Review:Aggregation-Induced Emission—A New Tool to Study Polymer Thermodynamics and Kinetics
2022-02-04,,,,
,,,,
(State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,College of Materials Science and Engineering,Donghua University,Shanghai 201620,China)
Abstract:Polymer thermodynamics and kinetics are important components in the basic theory of polymer physics,which provide critical support for polymer processing and molding.As an important thermal analysis technology,differential scanning calorimetry(DSC)is a key way to explore the molecular motion of polymer chains,molecular structure,and condensed structure,greatly promoting the development of polymer materials.However,this technique is limited by its ambiguous results,because of inaccurate heat flow measurement and high parameter dependence.As an alternative strategy,aggregation-induced emission luminogens(AIEgens)have been extensively applied in various targets analysis and process monitoring,owing to their weak intermolecular interactions and highly twisted conformation.The optical properties of AIEgens are highly sensitive to the variations of the polymer microenvironment,including characteristic transition,crosslinking reaction,crystallization behavior,and phase separation.In this review,the progress of AIE technology in visualizing polymer molecular motion and structure evolution is summarized,compensating for the limitation of the traditional DSC method to facilitate further research in polymer science and engineering.
Keywords:aggregation-induced emission,thermodynamics,kinetics,polymer physics,visualization
0 Introduction
The molecular motion of polymers has a significant influence on polymer processing and forming temperature,while polymer condensed structure is associated with final properties.These characteristics could be investigated by thermoanalytic techniques,such as differential thermal analysis,dynamic thermomechanical analysis(DMA),and differential scanning calorimetry(DSC).Among these,DSC is a fashionable tool.The primary signal measured during DSC detection is the difference in heat flux or power between the sample and the reference[1].DSC provides the results for analysis of polymer melting and crystallization[2-4],glass transition temperature[5-7],phase change[8-9],crosslinking reaction[10-11],crystallinity[12-13],compatibility[14-15],etc.This technique is especially critical for testing polymer materials at different temperatures in polymer science and engineering.It establishes a relationship between microscopic structure and macroscopic function of polymers and is important in the design of manufacturing processes.However,DSC technology encountered several problems:(i)The DSC results are highly dependent on a set of experimental parameters[16].For example,rapid heating rates result in an insufficient change of the sample during the measurement,compromising the accuracy of the results to some extent.The small size of the sample leads to an indistinguishable sample peak from any noise in the baseline,while a too large sample size causes a delay in heat transfer.(ii)The DSC results are sometimes equivocal[17].In some block copolymers,the heat flow involved in the glass transition of the minor component is likely to be absorbed by the major components,making it difficult for accurate detection.(iii)DSC technology is a destructive technique[18].The properties of the samples at the initial state could be hardly recovered after thermal analysis.Therefore,developing new technology to address the above limitations would be very rewarding.
Fluorescence technology is a desired tool that offers a sensitive,convenient,precise,and easyreadable choice for visual imaging and detection[19-21],especially in the field of monitoring polymer molecular motion and microstructure evolution[22-24].However,for most traditional fluorescent probes,their emissions are normally weakened or quenched in the condensed polymer matrix.This phenomenon is well recognized as aggregation-caused quenching(ACQ).Fortunately,aggregation-induced emission luminogens(AIEgens)show the opposite character.They are weak or nonemissive when isolated,but exhibit intense emission in aggregate,which is explained by the working mechanism of restriction of intramolecular motion(RIM)[25-26].Owing to the weak intermolecular interactions and highly twisted conformation of AIEgens,their optical properties are greatly sensitive to the alterations in the polymer microenvironment.Therefore,the information on the molecular motion of polymer chains and multiple condensed structures could be visualized.Compared with the traditional DSC technology,AIE fluorescence technology shows several advantages:(i)AIE probes embedded in the polymer matrix exhibit a highly sensitive,good reversible,and fluorescence-variable response to the polymer microenvironment,providing a direct visual way to distinguish the polymer state when exposed to excitation light.(ii)AIE provides a remote,touchless,and noninvasive sensing technique for polymer thermal analysis,showing great potential to study the internal state of polymer materials and the structural evolution process with high spatial and temporal resolution.(iii)AIE probes as“built-in”sensors in the polymer matrix have the capability to indicate the changes of the polymer at the molecular state,showing comprehensive information that might not be accessed by DSC technology(Fig.1).

Fig.1 Comparison between DSC technology and AIE technology in terms of polymer thermodynamics and kinetics
In this review,the focus is on the current developments in AIE technology for the thermal analysis of polymer materials.Specifically,the advances of AIEgens are introduced to visualize the polymer characteristic transition, crosslinking reaction,crystallization behavior,and phase separation.The merits of AIE technology are discussed compared with the traditional methods.We envision this review will inspire more AIE work to focus on the study of polymer thermal analysis,which will provide a new opportunity for smart polymer manufacturing.
1 Thermal Analysis of Polymers by AIE Technology
1.1 Characteristic Transition
The mechanical state of polymer will greatly change at some specific temperatures due to its unique multi-level structure,affecting the processing and use of polymer materials.For example,the glass transition temperature(Tg)is the temperature range where the polymer changes from a rigid glassy state to a soft(not melted)state owing to the molecular motions of polymer chains.Tgdetermines the end-use temperature and is related to the strength of polymer materials.DMA and DSC are common methods to detectTg.The former can determineTgby storage modulus curve,loss modulus curve,or loss factor curve,but strict requirements for sample preparation are imposed and the results are easily affected by parameter settings such as oscillation mode and frequency.The latter determinesTgbased on the baseline alteration caused by the change in heat capacity[27].In addition,for some block copolymers,like styrene-butadiene-styrene block copolymer(SBS),it is difficult to give the accurateTgof the minor component by DSC.Nevertheless,AIE technology can make up for these deficiencies well.Taking SBS as an example,Qiu et al.[28]incorporated AIE probes of DPA-IQ into the polystyrene(PS)block in the SBS matrix based on the higher structure similarity of DPA-IQ with PS.Then they used a camera to monitor fluorescence changes during the heating process(30-130℃)(Figs.2(a),(b)).BelowTgof the PS block,the fluorescence of DPAIQ shows intense emission because of the rigid properties of the PS block based on the RIM effect;aboveTgof the PS block,the fluorescence intensity gradually decreased due to the soft environment in the PS block(Fig.2(c)).The fluorescent images of DPA-IQ dopped SBS film were recorded at different temperatures and then analyzed by using a MATLAB program.As a result,an evident change was observed at 93.6℃(Tgof the PS block)by plotting a graph of grayscale of fluorescent images against temperature,which could not be easily obtained by DSC technology(Fig.2(d)).Similarly,Tgof other common polymers,such as PS,polymethyl methacrylate(PMMA),and polyvinyl chloride,has been also successfully detected.
Unlike thermoplastics discussed above,thermosets are polymers in which an extensive crosslinking reaction occurs to promote chemical bonding between polymer chains and create a threedimensional(3D)network.Thermosets are generally stronger than thermoplastic materials,which have been generally used in diverse areas including protective coating,seamless flooring,civil engineering construction,etc.However,they could not be reprocessed or recycled due to the chemical bonded 3D network.Vitrimers came to being.They can flow like viscoelastic liquids at high temperatures but behave like classical thermosets at low temperatures,during which,the bond-exchange reactions are dominated and vitrimers are allowed to be easily processed in a wide temperature range like a self-healing material[29-30].For vitrimers,the characteristic temperature of topology freezing transition temperature(Tv)is critical to determine the upper limit utilization temperature and reprocessing temperature.Generally,dilatometry test and stressrelaxation test measured by rheology or DMA are utilized to measureTv.However,all of these technologies need to apply an external force to the sample,which affects the effective activation energy of the exchangeable reaction.Thus,it is difficult to detect the intrinsicTv.Fortunately,Yang et al.[31]introduced AIEgen into the vitrimer network to achieveTvmeasurement in static-situation.As shown in Fig.2(e),when the temperature was lower thanTv,the exchangeable reaction in the vitrimer network was slow,which inhibited the intramolecular motion of TPE(inset of Fig.2(f))and resulted in comparatively strong fluorescence emission.Meanwhile,when the temperature was higher thanTv,the rearrangement of the vitrimer was activated to allow the intramolecular motion of TPE,leading to the quickly diminished fluorescence intensity.In the plot of temperature versus fluorescence shown in Fig.2(f),a turning point(~92℃)was indicated asTv.It is worth noting that this method has the merits of good reproducibility,high sensitivity,and independence of heating rate,resulting in reliable data for studying the characteristics of the vitrimer network as temperature varies.

Fig.2 Characteristic temperature detection.(a)Chemical structure of DPA-IQ.(b)Fluorescence images of SBS films doped with DPA-IQ at various temperatures.(c)Mechanism of AIEgen in Tg detection.(d)(Left)Fitting curve of relative grayscale of DPA-IQ-doped SBS films versus temperature and(middle)corresponding second derivative.(Right)DSC thermogram of SBS.(Reproduced with permission[28],Copyright 2017,American Chemical Society.)(e)Mechanism of AIEgen in Tv detection.(f)(inset)Relationship between the fluorescence intensity of TPE-doped vitrimer and temperature with chemical structure of TPE as the inset.(Reproduced with permission[31],Copyright 2019,Springer Nature.)
As another important characteristic temperature,melting temperature(Tm)is a temperature that changes in the physical state from solid to liquid,which is related to the polymer processing temperature.Likewise,AIE technology has also been demonstrated to be able to detect theTmof polymers[32].Song et al.[33]incorporated TPE-4COOH as a built-in sensor into the polylactic acid matrix by virtue of hydrogen bonding.Upon heating,the fluorescence intensity of TPE-4COOH decreased accordingly due to the RIM effect.BothTgandTmof polylactic acid were simultaneously detected by recording the changes in fluorescence intensity as the system temperature increased from 40℃to 200℃.Besides the RIM effect,the twisted intramolecular charge transfer(TICT)effect is also promising for studying the melting behavior of polymers.Xue et al.[34]used conjugated electron-donor(D)-acceptor(A)structured AIEgen of TICT@AIE to construct a sensitive thermometer with multiple emission colors(Fig.3(a)).For D-A structured AIEgen,the TICT effect is a general phenomenon in this system[35].Their emission properties are sensitive to the aggregation state and the surrounding polar environment(Fig.3(b)).Then TICT@AIE molecules were incorporated into a phase-change material mixed with lauric acid and stearic acid.When the temperature was higher than the melting point of the phase-change material(>39℃),TICT@AIE molecules were homogeneously dispersed in the material melt.According to the principle of“like dissolves like”,the isolated AIEgens in the local hydrophobic environment generated blue emission.Below the melting point,however,the high crystallinity of the phase-change material forced TICT@AIE to form aggregates in the system.TICT@AIE exhibited red-shifted emission because of the polar microenvironment created by themselves by virtue of their own dipole moment(Fig.3(c)).Thus,the relationship between fluorescence properties and temperature was established for phase change visualization(Fig.3(d)).This brings a very useful inspiration for the development of new methods to study the melting behavior of polymers.
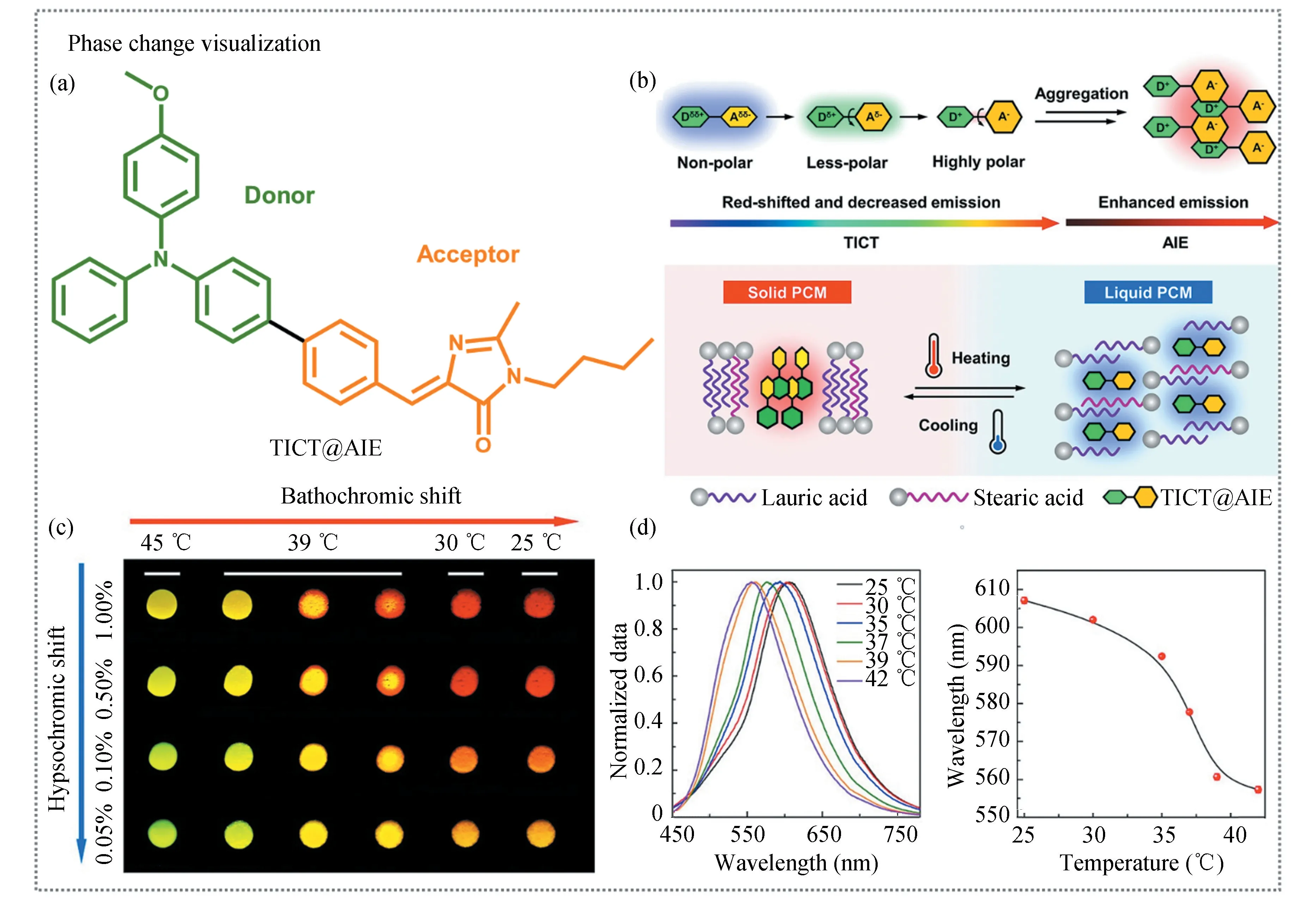
Fig.3 Phase change visualization.(a)Chemical structure of TICT@AIE.(b)Schematic illustration of TICT@AIE responding to the environment polarity and aggregation state.(c)Fluorescence images of the mixture of lauric acid and stearic acid doped with TICT@AIE at a series of temperatures.(d)(Left)Emission spectra of the mixture of lauric acid and stearic acid doped with TICT@AIE at various temperatures and(right)plot of the maximum emission wavelength varies with temperature.(Reproduced with permission[34],Copyright 2021,American Chemical Society.)
1.2 Crosslinking Reaction
The crosslinking reaction shows exothermal effects in DSC measurements and then the characteristic reaction temperature and the reaction enthalpy could be determined.In addition,the reaction kinetics could be indicated.The rheological test can also be used to study the crosslinking reaction according to the storage modulus curve and loss modulus curve.Another possible method of tracking crosslinking reaction is AIE technology.Wang et al.[36]used fluorescence imaging technology to study the crosslinking network evolution of chitosan in a lithium hydroxide-urea aqueous solution system.TPE was introduced onto chitosan chains by chemical labeling to form TPE-CS(Fig.4(a)),then the crosslinking state of TPE-CS was studied utilizing a confocal laser scanning microscope.As shown in Figs.4(b)-(d),the intramolecular motion of TPE was activated in the mobile polymer solution before gelation,generating a weak and uniform luminescence pattern in the fluorescence image.Upon heating,the homogeneous polymer solution underwent a sol-gel transition process,promoting the formation of intermolecular/intramolecular hydrogen bonds and the crystalline region of CS.Thus,the crosslinked network strongly restricted the intramolecular motion of TPE to activate the radiative decay with enhanced fluorescence emission.As a result,some bright emissive areas could be observed in the fluorescence image.The hydrogen bonds were further developed after the removal of lithium hydroxide and urea.In the fluorescence image,the bright areas were subdivided and contracted,eventually forming a network with alternating bright and dark areas.The crosslinking state of chitosan during the gelation process could be successfully visualized from the AIE fluorescence imaging.

Fig.4 Crosslinking reaction detection.(a)Chemical structure of TPE-CS.(b1,c1,and d1)Confocal laser scanning fluorescence microscope images of the(b1)TPE-CS solution,(c1)TPE-CS gel after thermal treatment,(d1)TPE-CS hydrogel after rinse procedure and(b2,c2,and d2)corresponding schematic illustration for the formation of crosslink network.Scale bar:250 μm.(Reproduced with permission[36],Copyright 2016,Springer Nature.)(e)Molecular structure of TPE-CS and PMFM.(f)Schematic diagram of“ON/OFF”fluorescence behavior caused by reversible crosslinking.(Reproduced with permission[37],Copyright 2020,Wiley-VCH.)
Different from the above-mentioned mechanism of RIM,Jiang et al.[37]achieved dynamic tracking of the crosslinking process by photoinduced electron transfer effect(PET).As shown in Figs.4(e),(f),TPE-2MI as a crosslinking agent was used to take a Diels-Alder reaction between the maleimide(MI)group of TPE-2MI and the furan group in the linear random copolymer of methyl methacrylate and furfuryl methacrylate(PMFM).As a result,the reversible crosslinked polymer network of PMFM/TPE-2MI was constructed.The as-prepared polymer emitted bright fluorescence at room temperature but showed gradually weakened emission when the temperature rose to 140℃.Upon heating,the inverse Diels-Alder reaction was activated,resulting in the gradual release of the MI group of TPE-2MI from the polymer network and then dissociation of the crosslinking network.Therefore,the fluorescence of TPE-2MI was quenched due to the PET effect.When returned to room temperature,the Diels-Alder reaction was gradually accelerated,and the MI group in TPE-2MI was re-bonded with the furan group in PMFM chains,inhibiting the PET effect and relighting the fluorescence.Through the above reversible“ON/OFF”fluorescence behavior,the dynamic tracking of the reversible crosslinking process of the system was visualized.Compared with DSC technology and rheological test,AIE technology provides a real-time,accurate,and more intuitive detection of the polymer crosslinking process,promoting the reaction kinetics study and expanding the application of reversibly crosslinked materials.
1.3 Crystallization Behavior
Crystallization of polymers is a process relevant to the partial alignment of their molecular chains.The crystallinity of a polymer,usually from 10% to 80%,refers to the fraction of the ordered molecules in the polymer.The crystallinity plays a vital role in determining the final properties of polymers[38-40]and could be evaluated by DSC.The DSC detection mechanism is based on the melting enthalpy,which is proportional to the crystallinity[41].In contrast to the destructive analysis of DSC by melting,AIE technology provides a noninvasive method for polymer crystallinity determination.For example,Khorloo et al.[42]used a polymorphic TPE-EP to distinguish the crystalline region from the amorphous region of poly(L-lactic acid)(PLLA)and then the intuitive crystallinity detection was achieved.By using solvent evaporation-induced crystallization process,TPE-EP molecules adopted thermodynamically crossed packing in PLLA amorphous region;while they were forced to show a metastable parallel packing when confined between two PLLA lamellas(Fig.5(a)).As a result,the amorphous PLLA region showed green emission,but the crystalline PLLA region emitted yellow emission(Fig.5(b,c)).The distinct emission arose from the different molecular packing modes of TPE-EP.In addition,the polymer crystallinity could be quantitatively calculated from the linear relationship between emission maximum and crystallinity(Fig.5(d,e)).
Besides the crystallinity visualization,the authors[43]also investigated the crystallization evolution of polyethylene glycol(PEG)using AIE technology.As shown in Fig.5(f),TPE-PEG was prepared by grafting the TPE unit at the end of the PEG polymer chain by a reversible additionfragmentation chain transfer polymerization.In PEG crystalline region,the intramolecular motion of TPE was restricted to exhibit intense fluorescence emission;in PEG amorphous region,the intramolecular motion of TPE was activated to emit weak fluorescence emission.It is noted that the soft amorphous phase could also facilitate the photocyclization reaction of TPE to further quench the emission(Fig.5(g))[44].Upon crystallization,PEG segmental molecular motion at the boundary of the crystalline and amorphous region could further accelerate the intramolecular motion of TPE and then generated a dark circle at the boundary region(Fig.5(h)).Finally,the fluorescence pattern could be stored during the crystallization process,that is to say,the crystallization evolution could be monitored accordingly.Compared with DSC and X-ray diffraction,AIE technology can not only achieve nondestructive crystallinity detection,but also realize real-time monitoring of the crystal formation process.

Fig.5 Crystallization behavior monitoring.(a)The arrangement of TPE-EP in the PLLA matrix.(b,c)(insets)Fluorescence images of(b)amorphous and(c)crystalline PLLA films doped with TPEEP and their corresponding magnified images.Inset scale bar:1 cm.(d)Fluorescence images of PLLA films doped with TPE-EP at different degrees of crystallinity.Scale bar:5 mm.(e)Functional relationship between the maximum emission wavelength and crystallinity.(Reproduced with permission[42],Copyright 2019,Royal Society of Chemistry.)(f)Preparation route of TPEPEG.(g)Schematic diagram of TPE moiety monitoring PEG crystallization behavior.(h)Fluorescence pattern of TPE-PEG formed by isothermal crystallization with periodic ultraviolet light irradiation.(Reproduced with permission[43].Copyright 2020,American Chemical Society.)
1.4 Phase Separation
Blending is commonly used in polymer engineering for more desirable structure and physical properties,which are beyond those of a single component.In this case,phase separation usually occurs,and could be investigated by DSC fromTgdetection.However,DSC technology is not intuitive.Although scanning electron microscope and optical microscope can be used to capture phase separation structure,the former offers an ambiguous morphology in poor contrast unless using chemical staining of heavy-metal compounds,and the latter cannot provide clear information on the intrinsic properties of blend composition(Fig.6(a)).Whereas,fluorescence imaging endows each component with different emission colors,not only theTgof each constituent of the polymer blends could be detected,but also their morphologies of phase separation could be visualized from a fluorescent microscope.For the polymer blend of soft polybutadiene(PB)and rigid PS,Han et al.[45]doped TPE into the system for monitoring phase separation structure.TPE exhibited stronger emission in PS than that in PB based on the RIM effect(Fig.6(a)).For polymer blends composed of different polar components,D-A structured AIE probes of TPABMO(Fig.6(b))were incorporated into the various blended systems.Due to the TICT effect,TPABMO in the polar polymer showed redshifted emission than those in the nonpolar polymer.As a result,the spatial distribution of each component for various blended systems could be visualized(Fig.6(c)),providing a convenient,time-saving,and powerful method for monitoring microphase separation in polymer blends.
In addition,the aggregation state of AIEgen could also be used to visualize polymer phase separation.Cheng et al.[46]incorporated the polymorphic AIEgen of TDHA into the PB/PS polymer blend.The rigid PS inhibited the intramolecular motion of TDHA and forced them to adopt an amorphous packing and a planar conformation in the polymer network,while the soft PB promoted TDHA molecules to move freely to take a crystalline packing in the flexible polymer network.A twisted conformation was adopted by TDHA in PB(Fig.6(d)).Due to the different emission of TDHA in amorphous-and crystalline-aggregation state,PS and PB were endowed with an orange and blue emission,respectively.Consequently,the dynamic tracking of phase separation structure evolution of PS/PB with the changes in PS mass ratio was realized.(Fig.6(e)).

Fig.6 Phase separation detection.(a)(Left)SEM image,(middle)bright-field,and(right)fluorescent image of TPE-doped PS/PB.(b)Chemical structure of TPABMO.(c)Fluorescent images of various TPABMO-doped polymer blend films.(Reproduced with permission[45],Copyright 2017,American Chemical Society.)(d)Chemical structure of TDHA and its fluorescent images of crystalline and amorphous aggregates.(e)Fluorescence images of phase separation structure of PS/PB blend at different mass ratios of PS(percentage in the upper left corner of each image).(Reproduced with permission[46],Copyright 2018,Royal Society of Chemistry)
2 Conclusions
The study of polymer thermodynamics and kinetics is the key to deepening the understanding of polymers.As a highly efficient research tool,DSC could characterize thermal properties and thermal reactions of materials,promoting the establishment of the relationship between the polymer structures and properties,and facilitating the development of polymer science and engineering.As an alternative strategy,AIE technology provides a more sensitive,accurate,and easy-readable method for polymer analysis.Based on the generated fluorescence of AIEgen in polymer systems,the characteristic temperatures(Tg,Tm,andTv),crosslinking reaction,crystallization behavior,and phase separation could be visualized and monitored.We disclose how AIEgens play a role in studying polymer thermodynamics and kinetics,endowing AIEgens as a built-in sensor for polymer research that might be inaccessible by DSC technology.
This holds great promise in the field of polymer materials processing visualization,such as monitoring the structure evolution of polymers during fiber spinning,extrusion molding,and blow molding.In addition,AIE technology may provide a new platform for evaluating their service performance owing to its capability for polymer microstructure visualization.
At the same time,it should be noted that the synthetic step of most AIEgens is relatively complicated,which limits their large-scale application.Moreover,some AIEgens possess poor thermal stability and could not be used for studying polymer structure at high temperature.The above limitations challenge the practical application of AIE technology.But we firmly believe that the above problems will be overcome consequently with the optimization of the synthesis route,reasonable molecular design,and the continuous development of AIE theory.We envision this work will stimulate more collaboration between AIE and polymers to promote the development of polymer science and engineering.
杂志排行
Journal of Harbin Institute of Technology(New Series)的其它文章
- Co-MOF Derived NiCo-LDH Three-Dimensional Nanostructure on Carbon Cloth for High-Performance Supercapacitors
- Review:Fabrication and Application of Zwitterion-based Functional Coatings
- Preparation and Characterization of Glass-Ceramic Basalt Fiber
- Review:Progress in Core-shell Rubber Particles for Efficiently Toughening Resins
- CuS Electrode for All-pH Electrolyte in Aqueous Batteries
- Boosting Hydrogen Evolution and Oxygen Reduction Performances of Pd/C upon Surface Phosphorization
