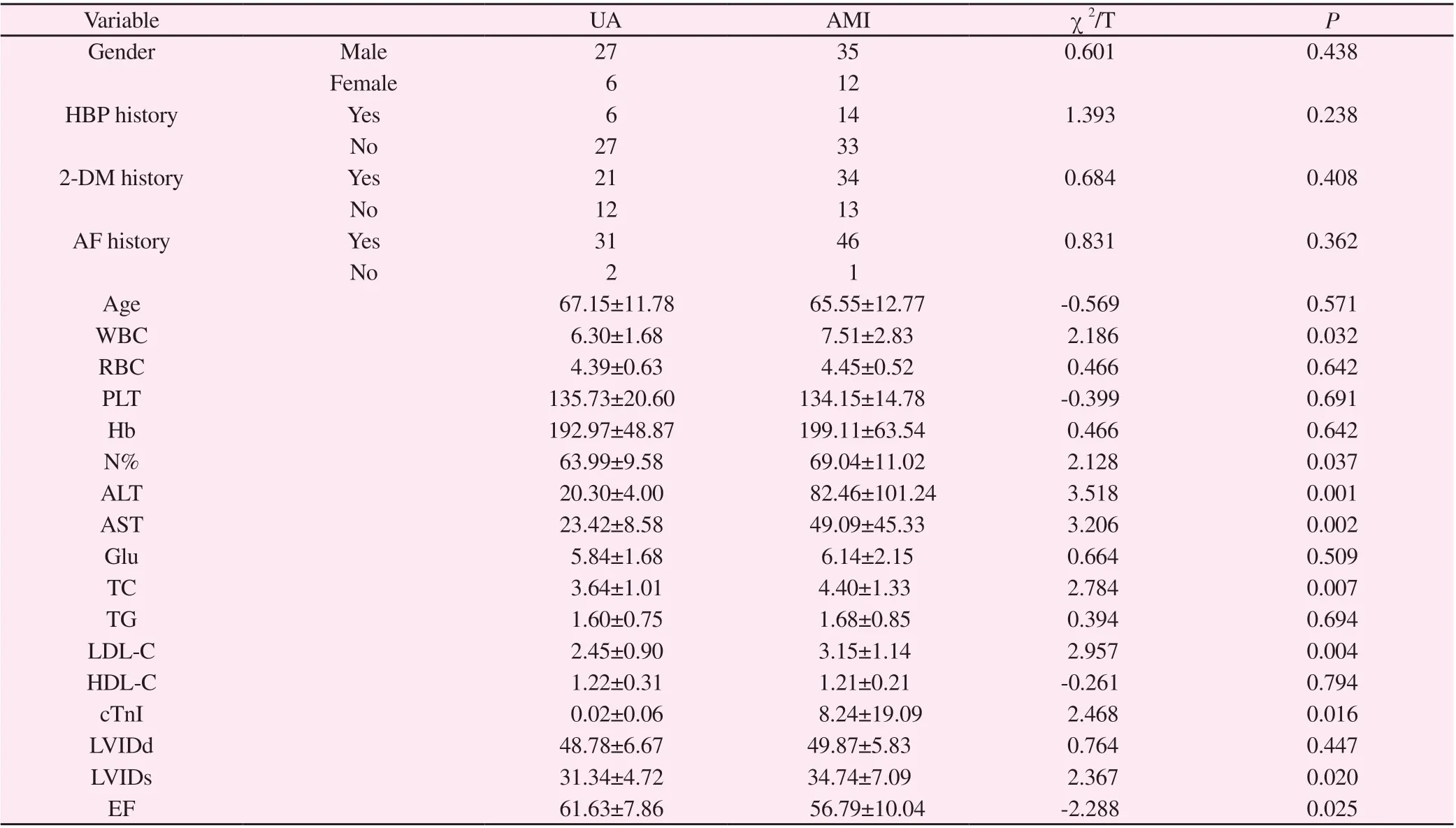
Tab 1 Comparison of general condition between UA group and AMI group
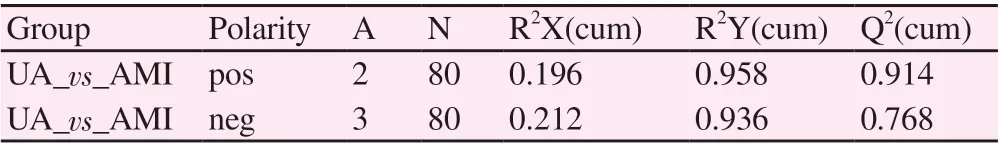
Tab 2 PLS-DA model parameters

Tab 3 OPLS-DA model parameters
With VIP>1.0 as the standard, combined with P value, the differential metabolites of UA group and AMI group were screened. As shown in Table 4, there are 54 metabolites with significant differences, including pantoprazole, acetyl-L-carnitine,palmitoylethanolamide, betaine, caprylic acid, bilirubin, histidine,oleic acid, and citrate acid, vanillin, etc. Among them, there are 32 metabolites with significant difference in positive ion mode and 22 in negative ion mode.

Tab 4 Different metabolites of positive and negative ion modes

Name Adduct Description VIP FC Trend M181T336 - 2-Oxoadipic acid 1.343 00 0.883 71 down M281T125 - Oleic acid 1.197 81 1.114 51 up M101T63 - Valeric acid 1.189 70 0.772 99 down M223T34 - Methyl linolenate 1.140 67 0.664 87 down M233T476 - Acetylglycine 1.089 53 0.717 29 down M149T158 - D-Lyxose 1.031 18 0.792 44 down M149T124 - D-Ribose 1.013 25 0.871 16 down

Fig 1 Scores of PLS-DA and OPLS-DA models
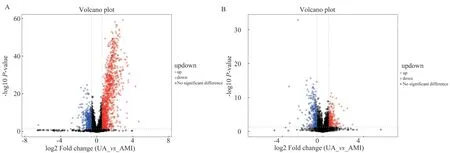
Fig 2 Volcano plot of positive and negative ion modes
3.6 KEGG pathway enrichment analysis of differential metabolites
KEGG pathway enrichment analysis was performed on significantly different metabolites by Fisher's exact test to identify the affected metabolic pathways. As shown in Figure 4, pathways such as ABC transporters, Aminoacyl-tRNA biosynthesis, and Central carbon metabolism in cancer were significantly changed.

Fig 3 Clustering heat map of significant different metabolites of positive and negative ion modes
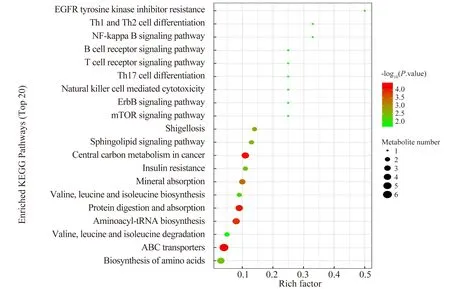
Fig 4 Enrichment analysis of KEGG pathway
4. Discussion
ACS is one of the common clinical critical illnesses. The current diagnosis relies on symptoms, electrocardiogram, myocardial markers, etc. However, for patients with less typical symptoms, it is easy to lead to misdiagnosis, suggesting that there may be other unknown factors in the pathogenesis of ACS to a certain extent,which needs to be further explored. In the past, there have been literatures [10-11] comparing the metabolomic characterization of ACS and non-ACS patients, however, the differential metabolites of UA and AMI have not been reported. In this study, ACS was divided into UA and AMI for the first time, and 33 UA patients and 47 AMI patients were retrospectively investigated and analyzed,whose general conditions, blood routine, blood biochemistry and cardiac function were compared. The results showed that there was no significant difference in gender, age, history of hypertension,history of diabetes, history of atrial fibrillation, RBC, PLT, Hb,Glu, TG, HDL-C and LVIDd between the two groups (P>0.05).But there were differences in WBC, N%, ALT, AST, TC, LDL-C,cTnI, LVIDs, and EF (P<0.05), suggesting that UA and AMI may have differences in inflammation, myocardial enzyme spectrum,cholesterol metabolism, and cardiac function.
Further non-targeted LC-MS metabolomic analysis of the plasma samples from the included patients showed that the metabolic levels of the two were different. With VIP>1.0 and P value<0.05 as indicators, 54 metabolites with significant changes were detected,such as pantoprazole, acetyl-L-carnitine, palmitoylethanolamide,betaine, caprylic acid, bilirubin, histamine Acid, oleic acid, citrate,vanillin, etc., involving lipids, phenols, keto acids, carboxylic acids,steroids, hydroxy acids and other categories. The results showed that substances with relatively high contents in the UA group included pantoprazole, acetyl-L-carnitine, betaine, caprylic acid, vanillin,L-lysine, proline, phenylalanine, valine, Valeric acid, acetylglycine,D-ribose, while relatively high levels of substances in the AMI group included palmitoylethanolamide, D-proline, ethylene glycol monoethyl ether, sarcosine, oxidized proline, bilirubin , L-leucine,histidine, oleic acid, citrate, lansoprazole, etc. At the same time,metabolic pathways derived from KEGG pathway enrichment analysis contained ABC transporters, aminoacyl-tRNA biosynthesis,and central carbon metabolism in cancer. Here, we briefly discuss the main differential metabolites and metabolic pathways.
Pantoprazole (PPZ), Acetylcarnitine (ALC), Betaine, Caprylic acid and Vanillin were up-regulated in the UA group. Among them, PPZ can reduce the release of pro-inflammatory cytokines and inhibit tissue damage associated with oxidative stress, inflammation and dyslipidemia [12]. In a rat experiment, PPZ was shown to reduce ischemia-reperfusion injury by inhibiting the intracellular signaling pathway MAPK (ERK1/2, JNK, p38)-NF-κB [13]. ALC is an endogenous molecule that participates in energy metabolism and has antioxidant properties, which can effectively protect free radicals,restore and stabilize mitochondrial activity, and regulate brain neurotransmitters, such as acetylcholine, dopamine, amino acids,lipids, etc. It can also protect tissue from ischemia-reperfusion injury,which may be related to its high expression in the UA group [14-15].Betaine can improve sulfur amino acid metabolism under oxidative stress, inhibit nuclear factor-κB activity and NLRP3 inflammasome activation, reduce endoplasmic reticulum stress and apoptosis, and play an anti-inflammatory effect [16-17]. At the same time, betaine can prevent the further development of isoprene-induced myocardial dysfunction and improve cardiac function by way of antioxidant and mitochondrial protection [18]. Caprylic acid, as a medium-chain fatty acid, has been found in the past to be effective in inhibiting the progression of atherosclerosis and endothelial dysfunction. Further in vitro experiments showed that caprylic acid could inhibit the expression of inflammatory cytokines in plasma through the TLR4/NF-κB signaling pathway and improve atherosclerosis in ApoEdeficient mice. It is speculated that caprylic acid may be one of the potential substances to prevent and improve atherosclerosis and related chronic inflammatory diseases [19]. In addition, multiple studies have revealed that vanillin and its main metabolites have certain therapeutic potential in regulating inflammatory response and oxidative stress, and it is expected to be promoted in inflammatory diseases in the future [20].
The expressions of Palmitoyl ethanolamide (PEA), Bilirubin,Histidine, Oleic acid and Citrate were up-regulated in the AMI group. Among them, PEA widely exists in nerve cells, microglia,immune cells and other tissue cells related to inflammation, and produces analgesic and anti-inflammatory effects by activating PPAR-α [21]. Besides, the increase of PEA level may be correlated with the deterioration of coronary artery function, and can predict coronary endothelial dysfunction to a certain extent, especially when PEA is less than 2.465 ng/mL, it can better predict endothelialdependent coronary artery expansion [22]. Bilirubin not only has the ability to scavenge overproduced reactive oxygen species and inhibit the proliferation of vascular smooth muscle cells, but also attenuate the chemotactic activity of monocytes and reduce the production of pro-inflammatory cytokines, the content of which is inversely proportional to cardiovascular disease and cardiovascular risk factors, so it is also a protective factor for atherosclerosis [23-24]. Histidine, an alpha-amino acid, is inversely related to markers of inflammation. When the content of histidine decreased, the concentration of pro-inflammatory cytokines continued to rise, and supplementation of histidine has the function of reducing oxidative stress, thereby alleviating myocardial injury caused by AMI [25].The anti-inflammatory and anti-apoptotic activities of oleic acid play an important role in the process of myocardial repair, including decreasing the lipid peroxidation level in cardiac tissue, enhancing antioxidant capacity, and reducing collagen fiber deposition [26]. In recent years, citrate has gradually become a target of much concern in inflammation [27]. Studies have confirmed that citrate pretreatment can improve myocardial ischemia-reperfusion injury in mice, reduce myocardial cell apoptosis, and increase left ventricular ejection fraction and cardiac output, thereby improving cardiac function [28].
As one of the pathways obtained in the KEGG enrichment analysis,ABC transporters are widely involved in various physiological processes such as cell metabolism, and are expressed in adipocytes,macrophages and various peripheral tissue cells [29-30]. The ABC transporter family is divided into 7 subtypes, among which ABCA,ABCG and other subfamilies play important roles in platelet production and activity [31-32]. Several studies have shown that ABC transporters have the function of promoting cholesterol efflux. Correcting platelet hyperactivity and reducing thrombosis by decreasing plasma membrane cholesterol accumulation may be a significant target for slowing the development of atherosclerosis and preventing atherothrombosis [33]. Aminoacyl-tRNA biosynthesis has been found to be related to gastric cancer in recent years.The aminoacyl-tRNA biosynthesis pathway in gastric tissue is significantly up-regulated relative to adjacent non-cancerous tissues.Targeting this pathway may be a new strategy for the treatment of gastric cancer. However, its application in cardiovascular disease has not yet been reported [34].
In conclusion, this study characterized the plasma metabolic levels of UA and AMI patients by non-targeted metabolomics method,and obtained 54 differential metabolites, which can be used to assist in the clinical differentiation and diagnosis of UA and AMI,and also provides a new idea for sorting out the pathogenesis of the two. However, due to the limited sample size and the failure to include blank controls in the study, there is a lack of qualitative and quantitative data. Therefore, it is uncertain how the differences in metabolites in the results affect the onset or progression of UA and AMI. On the other hand, considering that there are many interference factors, the age, disease course, past medical history,and cardiac function classification of the included patients should be further controlled in the screening process in the future, and the baseline of the included patients should be kept consistent as much as possible, so as to provide reference for the identification of UA and AMI. In addition, it is necessary to establish a prospective, largesample cohort study with blank controls in the future, and actively build in vivo and in vitro models to observe the effects of metabolites on cells or mice, providing scientific evidence for the application of metabolomics in the field of early prevention and treatment of cardiovascular diseases.
In Summary, as an emerging technology, metabolomics has provided new insights into the pathological mechanisms of many diseases, including cardiovascular diseases [35]. In this study, a nontargeted metabolomics method based on LC-MS technology was used to analyze the differential metabolites and related pathways in patients with UA and AMI. These differences may reflect the developmental process of atherosclerosis in ACS and may provide directions for early diagnosis of ACS. However, this preliminary conclusion is still limited by a variety of factors, and further validation and support are needed to identify more appropriate biomarkers to assist in the assessment of the progression of ACS, and to further identify targets for early intervention to improve prognosis.
Author’s Contribution:
YU Peng and SHEN Le were responsible for the analysis of relevant data and the writing of the article; YANG Zhe-jun and Shi Rui-jie participated in the collection and arrangement of the data;SUN Wei-xin was responsible for the conception and revision of the article. CHEN Xiao-hu guided the article. All authors have read and agreed to the final text.
All authors declare no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- Research advances in functional heartburn based on Rome Ⅳ criteria
- Research progress on modern pharmacological action of Radix bupleuri
- Tanreqing injection auxiliary in the treatment of heart failure with pulmonary infection: A systematic review
- Mechanism of total flavonoids in the treatment of rheumatoid arthritis based on network pharmacology
- Clinical characteristics of 72 cases with neuromyelitis optical associated optic neuritis
- Application of SOAT1 combined with multiple markers in the auxiliary diagnosis of hepatocellular carcinoma
