含萘基团的锆金属有机骨架材料对水中硝基芳烃爆炸物的荧光检测性能
2022-01-24乔珺一刘鑫垚刘云凌
李 文,乔珺一,刘鑫垚,2,刘云凌
(1.吉林大学化学学院,无机合成与制备化学国家重点实验室,长春 130012;2.中国中化控股有限责任公司,北京 100031)
1 Introduction
With the development of the modern chemical industry,the issue of global environmental protection has constantly been raising[1,2].As a class of important explosive chemical compounds,nitroaromatics have been widely synthesized and used[3,4].They are not only explosive but also toxic,even carcinogenic[5,6].For the concern of environmental protection,homeland security,and public health,the detection and removal of nitroaromatics are very important[7].Meanwhile,the present detective methods normally require expensive instruments which raises the cost[8].Thus,the development of highly efficient detective strategies of nitroaromatics is urgent.
Metal-organic frameworks(MOFs),which are constructed from metal ions or metal clusters and bridging organic ligands,have emerged as a very important family of porous materials since the 1990s[9,10].By the combination of different kinds of metal-based secondary building units(SBUs)and organic linkers,more than a million of various MOFs have been synthesized and reported[11].Benefiting from the unique porous structures and inorganic-organic hybrid composition,MOF materials have shown great performances in gas storage[12,13]and purification[14,15],catalysis[16—19],molecular capture[20,21],and other applications.Since the organic ligands can be diversely tailored,different functional groups can be implanted into the frameworks for specific purposes[22,23].Meanwhile,the introduction of fluorophores will endow MOFs with fluorescent properties,which renders MOFs potential candidates for the application in fluorescent detections[24].Reported works have proved that fluorescent MOFs have shown great performances in detections of different organic compounds or ions[25—28].
For practical applications,the detection should be conducted in water,thus the water stability of MOF materials is crucial[29,30].Among the reported MOFs,zirconium-based MOFs(Zr-MOFs)have shown great chemical and water stability[31].Another feature of Zr-MOFs is the structural guidance:the linker with a particular shape and connectivity will bridge the Zr-nodes into a corresponding framework with a distinct topology[32].This feature,plus the modulated synthetic strategy,provides a convenience for the design and synthesis of stable and functional Zr-MOFs[33—36].
In this work,a Zr-MOF,[Zr6(μ3-O)4(μ3-OH)4(NDBA)6]·7DMF·4H2O(JLU-MOF100,JLU is the abbreviation of Jilin University)which is constructed from a naphthalene-based linear organic ligand 4,4'-(9,10-naphthalenediyl)bis-benzoic acid(H2NDBA)is present.The synthesized JLU-MOF100 exhibits anticipated fcu topology and good water stability.It shows great fluorescent property which originates from the naphthalene group in the organic linkers.By quenching effect,JLU-MOF100 could efficiently detect different nitroaromatic explosives in water.
2 Experimental
2.1 Synthesis of JLU-MOF100
ZrOCl2·8H2O(20 mg,0.062 mmol),H2NDBA(10 mg,0.029 mmol),trifluoroacetic acid(120μL),andN,N-dimethylformamide(DMF,2 mL)were put into a 2-dram vial and sealed carefully.The mixture was ultrasonicated for 5 min,transferred into a pre-heated oven,and heated at 120℃for 48 h.Colorless transparent octahedral crystals were gathered and washed with fresh DMF for further activation.The yield of the activated product is 70%(calculated based on the dosage of ZrOCl2·8H2O).Elemental anal.(%)calcd.for JLU-MOF100:C 57.25,H 4.22,N 2.83;found:C 57.66,H 4.19,N 3.03.
2.2 Fluorescent Detection
Before measurement,JLU-MOF100 samples were finely ground.Typically,2 mg of ground JLU-MOF100 was added to a cuvette containing 2.5 mL of deionized water and then ultrasonically dispersed.The fluorescence upon excitation at 370 nm was measuredin situafter the addition of fresh analyte solution(1 mmol/L or 0.25 mmol/L,20µL addition each time).The mixture was stirred during detection to maintain dispersion.All the experiments were performed several times and consistent results were reported.
The fluorescent quenching efficiency can be quantitatively explained by the Stern-Volmer(SV)equation:I0/I=1+KSV[A],whereKSV(L/mol)is the quenching constant,[A](mol/L)is the molar concentration of used analyte,I0andIare the fluorescence intensities before and after the addition of analyte,respectively.
3 Results and Discussion
3.1 Structural Design,Description,and Characterization
To conduct fluorescent detection of nitroaromatics in water,the MOF material should possess two basic features:water stability and fluorescence.In the aspect of water stability,Zr-MOFs have been proved as a kind of highly water stable materials since the strong coordination bond between Zr4+and oxygen donor of carboxylic organic ligand[37—39].Based on the molecular building block(MBB)strategy[40,41],the bridging of Zr6-clusters by linkers with different shapes will form MOFs with various topologies such as fcu[42],reo[43],spn[44],ftw[45],csq[46,47],etc.In this work,we chose the fcu topology(UiO series)[Fig.1(G)]which requires a bidentate linear ligand as a case of study.Meanwhile,in order to introduce fluorescence into the MOF,we chose naphthalene as the fluorophore.Combining the above-mentioned points,we designed and synthesized a naphthalene-based linear organic ligand H2NDBA[Fig.1(B)and Fig.S1,see the Supporting Information of this paper]to build the functional MOF material with fluorescent detection property in water.As a result,the solvothermal reaction between ZrOCl2·8H2O and H2NDBA in DMF using trifluoroacetic acid as modulator yielded colorless transparent octahedral crystals[Fig.2(A)inset]of JLU-MOF100.
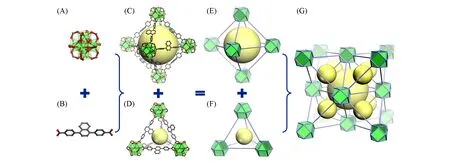
Fig.1 Schematic representation of the structure of JLU⁃MOF100
The single-crystal XRD analysis confirms that JLU-MOF100 crystallizes in the cubic space groupFm3ˉm(CCDC number:2108022).Each Zr4+ion is coordinated by eight O2-donors from four distinct—COO-of four independent NDBA2-ligands and fourμ3-O2-(orμ3-OH-)groups.Six Zr4+ions are bridged by fourμ3-O2-groups,fourμ3-OH-groups,and twelve carboxyl groups to form[Zr6(μ3-O)4(μ3-OH)4(—COO-)12](Zr6-cluster)[Fig.1(A)],a twelve-connected SBU.Each Zr6-cluster is linked by twelve NDBA2-ligands,and each ligand is connected by two isolated Zr6-SBUs,to construct the final 3D structure.There are two kinds of cages in the framework of JLU-MOF100.One is the octahedral cage built of six Zr6-clusters and twelve ligands[Fig.1(C)and(E)],the other is the tetrahedral cage built of four Zr6-clusters and six ligands[Fig.1(D)and(F)].For topological analysis,the carbon atoms of the carboxyl group act as vertices and points of extension.As a result,the Zr6-cluster can be simplified as cuboctahedron(cuo),and the connections of cuo SBUs by linear linkers form the anticipated quasiregular face-centered cubic(fcu)net[Fig.1(G)].
The PXRD patterns[Fig.2(A)]prove the phase purity and water stability of synthesized JLU-MOF100.The thermogravimetric analyses(TGA,Fig.S3,see the Supporting Information of this paper)reveal that a mass loss ofca.15%occurs before 350℃,which could be attributed to the removal of DMF and H2O molecules,and JLU-MOF100 could maintain its crystallinity up to 400℃.The N2adsorption-desorption isotherm at 77 K[Fig.2(B)]reveals the permanent porosity of JLU-MOF100.The BET surface area and pore volume were calculated to be 3650 m2/g and 1.51 cm3/g,respectively.The DFT pore size distribution[Fig.2(B)inset]shows two main pore sizes for JLU-MOF100,which are 1.88 nm and 2.22 nm.
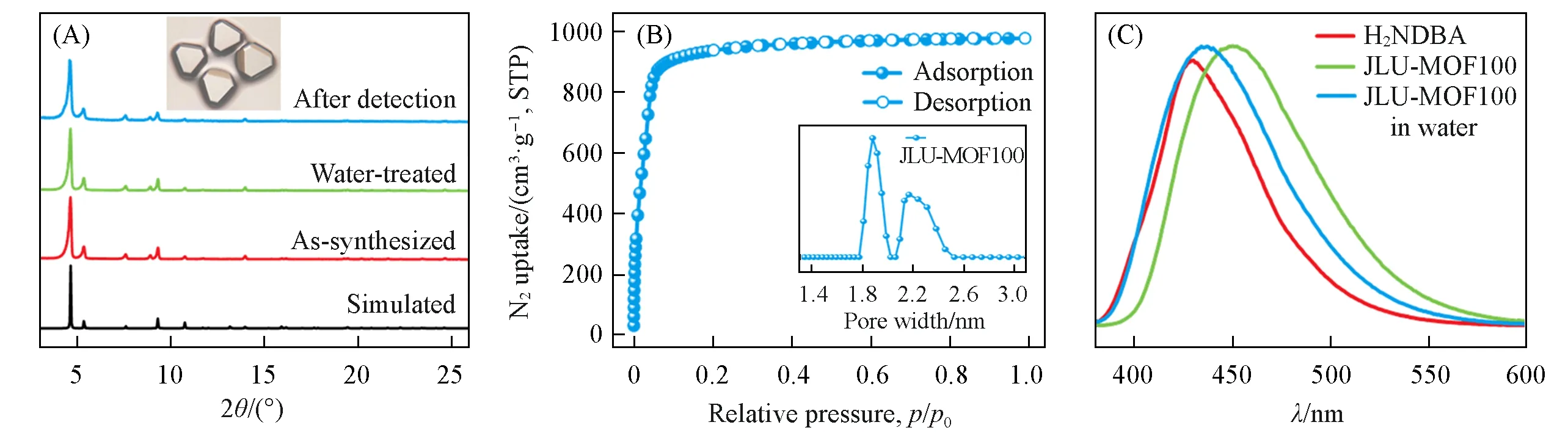
Fig.2 PXRD patterns and crystal image(inset)of JLU⁃MOF100(A),N2 adsorption⁃desorption isotherm at 77 K and pore size distribution(inset)of JLU⁃MOF100(B)and fluorescence patterns of H 2NDBA,JLU⁃MOF100,and JLU⁃MOF100 in water(C)
Before testing the detection ability,we investigated the fluorescence properties of JLU-MOF100.As shown in Fig.2(C),JLU-MOF100 exhibits blue fluorescence upon excitation at 370 nm.Compared with the emission pattern of the free H2NDBA ligand,we can conclude that the fluorescence of synthesized MOF is originated from the organic ligand.Moreover,JLU-MOF100 shows similar strong emission in water,which proves the stability of MOF and also provides the feasibility of fluorescent detection in water.
3.2 Fluorescent Detection
To investigate the detection properties of JLU-MOF100,we chose six different nitroaromatics as analytes(Fig.S2,see the Supporting Information of this paper):trinitrophenol(TNP),2,4-dinitrophenol(2,4-DNP),4-nitrophenol(4-NP),2,4-dinitrotoluene(2,4-DNT),4-nitrotoluene(4-NT),and 3-nitrotoluene(3-NT).The studies of fluorescent detection of JLU-MOF100 were carried out by fluorescence-quenching titrations with piece-by-piece additions of analytes into MOF-water dispersion.
As shown in Fig.3(A)—(F),the analytes(1 mmol/L aqueous solution)can effectively quench the fluorescence of JLU-MOF100 in water.Especially for the TNP,the quenching phenomenon can be visually observed under the excitation of UV-light[Fig.4(A)].Analytes have different quenching efficiencies that range from 34.7%(3-NT)to 77.5%(TNP)[Fig.4(B)].Interestingly,an obvious red-shift occurs in the emission band of JLU-MOF100 with the additions of TNP analytes from 0 to 200μL,which may be due to the formation of exciplexes(excited complexes)[48].In order to evaluate the fluorescent detection,theKSVvalue can numerically present the performances.Due to the self-absorption and energy transfer processes[26,49—51],the SV plots are deviated from linear diagrams when using analytes solution with higher concentrations.So,we conducted the quenching titrations using 0.25 mmol/L analytes aqueous solution(Fig.S4,see the Supporting Information of this paper)to calculate the preciseKSVvalues.

Fig.3 Fluorescence spectra and SV plots(inset)of quenching titration using JLU⁃MOF100 towards 1 mmol/L solutions of TNP(A),2,4⁃DNP(B),4⁃NP(C),2,4⁃DNT(D),4⁃NT(E)and 3⁃NT(F)

Fig.4 Fluorescent images of JLU⁃MOF100 water dispersion before(left)and after(right)the addition of 200μL of 1 mmol/L TNP aqueous solution(A)and quenching efficiencies towards six nitroaromatics(B)
As shown in Fig.5(A),the SV plots plotted from the data of low-concentration titration are nearly linear.TheKSVvalues of six analytes ranging from 5.9×103L/mol(3-NT)to 5.27×104L/mol(TNP)are presented in Fig.5(B).Besides,the limit of detection(LOD)of JLU-MOF100 by the equation LOD=3σ/mwas tested[52],and the detection limit was calculated to be 0.22 mg/L for TNP(Fig.S5,see the Supporting Information of this paper).To prove the advantage of detection by JLU-MOF100 over the free ligand,the detective ability of free H2NDBA ligand was also investigated(Fig.S6,see the Supporting Information of this paper).The quenching efficiency andKSVof H2NDBA ligand were calculated to be 61%and 2.1×104L/mol,respectively,much lower than JLU-MOF100,indicating that the MOF framework may provide more interaction with analytes and enhance the sensing ability.Compared to the reported iso-structural MOF(UiO-68-mtpdc/etpdc,KSVvalues towards TNP is 2.8×104L/mol)[53],JLU-MOF100 shows better performances.It should be noted that the fluorescent detections of some reported MOF materials with similar detection abilities are conducted in an organic solvent such as DMF[54],CH3OH[53,55],and CH3CN[25,56],by contrast,JLU-MOF100 can detect nitroaromatics in water.Meanwhile,JLU-MOF100 shows a good cyclic performance of detection,there is only a small decrease of quenching efficiency after five detection-washing cycles(Fig.S7,see the Supporting Information of this paper).Moreover,the PXRD result proves that the structure of JLU-MOF100 maintains integrity after detection[Fig.2(A)].Based on the aforementioned data,the performances of JLU-MOF100 are comparable among the reported MOF materials[57—62].

Fig.5 SV plots of titration using JLU⁃MOF100 towards 0.25 mmol/L analytes solution(A)and K SV values of six nitroaromatics(B)
The quenching mechanism can be described in two aspects.One is the short-range electron transfer mechanism.For a better understanding,DFT calculations were carried out at B3LYP/6-31G*level with Gaussian 09 program[63].As the fluorescence of JLU-MOF100 generates from the fluorescence of the ligand,the valance band(VB)and conduction band(CB)of JLU-MOF100 could be simplified as the molecular orbitals(MOs)of the ligand.As displayed in Fig.S8(see the Supporting Information of this paper),the CB energy of JLU-MOF100 is higher than the energy of the lowest unoccupied molecular orbitals(LUMOs)of the analysts,accessible for electron transfer from JLU-MOF100 to these six analytes and giving rise to fluorescence quenching.However,the quenching efficiencies andKSVvalues are not in accordance with the LUMO energies of the analytes.Therefore,another rational energy transfer mechanism is also proposed here,which might dominate the overall quenching process of JLU-MOF100.As shown in Fig.S9(see the Supporting Information of this paper),compared with the other four analytes,the absorption bands of TNP and 2,4-DNP have the highest degree of overlapping with the emission band of JLU-MOF100,resulting in the highest quenching efficiencies andKSVvalues.As for the detections of nitrotoluenes without observed overlap,the detection behaviors could be attributed to electron transfer mechanism.
4 Conclusions
Overall,we designed and synthesized a naphthalene-based Zr-MOF,JLU-MOF100,with fcu topology.It showed significant water stability and great fluorescence in water that originated from the naphthalene group in the pre-designed organic ligand.JLU-MOF100 exhibited good fluorescent detection ability towards nitrophenols and nitrotoluenes in water by quenching effects.Among the tested analytes,JLU-MOF100 showed the greatest detection performance towards TNP with the quenching efficiency andKSVvalue of 77.5%and 5.27×104L/mol,respectively.Moving forward,we anticipate that this work will provide an ideal strategy for the design and synthesis of stable MOF materials with fluorescent detection abilities.
We sincerely thank Omar K.Farha and Xuan Zhang from Northwestern University for their help in the collection and analysis of single-crystal XRD data of JLU-MOF100.The single-crystal XRD data were collected in the Integrated Molecular Structure Education and Research Center(IMSERC)at Northwestern University.
Liu Xinyao gratefully acknowledges the support from the China Scholarship Council(CSC)during his visit to Northwestern University(No.202006170134)for crystallography study in this work.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20200626.
