COVID-19 vaccine candidates and vaccine development platforms available worldwide
2022-01-19NilunDumnZhrALziiBusrAynkinDuyuTskinBusrDmirorsAulkiYilirimIzmOlyShinFikBililiThirTurnliTommsoBriMttoBrtlliMunisDunr
Nilun Dumn,Zhr ALzii,Busr Aynkin,Duyu Tskin,Busr Dmirors,Aulki Yilirim,Izm Oly Shin,Fik Bilili,E Thir Turnli,Tommso Bri,Mtto Brtlli,Munis Dunr,*
aDepartment of Medical Genetics,Dragos Hospital Istanbul,Bezmialem Vakif University,Istanbul,34854,Turkey
bDepartment of Biotechnology,Faculty of Applied Science,Cukurova University,Adana,01380,Turkey
cDepartment of Medical Genetics,Medical Faculty,Erciyes University,Kayseri,38030,Turkey
dDepartment of Economics,Faculty of Economics and Administrative Sciences,Erciyes University,Kayseri,38030,Turkey
eDepartment of Molecular Biology and Genetics,Faculty of Science,Acibadem University,34684,Istanbul,Turkey
fDepartment of Pharmaceutical Sciences,University of Perugia,Perugia,06123,Italy
gMAGI’S LAB,Rovereto(TN),Trentino,38068,Italy
Keywords:
COVID-19 vaccine
Vaccine development platform
Coronavirus outbreak
SARS-CoV-2 variants
Treatment
Peer review under responsibility of Xi'an Jiaotong University.
A B S T R A C T
The pandemic caused by the worldwide spread of the coronavirus,which first appeared in 2019,has been named coronavirus disease 19(COVID-19).More than 4.5 million deaths have been recorded due to the pandemic caused by severe acute respiratory syndrome coronavirus 2(SARS-CoV-2),according to the World Health Organization.COVID-19 Dashboard in September 2021.Apart from the wildtype,other variations have been successfully transmitted early in the outbreak although they were not discovered until March 2020.Modifications in the SARS-CoV-2 genetic material,such as mutation and recombination,have the ability to modify the viral life span,along with transitivity,cellular tropism,and symptom severity.Several processes are involved in introducing novel vaccines to the population,including vaccine manufacturing,preclinical studies,Food and Drug Administration permission or certification,processing,and marketing.COVID-19 vaccine candidates have been developed by a number of public and private groups employing a variety of strategies,such as RNA,DNA,protein,and viral vectored vaccines.This comprehensive review,which included the most subsequent evidence on unique features of SARS-CoV-2 and the associated morbidity and mortality,was carried out using a systematic search of recent online databases in order to generate useful knowledge about the COVID-19 updated versions and their consequences on the disease symptoms and vaccine development.
1.Introduction
A novel coronavirus of unidentified source, severe acuterespiratory syndrome coronavirus 2 (SARS-CoV-2), emerged inDecember 2019. The virus is the source of the current pandemic,which is identified as 2019-nCoV, better known as coronavirusdisease 19 (COVID-19) [1]. On March 11, 2020, the COVID-19epidemic was first classified as a “pandemic” by the World HealthOrganization (WHO) [2]. Infected patients show common symptoms,such as gastrointestinal infection, fever, cough, and fatigue. Absence of any vaccine at the beginning of the epidemic increased the severityof the epidemic, worsening outcomes worldwide [3,4]. To end theepidemic and prevent further suffering, it is necessary to increase thenumber of people vaccinated by applying a predictive-preventivehealth strategy. This health strategy should comply with humanrights principles and ethical rules [5].
In studies on the development of individual-specific antibodies,it has been observed that some patients have a different immune response,which is seen veryearly,while in others too late,and that varies from person to person.It has been suggested that the incubation period for infected patients is 2-14 days(approximately 5 days on average).It has been reported that antibodies appeared within an average of 8 days after coronavirus exposure,reaching their highest levels in 14 days[6].
According to general genomic components, coronaviruses can bedivided into four subgroups: alpha-coronavirus, beta-coronavirus,gamma-coronavirus, and delta-coronavirus [7] (Fig. 1). These subgroups are further divided into groups. Beta-coronaviruses (SARSCoV, Middle East respiratory syndrome coronavirus (MERS-CoV),and SARS-CoV-2) have been passed from species to species andwere the source of SARS-CoV outbreaks in 2002, MERS-CoV in 2011,and SARS-CoV-2 in 2019 [8]. A recent mutational and genetic studyof the newly developed SARS-CoV-2 and the previously emergentSARS-CoV revealed that the two viruses were 79% similar to eachother [9]. With a genome of approximately 30 kb, coronaviruses have6-11 open reading frames (ORFs) [10]. The first of these ORF codesfor 16 types of non-structural proteins (NSPs). This ORF, which makes up two-thirds of the viral genome,is also known as ORF1a/b.Other ORFs code for the envelope(E)protein,nucleocapsid(N)protein,spike(S)glycoprotein(including S1 and S2 subunits),transmembrane(M)glycoprotein,and additional,structural proteins of the virus(Fig.2).S glycoprotein and M glycoprotein are the two main E proteins[11].The S glycoprotein is an antigen that binds to receptors and is responsible for cell fusion.M glycoprotein plays a role in the formation of virions and envelopes.
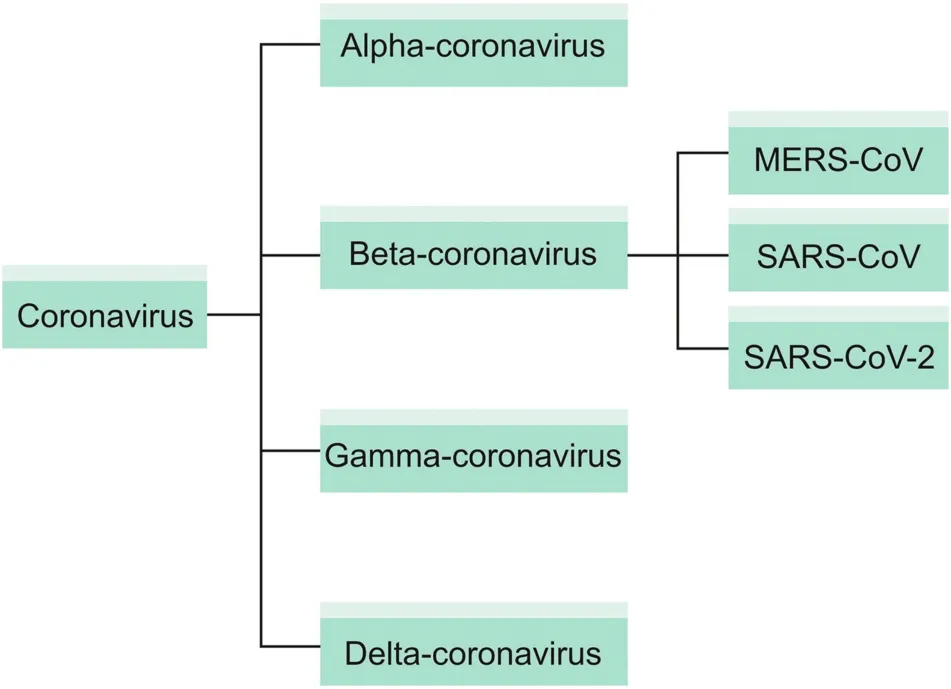
Fig.1.Phylogenetic tree of coronavirus subfamilies.MERS-CoV:Middle East respiratory syndrome coronavirus;SARS-CoV:severe acute respiratory syndrome coronavirus.
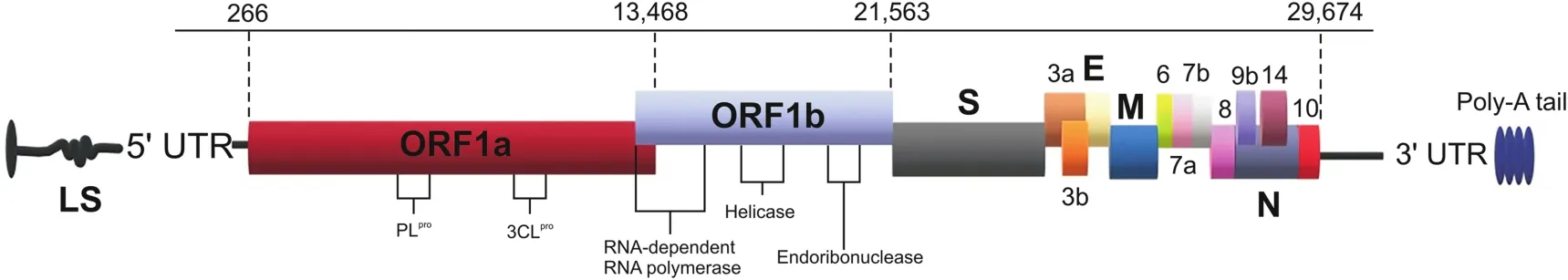
Fig.2.Coronavirus genome structure.UTR:untranslated region;ORF:open reading frame;3CLpro:3C-like protease;S:spike protein;E:envelope protein;M:transmembrane glycoprotein;N:nucleocapsid protein.
Virus-membrane fusion occurs primarily through the S2 subunit of S glycoprotein,once SARS-CoV-2 attaches to the angiotensin converting enzyme-2(ACE2)receptor through the S1 subunit[12].In this way,viral genomic RNA passes to the cytoplasm to form a replication-transcription complex and synthesizes all structural and auxiliary proteins and new genomic RNAs.The new genomic RNA is coated with glycoproteins and N proteins.The resulting virus particles interact with the endoplasmic reticulum and Golgi and then connect with the plasma membrane of the host cell to unleash the virus[13].Recent studies have shown that S glycoprotein,as a viral adhesion molecule in the outer membrane,interacts with ACE2,ezrin,dipeptidyl peptidase-4(DPP4),cyclophilin,and other cell-adhesion molecules[14].
Meta-transcriptomic sequences revealed similarity in terms of S protein and receptor binding domains(RBD)between SARS-CoV and SARS-CoV-2[15].SARS-CoV and SARS-CoV-2 viruses enter the cell through the human ACE2 receptor;however,small differences between genomes result in stronger binding of ACE2 receptor to SARS-CoV-2 than to SARS-CoV(Fig.3).In contrast,MERS-CoV is linked to the DPP4 receptor[16].The properties of the coronaviruses types are summarized in Table 1[17,18].
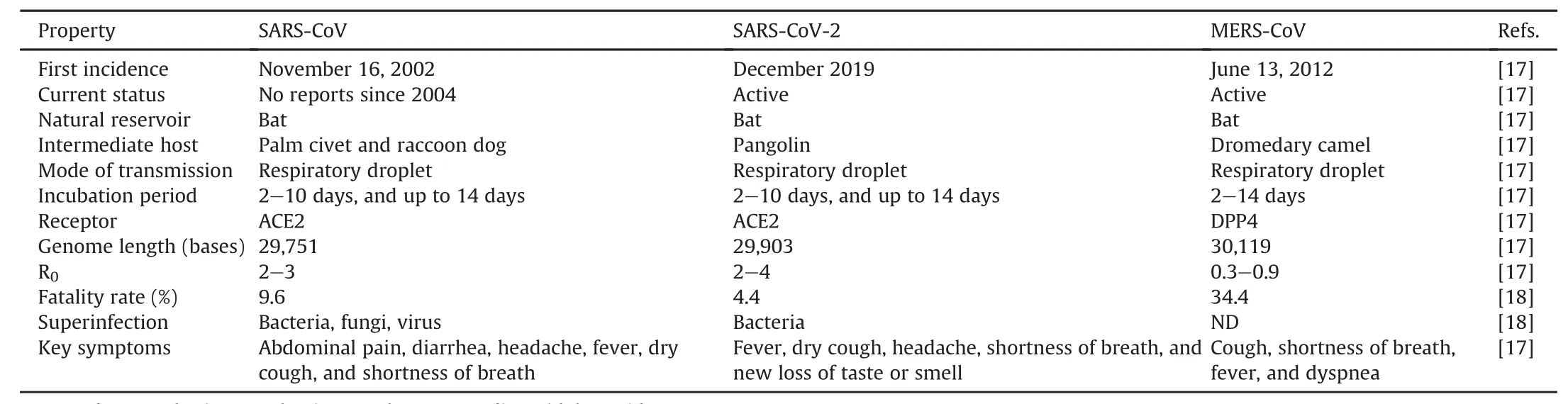
Table 1SARS-CoV,MERS-CoV,and SARS-CoV-2 properties.
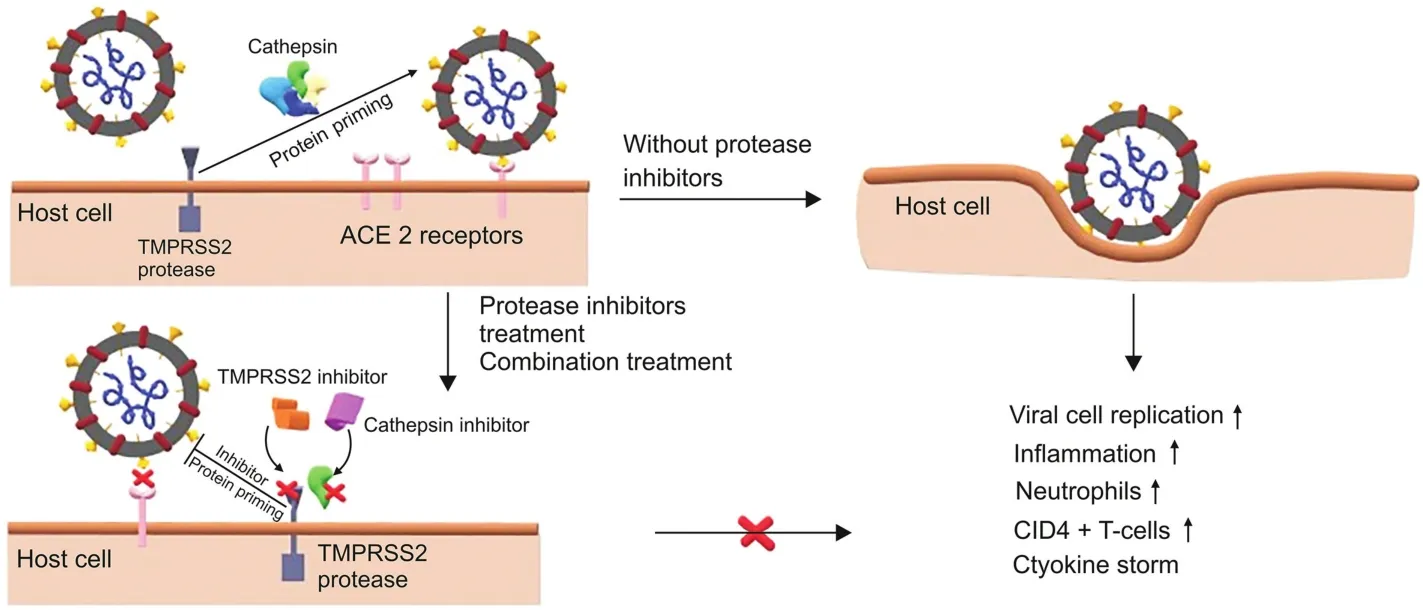
Fig.3.The figure demonstrates the binding of the SARS-CoV-2 S protein to the angiotensin converting enzyme-2(ACE2)receptors upon the host cell surface.Transmembrane serine protease 2(TMPRSS2)and cathepsin are actively engaged in S glycoprotein preparatory work and promote viral invasion,leading to increased immune reaction,intensification of the disease,and imbalance.Attacking proteases with unique protease inhibitors,separately or in combination,may have better therapeutic effects to inhibit SARS-CoV-2 infections.
In humans with high binding specificity through RBD,Lu et al.[19]proposed that the S protein links to the ACE2 receptor to stimulate entry of the virus into host cells.Furthermore,enzymes including papain-like cysteine protease and 3C-like protease(3CLpro)are positioned close to the 5’end of the ORF and contribute to the proteolytic degradation of polyprotein 1a(pp1a)and pp1ab to create NSPs such as RNA polymerase,helicase,and endoribonuclease.Such proteins make viral replication and transcription more possible[20].
2.Variety of COVID-19 vaccine candidates
Biotechnology has permeated all aspects of research,from basic sciences to small-and medium-sized businesses and large industries[21].Technologies that can be used together,such as epigenetics,PCR,bioinformatics,transcriptomics,and new sequencing tools,are at the forefront of biotechnological progress[22].
It has been suggested that SARS-CoV-2 is biotechnologically artificial.In bioinformatics studies that assess this idea,this virus was compared with strains prepared in the laboratory and other natural strains to estimate the genetic relationships of SARS-CoV-2.In these studies,it became clear that the artificial origin stories of SARS-CoV-2 were unfounded.Biotechnology,a powerful tool for advancing medical research,should not be abandoned because of these speculations[23].Biotechnology makes possible a wide variety of solutions that will bring health,food security,and wellbeing to humanity for centuries,offering new treatment options for diseases and significantly impacting our society and lifestyle[24,25].Previously unused vaccine-development techniques have also been adopted,and a wide variety of vaccines have been generated[26].Vaccines,including those based on inactivated viruses,live attenuated viruses,protein subunits,viral vectors,DNA,RNA,and nanoparticles,are being developed using a variety of different methods throughout the world to combat the global pandemic(Fig.4).These techniques should be studied in specially qualified laboratories[27].Various vaccine techniques related to COVID-19 are shown in Fig.5.

Fig.4.Adjuvant types platform of vaccine candidates for coronavirus.
2.1.Live attenuated vaccines(LAV)
Live vaccines use a weakened(or attenuated)SARS-CoV-2 form.This technique utilizes reconfiguration of the S protein engagement in the SARS-CoV-2 membrane with the RBD by creating mutations such as deletions in the virus genome.Live vaccines are developed by growing attenuated forms in host cells(chick embryo and kidney)[9].
Weakened vaccines trigger the stimulation of cells and antibodies,such as T lymphocytes,CD4+,and CD8+.These cells and antibodies can reduce or prevent infection by producing interleukins and killing infected cells.The specific induced effectors may differ depending on the vaccine used.LAVs attempt to assist in the generation of CD8+and T-linked antibody responses of cytotoxic T lymphocytes.The vaccine is effective as long as the body maintains its internal balance;it can stimulate and regulate cellular immune responses,including immune cells such as cytotoxic Tcells or macrophages,which can provide long-term and possibly lifelong immunity without requiring multiple vaccine doses[28].Vaccines developed consist of 14% inactivated virus vaccine and 2% live attenuated virus vaccine(Fig.5)[29].
2.2.Protein subunit vaccine
Protein-based vaccines are based on synthetic peptides or recombinant antigenic proteins,which induce a protective and therapeutic immune response.To improve the immune system,certain vaccines require adjuvant assistance;adjuvants are used to overcome the shortcomings of these vaccines.Since the virus uses the ACE2 receptor and the S protein-mediated endocytic pathway,its S protein and antigenic fragments are usually the main targets for protein-based vaccine design[30].
The S protein is an important target for the development of protein-based vaccines against beta-coronaviruses.Both the viral S protein and its antigenic fragments contain the S1 subunit(on RBD),its surrounding region,a receptor-binding motif,and an N-terminal domain(NTD).The S2 subunit has heptad repeat 1,heptad repeat 2,fusion peptide,and central helix and junction sites.These regions serve as important targets for the development of subunit vaccines for cellular proteins.For example,when binding to the ACE2 receptor via the RBD of the S1 subunit,the S1 protein is divided into subunits and creates conformational changes.The S2 protein acts as a delivery agent by connecting the virus to the membrane of the target cell,while the S1 subunit is critical for the formation of various types of SARS-CoV-2 variants.However,the S2 protein is the main target for developing a universal vaccine for different types of SARS-CoV-2 variants.Vaccines targeting the NTD,a subunit of the S1 protein,do not directly block receptor binding;however,vaccines for NTD are preferred,as they minimize the conformational change of the S protein[31].
In addition,vaccines targeting M,N,and E proteins have been developed.Short peptides are transferred to the infected host cell,triggering the release of B and T antibodies that stimulate a rapid immune response against viral infection.
Protein subunit vaccines are being developed directly for S1 and S2 proteins,or specific subregions of these viral membrane proteins.Since viral membrane proteins have many domains,the most widely used protein platform among vaccine studies is subunit vaccines[32,33](Fig.5).This vaccine development technique covers 34% of vaccines produced using available techniques(Fig.5).
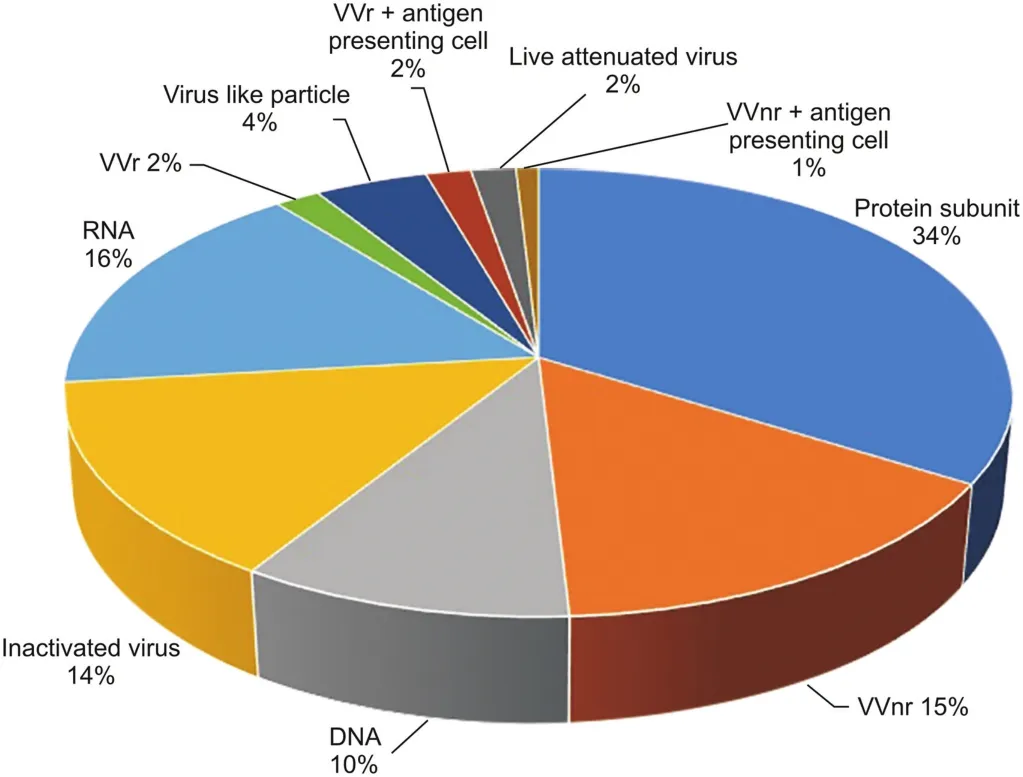
Fig.5.Vaccine development techniques used for COVID-19[29].LAV:live attenuated vaccines;VVr:viral vector(replicating);VVnr:viral vector(non-replicating).(Reprint with permission from Ref.[29]).
2.3.Viral vector vaccines
The safest strategy against this pathogen is to produce a vaccine based on viral vectors.The high efficacy of these vaccines is primarily derived from their ability to transport the viral vector gene into the target cell,which stimulates the immune response through gene transduction because it triggers cytotoxic Tcells and provides a long-term high level of protection[34].
Virus-like particle(VLP)vaccines are useful tools.They provide high-dose delivery of viral surface proteins that trigger immune responses and elicit conformational viral epitopes,creating durable memory B cells and T cells.VLPs have a radius of approximately 20-200 nm and are safer than attenuated viruses because they cannot be reproduced.VLPs have been used to develop commercially available human papillomavirus and U.S.Food and Drug Administration-approved vaccines for hepatitis B[35].Vaccine development techniques consist of 4% VLP,2% replicating viral vector,and 15% non-replicating viral vector techniques(Fig.5).
2.4.DNA vaccines
DNA vaccines aim to generate an immune response against themselves through injection of a genetically engineered plasmid that encodes the antigen.The antigen encoded by the DNA vaccine is injected into the cells by introducing an adjuvant that induces a compatible immune response.These cells act like living viruses by expressing proteins specific to the viral genome[36].
By causing a protective immunological response,this vaccine produces antigens and can induce a wide range of immune responses.It has advantages and disadvantages.Since August 2016,DNA vaccines have not been released for public use in the USA.When these vaccines are injected with DNA encoding certain antigens,and(depending on the existing genetic code in the plasmid)when they encounter amino acid sequences not included in the current code,they are considered foreign and trigger the body's defense system[37,38].This vaccine development technique covers 10% of the available techniques(Fig.5).
2.5.mRNA vaccines
The newly emerging mRNA vaccine technique is advantageous because it is not infectious.It is a strategic method that works with viral RNA or non-replicating RNAs.This technique is used as an immunogenic genetic vector,behaving like the antigen seen in natural infections[39].This technique is also helpful for targeting proteins involved in viral RNA transcription and replication.Moreover,it can be adapted to overcome viral variants[40].
mRNA vaccines enable production of viral antigens by sending an artificially designed part of the RNA sequence of the virus directly into the human cell(transfection),where it is translated.It also triggers the immune system by activating T cells that recognize targeted peptides on major histocompatibility complex molecules.
Vaccines on many platforms in development present individual advantages and disadvantages.Although inactivated virus vaccines have advantages,such as being generally more stable and safe,the integrity of immunogenic particles must be preserved,and a large amount of virus is used[41].LAVs can stimulate the immune system uniquely;however,there is a probability of nucleotide replacement during viral replication.Subunit-based vaccines do not contain any live components of the viral particle and have fewer side effects;however,there are concerns about the “memory”of the immune response.Viral vector-based vaccines have also been used for MERSCoV;they inhibit a strong immune response and infectious particle transport.These vaccines have serious disadvantages,such as a decrease in efficacy if the host was previously exposed,as well as the possibility of causing cancer as a result of the viral genome's incorporation into the host genome.While DNA vaccines possess critical advantages such as temperature resistance,absence of cold-chain requirements,and rapid development,the disadvantages are that foreign DNA penetration into the host genome usually triggers cell abnormalities and potentially produces antibodies against it.RNA vaccines eliminate the risk of integration into the host genome because mRNA transcription occurs in the cytoplasm.However,these vaccines have the potential to become unstable,and reactogenicity issues can arise.The RNA-based vaccine development technique covers 16% of the available techniques(Fig.5).
3.Standard vaccines compared with COVID-19 vaccine development
The production of standard vaccines is a lengthy process,and experiments are carried out in sequential steps.To evaluate and improve the manufacturing process,companies first create small quantities to perform limited-scale studies.They determine an effective formulation that optimizes the stability of the vaccine component through the end of its shelf life.The company then determines whether to continue developing and scaling-up production,or to stop the production procedure.In addition,to ensure that the vaccine meets its expected quality profile and regulatory requirements,the company implements an acceptable and efficient quality management plan,which comprehensively analyzes the different components of the vaccine,the final formulation to be used,and the entire production process.The vaccine developer performs several experimental methodologies using in vitro or animal models(in vivo and pre-clinical studies)to demonstrate how the vaccine causes an immune reaction and acts to prevent infections.The vaccine manufacturer then tests the vaccine in three stages of clinical trials,with an increased number of participants in each stage(Fig.6).

Fig.6.Comparison in development schemes between standard vaccines and COVID-19 vaccines.
Research and development of COVID-19 vaccines within such a limited time necessitated extensive collaboration between pharmaceutical companies,researchers,and governments.Some companies use existing manufacturing processes and infrastructure that are currently widely used for the safe and efficient production of vaccines.A few other COVID-19 vaccines are now being produced using innovative methods proposed to increase product stability,provide effective immune responses,and boost manufacturing capacity and speed compared to other vaccine categories.In this regard,the COVID-19 vaccine development process has progressed much faster than the standard vaccine development process.
4.Recent challenges for COVID-19 vaccine development
There has been a rapid increase in COVID-19 cases in the United Kingdom,particularly noticeable in Southeast England.This rise led to an intensified analysis of the epidemiology and virology of the virus.In October 2020,a new variant was first identified in the United Kingdom from a sample obtained at the beginning of the month,and it rapidly began to spread from mid-December[42].A considerable percentage(>50%)of cases related to the latest single phylogenetic cluster were identified using complete viral genome statistical analysis.It has been designated SARS-CoV-2 VUI 202012/01[variant under investigation,2020(year),12(month),variant 01]and is often referred to as lineage B.1.1.7.7.The identified COVID-19 cases linked to the variant of VUI 202012/01 were observed in Kent and South East England,along with London and East England[43].On December 20,2020,the BBC announced that the WHO declared nine additional variant incidents had already been confirmed in Denmark,with others in the Netherlands and Australia[44].
Another new variant of SARS-CoV-2,documented as 20H/501Y·V2 and B.1.351,originally detected in South Africa,corresponds to the next strain clade,20C,which emerged independently of B.1.1.7.This variant shares several mutations with the B.1.1.7.On February 11,2021,variant B.1.351 was reported in 40 countries worldwide according to official reports,and almost 1,400 incidents were registered worldwide[45].
Variant P.1 was first recorded by Japan in the passengers who had returned from Brazil,which relates to the next strain clade 20 B.On January 6,2021,the National Institute of Infectious Diseases of Japan first observed this variant of SARS-CoV-2 in four individuals who had landed in Tokyo after visiting Brazil[46].
In October 2020,CAL.20C,also known as lineage B.1.429,was observed in southern California,USA,and at the beginning of November,30 patients were detected in the northern part of the state,and five other states had reported the case.CAL.20C,as well as those initially reported in the United Kingdom(B.1.1.7),South Africa(B.1.351),and Brazil(B.1.1.248),is one of numerous other commonly circulated variants[47].In Tables S1-S4[29],information on the latest vaccine candidates for the Asian,American,African/Oceanic,and European continents is summarized.
In March 2021,a new variant was discovered and named delta(B.1.167.2)that reversed the decline in worldwide.As of June 21,2021,92,056 cases were detected in England,the country where the variant was first detected,and 53,822 of the cases were found to be unvaccinated[48].In addition,in a cohort study examining 43,338 COVID-19 positive patients,it has been found that the Delta variant might cause more burden on healthcare services compared to the Alpha variant[49].The effects of existing vaccines on the Delta variant are still under investigation.
4.1.The genomic properties of the latest version of SARS-CoV-2
Although the accuracy of SARS-CoV-2 RNA polymerase activity is high in many epidemiological studies,many mutations have been identified[50].SARS-CoV-2 is characterized by numerous S protein mutations,of which more than 4,000 have been reported[51].A typical way to manage the transmission of the virus is to concentrate on mutations.Seventeen mutations,along with some in the S glycoprotein,characterize the VUI-202012/01 variant(deletion 69-70,deletion 144,N501Y,A570D,D614G,P681H,T716I,S982A,D1118H)[52].The N501Y mutation,positioned within the RBD,appears to be among the most significant mutations,initiating a change in amino acid position 501 from asparagine(N)to tyrosine(Y).Its importance mainly comes from the fact that it is the location within the RBD of the S glycoprotein, which attaches to human and murine ACE2.On December 18,2020,the New and Emerging Respiratory Virus Threats Advisory Group informed the government of the United Kingdom about the possibility of RBD mutations that can promote the virus becoming much more contagious[53].
Multiple S protein modifications occurring overall viruses in the cluster(amino acid shifts D80A,D215G,E484K,N501Y,and A701V)were identified as B.1.351.Quite recently,viruses have demonstrated additional extra changes(amino acid change L18F,R246I,K417 N,and deletion 242-244)[45].
Compared to its ancestral lineage B.1.1.28,the P.1 variant has 11 amino acid changes in the S protein.Three of these proteins are located in the RBD.L18F,T20 N,P26S,D138Y,R190S,K417T,E484K,N501Y,H655Y,T1027I,and V1176F are the complete set of S protein modifications for the variant[46].
The latest CAL.20C variant,which emerged from the 20C clade,has five mutations:ORF1a:I4205V;ORF1b:D1183Y;S:S13I,W152C,and L452R[47].
The characteristics of the new SARS-CoV-2 variants are summarized in Table 2[53,54].In New York City,another category of coronaviruses,identified as B.1.526,has been spreading rapidly.The variant was initially reported in November 2020,and by mid-February 2021,it had accounted for nearly a quarter of all New York City sequences.There are two forms of this variant:one containing the E484K spike mutation,which may enable the virus to resist antibodies,and the other with the S477 N mutation,which may also help the virus attach more closely to human cells[55].

Table 2Characteristics of new SARS-CoV-2 variants.
4.2.Vaccine effectiveness
A number of COVID-19 vaccines were developed or produced in late 2020.According to the WHO Coronavirus(COVID-19)Dashboard on September 6,2021,a total of 5,289,724,918 vaccine doses were administered[55].Although the latest version consists of S glycoprotein mutations affected by the three major vaccines,the immune response to the vaccine develops antibodies to many domains of the protein.Therefore,it is suggested that a single mutation will not render vaccines less effective[56].German,British,and American health organizations and experts agree that current vaccines will be as potent against the latest VUI-202012/01 strain as they are to existing strains obtained by the end of 2020.Public Health England reported that as of December 20,2020,there was still“no proof”to indicate that the latest strain would be highly resistant to the Pfizer-BioNTech vaccine presently being used in the UK vaccine schedule,and that citizens should still be immunized[57].Table 3[58]summarizes the most crucial points,including the efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 new variants.
5.Conclusion
From the influenza pandemic that emerged at the beginning of the 20th century to the new coronavirus pandemic that has emerged today,an effective method for the early detection of these microorganisms has not yet been demonstrated.Even with the methods used today,false positive or negative results can still occur.Today,however,it is very important for every individual to become immune to the virus.Although mutant variants have emerged,vaccination studies are continuing rapidly all over the world.
As seen in Tables S1-S4,vaccine studies carried out in countries on all continents of the world continue at different clinical stages(preclinical,stage I,stage II and stage III).It is estimated that regions with weak economy,such as the majority of African countries,will be severely affected by the epidemic due to the cost of obtaining the developed vaccines.In this context,the equitable distribution of successful vaccines worldwide is particularly important in such countries.
All vaccine studies in Tables S1-S4 are based on the wild-type SARS-CoV-2 genome that has been genomically sequenced and characterized.But SARS-CoV-2 is mutating every day,and new variants have emerged and will continue to emerge.The type of variation to which the virus is exposed can change the rate of spread according to the function of the cellular component that this variation affects.While some of the vaccine development methods currently used are advantageous for wild-type SARS-CoV-2,a new variant may result in altered efficacy of the vaccine product.Existing vaccine candidates need to be reformulated according to viral variant types,as new variant types may emerge as the virus mutates.
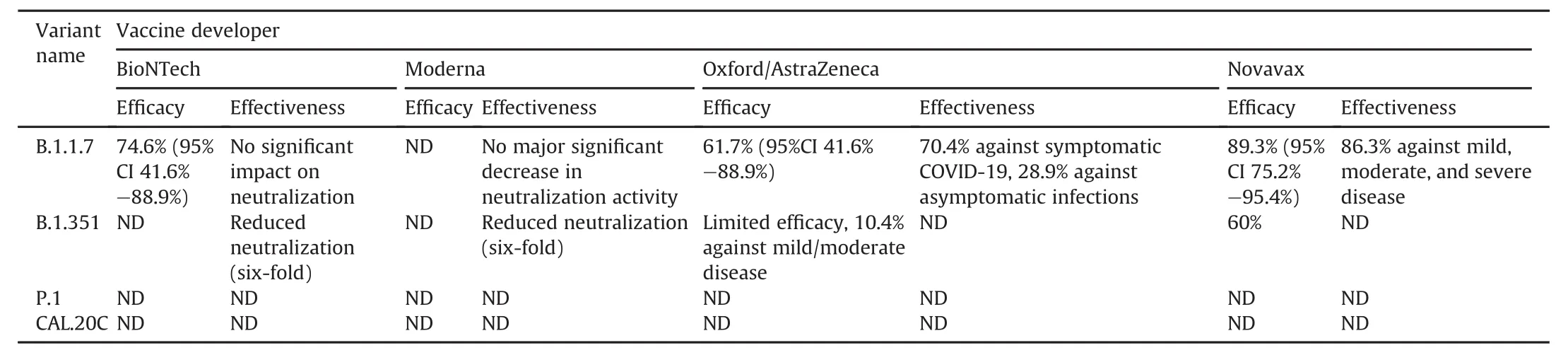
Table 3Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 new variants[58].
VUI 202012/01 variant SARS-CoV-2,known as Alpha variant,has changed the rate of spread of the epidemic.For this reason,a second or even a third wave has been observed in some countries.The virus is the main target for protein subunit-based vaccines,as it interacts with host cell ACE2 receptors via the S protein to enter the cell.At this point,the receptor binding affinity of the new VUI 202012/01 type increased as the conformational structure of the S protein changed.Therefore,protein subunit-based vaccines have become more advantageous together with vaccines targeting the S protein.In addition,due to the more severe effects of the Alpha variant,some existing vaccines have been reformulated to be effective against new variants.
The B.1.167.2 variant,also known as the Delta variant,which emerged in March 2021 in England,reversed the declining number of cases in some countries.According to some studies,about 60% of those infected with the new variant are unvaccinated.These studies have shown that the Delta variant doubles the risk of hospitalization compared to the Alpha variant.More research needs to be done to understand how severe the effects of the new variants are compared to previous variants.As the virus continues to spread among living things,there is a possibility that new variants with more severe effects will emerge.As variants change,more work is needed to make existing vaccines more effective against emerging new variants.
Declaration of competing interests
The authors declare that there are no conflicts of interest.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.09.004.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Effect of Shengmai Yin on the DNA methylation status of nasopharyngeal carcinoma cell and its radioresistant strains
- Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
- Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide
- Evaluation of the gastrointestinal anti-motility effect of Anacardium occidentale stem bark extract:A mechanistic study of antidiarrheal activity
- Synergistic effects of methyl 2-cyano-3,11-dioxo-18beta-olean-1,-12-dien-30-oate and erlotinib on erlotinib-resistant non-small cell lung cancer cells
- A living cell-based fluorescent reporter for high-throughput screening of anti-tumor drugs
