Reversible heart failure in a patient with hypocalcemic cardiomyopathy
2022-01-14MehrdadJafariFesharakiNooshinAhmadiKimiaKarimiTaheri
Mehrdad Jafari Fesharaki, Nooshin Ahmadi, Kimia Karimi Taheri✉
1. Department of Cardiology, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2. School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3. Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
The parathyroid gland is essential to maintaining the serum calcium level within the normal range by secreting the parathyroid hormone (PTH). Significant incidences of increased blood pressure, arrhythmia, left ventricular (LV)hypertrophy, and congestive heart failure (CHF)can occur in patients with parathyroid disease. Hypocalcemia can cause dilated cardiomyopathy,whether this is infrequent. Hypoparathyroidism caused by thyroidectomy and primary hypoparathyroidism are the greatest causes of hypocalcemic heart failure (HF).
The findings from our case may help clinicians to quickly diagnose hypocalcemic cardiomyopathy caused by hypoparathyroidism. Hypocalcemia can lead to severe LV contractile dysfunction irrespective of its cause. The direct action of the PTH on the heart and changes in calcium homeostasis, both hypocalcemia and hypercalcemia, comprise two primary mechanisms whereby disorders of the parathyroid gland affect the cardiovascular system.[1,2]The role of calcium in the myocardium is wellknown, and hypocalcemic HF owing to severe vitamin D deficiency, albeit a rare presentation, is welldefined, especially in infants.[3–6]
We herein describe a patient who presented to Department of Cardiology, Shahid Beheshti University of Medical Sciences, Tehran, Iran with acute CHF but without a history of underlying cardiac disease. We found that hypocalcemia and hypoparathyroidism were the potential cause of HF in this patient. A diagnosis of hypocalcemic cardiomyopathy was eventually established, and the symptoms were relieved after the treatment of hypocalcemia, thus, recommend that hypocalcemia and hypoparathyroidism be included in the differential diagnosis of all patients with cardiac dysfunction.
The patient was a 62-year-old man who came to Department of Emergency, Shahid Beheshti University of Medical Sciences, Tehran, Iran with a history of progressive pitting edema in the lower limbs,dyspnea on exertion, and paroxysmal nocturnal dyspnea of one week duration. His past medical history was chronic obstructive pulmonary disease and hypothyroidism with no previous history of cardiac problems. This patient had been diagnosed with hypothyroidism one week earlier and was on levothyroxine (100 μg/d) at hospital admission.
Physical examination revealed a respiratory rate of 18 breaths/min, a heart rate of 60 beats/min, a blood pressure of 120/75 mmHg, an oxygen saturation level of 93% in room air, and a body temperature of 37 ºC. Pulmonary auscultation showed a decreased lung sound at the base of both lungs and diffuse bilateral wheezing. Cardiac auscultation demonstrated normal S1 and S2 without any murmurs. In the examination, the thyroid size was 2 cm ×2 cm without any nodules and tenderness. The Trousseau’s sign was negative, but the Chvostek’s sign was positive. The jugular veins were distended, the peripheral pulses were normal, and the lower limbs were symmetrically edematous. Color Doppler sonography of the lower limbs was normal without any evidence of deep vein thrombosis.The chest X-ray showed signs of pulmonary stasis with hilar enlargement due to the involvement of vascular elements and an increased cardiothoracic index. Computed tomography scanning illustrated nodule-like ground-glass opacity, septal thickening in the basal segments of the right lung, and bilateral pleural effusion. The polymerase chain reaction test for COVID-19 was negative. The 12-lead electrocardiogram (ECG) exhibited a long QT interval. In the first ECG measurement, the corrected QT (QTc) was 0.563 s (normal ≤ 0.44 s) (Figure 1).
Because of the long QT interval in the ECG, heart consultation was requested. Echocardiography revealed an enlarged LV, global hypokinesia, moderate LV systolic dysfunction [ejection fraction (EF) =40%], moderate LV diastolic dysfunction, mild-tomoderate mitral regurgitation, and mild pulmonary hypertension (systolic pulmonary arterial pressure = 40 mmHg) (Figure 2).
During emergency internment, serum electrolytes and laboratory blood tests disclosed severe hypocalcemia with a total serum calcium level of 5.2 mg/dL (Table 1). Based on the low serum calcium level and echocardiographic findings, a diagnosis of HF due to hypocalcemia was suspected. The administration of two vials of calcium gluconate 10% with an infusion of 500 mL of dextrose water in one hour, followed by eleven vials of calcium gluconate in 1,000 mL of normal saline, was immediately started, which elevated the calcium level to 6.5 mg/dL.
The patient’s hemoglobin level was about 7.3 mg/dL (Table 1), which was less than its normal value (13.5−18.0 mg/dL). A peripheral blood smear showed no basophilic stippling. All rheumatologic markers, including antinuclear antibodies, rheumatoid factor, and anti-double-stranded DNA, were within the normal range. Moreover, the prothrombin time, the partial thromboplastin time, the international normalized ratio, the bilirubin (total and direct) level, the liver function test, and the stool exam were also normal. In abdominal sonography, the splenic span was 112 mm, and the liver had a normal size with normal parenchymal echo. The lead level was requested, and it was within the normal range, too. It was, therefore, concluded that the patient’s anemia was due to HF (anemia of chronic disease).
The patient was admitted to the Cardiac Care Unit, where calcium therapy was continued in tandem with daily checks of serum calcium, magnesium,and phosphorus. Moreover, the patient received one isogroup isoRh pack cell. Initially, the magnesium level was low (1.8 mg/dL). However, magnesium sulfate therapy raised it to the normal range, and it did not have any effect on the serum PTH. After five days, the calcium level rose to 8.8 mg/dL, the hemoglobin level reached 10.3 g/dL (Table 1), and the QTc interval decreased to 0.48 ms (Figure 3).
The patient was discharged after one week with improvements in cardiac symptoms and lower limb edema and with no manifestation of hypocalcemia.Three weeks later, echocardiography was performed,and the results revealed improvements in the LVEF(= 50%) and LV dimensions. For the evaluation of coronary artery disease, a myocardial perfusion scan was done, and it exhibited no significant ischemia.

Figure 1 The first electrocardiogram measurement. It shows an increased QTc interval.

Figure 2 Echocardiography in the long-axis. The echocardiography that shows an increased left ventricular diastolic dimension (6.20 cm).
Primary hypoparathyroidism is described as the abnormally low secretion of PTH. Given the reversibility of some cases of hypocalcemic cardiomyopathy, it is vital to determine the best therapy.[3]In healthy persons with a normal blood calcium level,the reported Chvostek’s sign and Trousseau’s sign are up to 25% and 4%, respectively.[7,8]We routinely use the Chvostek’s sign, which has a sensitivity of 29% and a specificity of 75%,[9]for patients suspected of hypocalcemia.
PTH plays an essential role in cardiac function,not only because it maintains calcium homeostasis but also because it exerts a direct positive effect on cardiac muscles as has been revealed in recent molecular research.[10]Although the accurate mechanisms have yet to be elucidated, hypocalcemia may reduce cardiac contractility, while PTH itself seems to directly influence cardiomyocytes.[11]

Table 1 The results of the blood laboratory test of the patient.
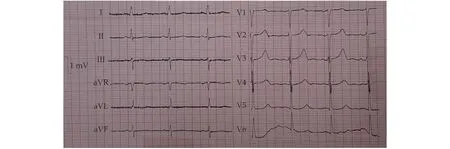
Figure 3 The last electrocardiogram measurement. It shows a decreased QTc interval after treatment.
Calcium homeostasis is disturbed in hypoparathyroidism. This impairment is associated with poor cardiovascular findings such as cardiomyopathy, CHF, and arrhythmia.[12–14]Also, at an increased risk of cardiovascular disease are patients with nonsurgical hypoparathyroidism and impaired calcium homeostasis.[15,16]
In a previous investigation, patients with chronic hypoparathyroidism had a remarkably higher incidence of cardiovascular disease than subjects without chronic hypoparathyroidism (19.4%vs.9.5%). Additionally, compared with the non-chronic hypoparathyroidism group, the chronic hypoparathyroidism group had higher incidence rates of atrial fibrillation (6.0%vs.2.7%), tachyarrhythmia (0.7%vs.0.4%), myocardial infarction (1.9%vs.1.3%), coronary artery disease (9.6%vs.5.3%), HF (5.9%vs.2.4%),stroke (4.6%vs.2.4%), and cerebrovascular disease(6.0%vs.3.0%) (allP< 0.001).[17]
In this case, we reported that three weeks of treatment with calcium supplements augmented the LVEF to almost a near-normal level. Nevertheless,previous studies have reported that the recovery of LV function can take up to six months in patients with HF due to hypoparathyroidism. For instance,in a prior investigation, a 40-year-old woman with a history of thyroidectomy for Graves’ disease was hospitalized for syncope and symptoms of HF. The patient had a long QT interval on ECG and a calcium level of 3.6 mg/dL. It took one year of treatment to improve her symptoms.[3]In another study,following one year of treatment, the symptoms of the patient improved, and LV dilation, which had been depicted by the initial echocardiogram, disappeared.[1]
Hypocalcemia is a rare but treatable cause of cardiomyopathy and should, therefore, be considered in all unexplained cases of LV dysfunction.[17–19]This etiology must be suspected in a patient with signs and symptoms of CHF, as was the case in our patient, who presented with lower limb edema, dyspnea, and LV dysfunction. Early calcium supplementation, usually along with vitamin D, can reduce and even reverse systolic and diastolic dysfunction and conduction abnormalities.[2]
杂志排行
Journal of Geriatric Cardiology的其它文章
- Echocardiography and impedance cardiography as determinants of successful slow levosimendan infusion in advanced older heart failure patients
- Association between serum cystatin C level and hemodynamically significant aortic stenosis: a prospective cohort study
- Lipoprotein(a) is associated with coronary atheroma progression:analysis from a serial coronary computed tomography angiography study
- A different cardiac resynchronization therapy technique might be needed in some patients with nonspecific intraventricular conduction disturbance pattern
- MiR-21-5p-expressing bone marrow mesenchymal stem cells alleviate myocardial ischemia/reperfusion injury by regulating the circRNA_0031672/miR-21-5p/programmed cell death protein 4 pathway
- Takotsubo syndrome with underlying pheochromocytoma
