A Highly Selective Colorimetric Naked-Eye Probe for Hypochlorite Detection in Water
2022-01-12YUQingCHENXiaoliZHANGQilongLIUHuaYANGXianjiongXUHongHUANGYaliFENGXingREDSHAWCarl
YU Qing,CHEN Xiao-li,ZHANG Qi-long,*,LIU Hua,YANG Xian-jiong,XU Hong,HUANG Ya-li*,FENG Xing,REDSHAW Carl
1.School of Public Health,the Key Laboratory of Environmental Pollution Monitoring and Disease Control,Ministry of Education,Guizhou Medical University,Guiyang 550004,China 2.School of Clinical Medicine,Guizhou Medical University,Guiyang 550004,China 3.School of Basic Medical Science,Guizhou Medical University,Guiyang 550004,China 4.Guangdong Provincial Key Laboratory of Functional Soft Condensed Matter,School of Material and Energy,Guangdong University of Technology,Guangzhou 510006,China 5.Department of Chemistry &Biochemistry,University of Hull,Cottingham Road,Hull,Yorkshire HU6 7RX,UK
Abstract The real-time detection and monitoring of hypochlorites (ClO-)in water is highly challenging.An excellent colorimetric “naked-eye”probe photoacid (PAH)was synthesized.PAH was comfirmed using High-Resolution Mass Spectrometry (HRMS),1H NMR and 13C NMR.The interaction between PAH and ClO- was investigated via UV-Vis absorption spectrophotometry under different pH conditions.The results indicated that PAH was completely soluble in water,PAH displayed a yellow solution in a phosphate buffer with a pH of 2.0 to 5.0,and the maximum absorption peak was at 424 nm.PAH displayed a purple solution in a phosphate buffer with a pH of 6.0~12.0,and the maximum absorption peak was at 532 nm.After adding ClO- to different pH systems,PAH discoloration and the UV-Vis absorption peak disappeared.The probe PAH exhibited specific selectivity and sensitivity for ClO- detection with a low detection limit in the pH 5.0 phosphate buffer.After PAH reacted with ClO-,the absorption peak of the probe at 424 nm gradually decreased,and a new absorption peak appeared at 532 nm.The probe displayed a vivid color-tunable process from yellow to purple then to colorless with a fast response time for ClO- detection.However,other common 33 substances such as metal ions(Li+,Co2+,Cr3+,K+,Cd2+,Pb2+,Ca2+,Hg2+,Ba2+,Cu2+,Mg2+,Ni2+,Zn2+,Al3+ and Fe3+),anions I-,AcO-, CN-,Br-, and F-),reactive oxygen species (ROO·,·OH,H2O2, tBuOOH,tBuO· and 1O2)and reactive nitrogen species (ONOO- and NO·),did not cause changes in the color of the probe solution and the UV-Vis absorption spectrum.The above species had only a limited effect on detecting the ClO- anion.When they coexisted with ClO-,the probe also showed a similar solution color change,and the absorption peak at 424 nm disappeared.Meanwhile,the probe PAH could quantitatively detect the content of ClO- with a detection limit of 5.39 μmol·L-1 (y=1.586 78-0.524 51x,R2=0.998 52).Furthermore,ClO- concentration in the water system (84 disinfectant and tap water)was analyzed.The average concentration of ClO- ion in the tap water measured by three parallel tests was 7.96 μmol·L-1 with high recoveries rate.It showed that PAH could also be utilized to detect ClO- quantitatively in real water systems.
Keywords Colorimetric probe;Hypochlorite anion;Rapid response;High selectivity
Introduction
Hypochlorous acid (HClO)or hypochlorite (ClO-),as typical reactive oxygen species (ROS),play several fundamental roles in the human body and are biologically produced by the reaction of chloride ions (Cl-)and hydrogen peroxide (H2O2)via catalysis of myeloperoxidase (MPO)in the immune cell[1].Moreover,an appropriate amount of ClO-can protecting the immune system against the invasion of pathogens.Nevertheless,excess production of ClO-may lead to ageing and an increased risk of cell membrane damage[2],Alzheimer’s disease[3]and cardiovascular disease[4].
Hypochlorite is ubiquiuous in daily human life and appears in many applications,such as the sanitization of tap water and swimming pools.Traditional methods for the detection of ClO-include iodine reduction titration,electrochemical methods[5],chemiluminescence methods[6]and ion chromatography[7]as well as spectroscopic (colorimetry and fluorescence)detection.Commonly,HClO/ClO-concentration in the standards for drinking water quality in China ranges from 0.05 to 4 mg·L-1[8].Theoretically,the detection mechanism of HClO is involved in common oxidation reactions[9],cleavage reactions[10].However,the hydrophobic nature of the probe has limited their practical application environment.Water-solubility is a crucial criterion for the practical use of a probe;and the development of water-solubility colorimetric probes with a specific response to ClO-and HClO in a water environment still remain challenging.There are few reports on the detection of ClO-and HClO in a water environment by use of colorimetric probes.Therefore,it is important to design and synthesize hypochlorite colorimetric probes with good water-solubility,high selectivity and sensitivity.More importantly,this type of probe with excellent characteristics can be used for rapid and selective detection of HClO/ClO-in water environments.
In this paper,for portable purposes,a simple-to-use and naked-eye diagnostic tool were explored for ClO-detection in an aqueous solution with the advantage of a rapid response,high sensitivity and a low detect limitation.Herein,we present an example of a colorimetric “naked-eye”probe photoacid (PAH)for ClO-detection with high selectivity in aqueous solution,which was designed and synthesized via a Knoevenagel reaction between indolinium and salicylic aldehyde in good yield[11](Scheme 1).The structures of the synthesized compounds were fully characterized by1H/13C NMR spectroscopy and High-Resolution Mass Spectrometry (HRMS);the spectra are shown in the Supplementary Information (Fig.S1—S2).

Scheme 1 The synthetic route to probe PAH

Fig.S1 1H NMR spectrum of PAH (400 MHz,d6-DMSO)

Fig.S2 13C NMR spectrum of PAH (d6-DMSO)
1 Experimental
1.1 Materials and instruments
All reagents and solvents were of analytical grade and were used without further purification.Ultrapure water was used throughout the experiments.The solutions of metal ions were prepared from their nitrates,and the solutions of anions were prepared from their sodium salts.The UV-Vis absorption spectra were determined at room temperature on a Shimadzu UV-2600 spectrophotometer in a 1 cm quartz cell.The pH values were determined with a model pHS-25 pH meter.High-resolution mass spectrometry (HRMS)were performed in a micro TOF-QⅡ mass spectrometer (USA).1H/13CNMR (400 MHz)spectra was recorded on a Bruker Advance 400 spectrometer(Germany),with DMSO-d6 used as a solvent and tetramethylsilane (TMS)as an internal standard.
1.2 Experimental method
1.2.1 Synthesis of probe PAH
Probe PAH was synthesized according to the reported procedures[12].A mixture of 2,3,3-trimethylindolenine 1 (1.65 g,0.01 mmol)and 1,2-oxathiolane 2,2-dioxide (1.26 g,0.01 mmol)in toluene and stirred at 90 ℃ for 4 h under N2.The purple solid was collected by filtration,washed with cold ethylether,and dried in vacuo to afford 2,3,3-trimethyl-1-(3-sulfonatepropyl)-3H-indolium 2 (2.61 g,89% yield).Without further purification,the synthesized compound 2 (100 mg,0.36 mmol)and 2-hydroxybenzaldehyde (48 mg,0.39 mmol)were added into anhydrous ethanol (2 mL).The mixture was allowed to reflux overnight.The orange solid was obtained by filtration (110 mg,80% yield);1H NMR (400 MHz,d6-DMSO):δ8.6~8.48 (m,1H),8.24 (d,J=7.9 Hz,1H),7.99 (d,J=8.2 Hz,1H),7.90~7.78 (m,2H),7.65~7.53 (m,2H),7.44 (t,J=7.7 Hz,1H),7.04~6.89 (m,2H),4.90~4.66 (m,2H),2.62 (t,J=6.4 Hz,2H),2.22~2.05 (m,2H),1.84~1.62 (m,6H);13C NMR (100 MHz,d6-DMSO)δ182.27 (s),159.55 (s),149.19 (s),144.01 (s),141.45 (s),136.28 (s),130.28 (s),129.66 (s),123.52 (s),121.86 (s),120.59 (s),117.15 (s),115.61 (s),111.97 (s),52.43 (s),47.87 (s),46.06 (s),26.97 (s),25.12 (s).HRMS:m/z[M+Na]+=408.126;Calcd:407.120.The1H/13C NMR data is consisting with the previously reported data[12].
1.2.2 Preparation of solutions
Phosphate buffer saline (PBS)with different pH from 2.0 to 12.0 was prepared from disodium hydrogen phosphate,dihydrogen phosphate and sodium chloride in a certain proportion with ultrapure water[13].The 0.5 mmol·L-1stock solution of probe PAH was prepared in an aqueous solution of PBS (0.01 mol·L-1,pH 5.0).The 0.01 mol·L-1stock solutions of the metal ions and various anions were dissolved in an aqueous solution of PBS (0.01 mol·L-1,pH 5.0).The 0.01 mol·L-1stock solutions of reduced vitamin C (Vc)and glutathione (GSH)were prepared directly from the solids in aqueous solution of PBS (0.01 mol·L-1,pH 5.0).Hypochlorites were derived from sodium hypochlorite.The stock solution of ClO-was prepared with 0.01 mol·L-1sodium hydroxide solution and standardized at 292 nm using an extinction coefficient 350 M-1·cm-1at pH 12.0[14],then diluting the stock solution of ClO-to 8 mmol·L-1.The 0.01 mol·L-1stock solution of other reactive oxygen species (ROS)and reactive nitrogen species (RNS)were prepared according to the literature[15].
1.2.3 General procedure for analysis
Before conducting the spectroscopic measurements,the corresponding solutions of probe PAH and the reactive species (metal ions,anions,ROS and RNS)were freshly prepared.For UV-Vis selective experiments,test solutions were prepared by placing 0.6 mL of the probe PAH solution (0.5 mmol·L-1)and 0.12 mL of reactive species solutions in the absence and presence of 0.12 mL of ClO-solutions (8 mmol·L-1)into a 3 mL cuvette,and then diluting the solution to 3 mL with PBS (0.01 mol·L-1,pH 5.0).For UV-Vis titrations,0.6 mL of the probe PAH solution and different amounts of ClO-was added into a 3 mL cuvette.These solutions were diluted with PBS (0.01 mol·L-1,pH 5.0)to 3 mL and mixed,then the spectra of these solutions were immediately recorded through the UV-Vis method.
2 Results and discussion
2.1 The pH effect to PAH


Fig.1 The change of UV-Vis spectra (a)and line graph (b)of probe PAH (0.1 mmol·L-1)in the range from pH 2.0~12.0 using PBS as buffer solution;The color change of photography of probe PAH with the pH value increases under natural light (inset)
2.2 Selectivity recognition toward ClO-


Fig.2 Absorption intensity (a—c)at 424 nm of probe PAH (0.1 mmol·L-1)with addition of different species (0.4 mmol·L-1)in the absence/presence of ClO- (0.4 mmol·L-1)in PBS solution (0.01 mol·L-1,pH 5.0),Error bar=RSD (n=3);Visible color (d—f)of probe PAH toward ClO- (0.4 mmol·L-1)in different species under sunlight

Fig.S3 UV-Vis spectra of PAH (0.1 mmol·L-1)with addition of nitrate salts of Li+,Co2+,Cr3+,K+,Cd2+,Pb2+,Ca2+,Hg2+,Ba2+,Cu2+,Mg2+,Ni2+,Zn2+,Al3+ and Fe3+(0.4 mmol·L-1),sodium salts of I-,AcO-, CN-,Br-, and F- (0.4 mmol·L-1),ClO- (0.4 mmol·L-1),GSH,Vc and ROS/RNS of ONOO-,ROO·,·OH,H2O2, tBuO·,1O2 and NO· (0.4 mmol·L-1)in PBS solution (0.01 mol·L-1,pH 5)


Fig.3 UV-Vis absorption spectra (b)of PAH (0.1 mmol·L-1)in the presence of I- toward various concentration of ClO- in PBS buffer solution (0.01 mol·L-1,pH 5.0);The change of visible color (a)of probe PAH toward ClO- under sunlight

Fig.S4 UV-Vis absorption spectra (a—d)of PAH (0.1 mmol·L-1)with the addition of GSH,Vc,ONOO- toward various concentration of ClO- in PBS buffer solution (0.01 mol·L-1,pH 5.0);The change of visible color of probe PAH toward ClO- under sunlight (inset)
2.3 UV-Vis absorbance response of probe PAH towards ClO-
The recognition ability of probe PAH toward ClO-was measured using UV-Vis titrations in buffered aqueous solution (PBS,0.01 mol·L-1,pH 5.0).As shown in Fig.4,with increasing ClO-concentrations,the absorption peak of probe PAH at 424 nm gradually decreased and the short absorption wavelength centering around 296 nm gradually increased,accompanied by a change in the color of the solution from yellow to colorless.Moreover,a well-defined isosbestic point was observed at 315 nm,indicating the formation of a single new species.In addition,the UV-Vis titration experiments between the corresponding absorbance values at 424 nm and ClO-concentrations exhibited an excellent linear correlation with a high coefficient (y=1.586 78-0.524 51x,R2=0.998 52)in the range of 0~0.28 mmol·L-1[Fig.4(c)].Thus,the detection limit of probe PAH for ClO-detection was determined to be 5.39 μmol·L-1according to the IUPAC definition (CDL=3Sb/m,Sbis the standard deviation of the blank samples,mis the slope of the linear equation)from 10 blank solutions[18].These results illustrated that probe PAH has the excellent capability for the qualitative and quantitative determination of ClO-with high sensitivity in total aqueous solutions.

Fig.4 UV-Vis spectra (a)of probe PAH (0.1 mmol·L-1),changing of the absorption intensity (b)at 424 nm of probe PAH upon addition of increasing concentrations (0~1.4 mmol·L-1)of ClO- in PBS (0.01 mol·L-1,pH 5.0)and linearity of absorption intensity (c)of probe PAH with the addition of ClO- from 0~0.28 mmol·L-1. Error bar=RSD (n=3)
It is noteworthy that on the addition of a small amount of ClO-to the solution of probe PAH,a new absorption band appeared in the range from 500~570 nm,while following an increase in the concentration of ClO-,the absorption gradually disappeared again.We hypothesized that the new long absorption band originated from the degraded compound of indolinium,which strongly agrees with our experiment results (Fig.S5).
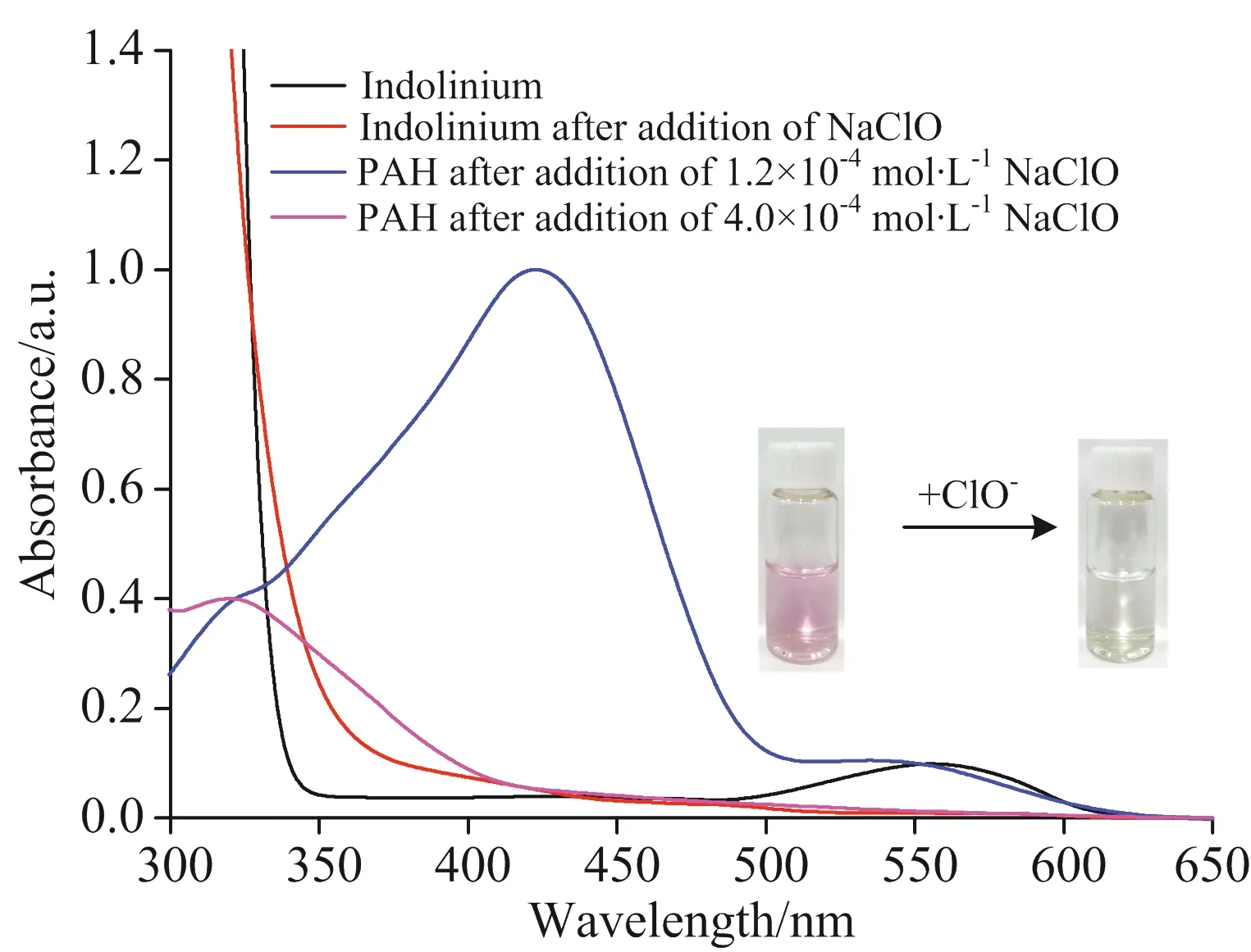
Fig.S5 UV-Vis absorption spectra of indolinium in the absence/presence of ClO- in aqueous solution and UV-Vis absorption spectra of probe PAH (0.1 mmol·L-1)in the presence of ClO- (0.12 mmol·L-1 or 0.4 mmol·L-1)in PBS (0.01 mol·L-1,pH 5.0);Photography of indolinium in the absence/presence of ClO- (inset)
2.4 The pH effect toward ClO- detection
The pH value of the environment is a crucial parameter that can affect the selectivity,sensitivity,and detection limit of a probe[19].Carefully,the absorption behavior of probe PAH toward ClO-was investigated over the relevant pH range from 2.0 to 12.0,respectively (Fig.5 and Fig.S6).The maximum absorption peak of probe PAH is at 424 nm within a pH range of 2.0~5.0 with a yellow color solution,but when treated with ClO-,the solution color dramatically changed from yellow to colorless,and the maximum absorption peak of 424 nm disappeared.On the other hand,the neutralized PAH at pH>6.0,displayed two absorption peaks at 424 and 550 nm,respectively.However,when treated with ClO-from 0 to 1.4 mmol·L-1,both absorption peaks gradually decreased,and even disappeared,and the solution containing PAH turned from purple to colorless.These results indicated that the PAH is a highly sensitive,“naked-eye”colorimetric sensor for ClO-detection in aquatic environments.More importantly,the presence of metal ions and anions,ROS and RNS only provide a limited ClO-detection disruption.Thus the excellent probe PAH can be applied for detecting the ClO-in natural water samples with significantly more complex compositions.

Fig.5 UV-Vis spectra of probe PAH (0.1 mmol·L-1)with the addition of ClO- (0~1.4 mmol·L-1)in PBS (0.01 mol·L-1)in pH 2.0 (a),pH 7.4 (b),respectively;The photography change of visible color of probe PAH toward ClO- under sunlight (inset)

Fig.S6 UV-Vis spectra of probe PAH (0.1 mmol·L-1)with the addition of ClO- (0~1.4 mmol·L-1)in PBS (0.01 mol·L-1)in pH 3.0 (a),pH 4.0 (b),pH 5.0 (c),pH 6.0 (d),pH 7.0 (e),pH 8.0 (f),pH 9.0 (g),pH 10.0 (h),pH 11.0 (i),pH 12.0 (j),respectively;The photography change of visible color of probe PAH toward ClO- under sunlight (inset)
2.5 Recognition mechanism


Scheme 2 The proposed recognition mechanism of probe PAH toward ClO-

Fig.S7 Comparison of the interaction time between PAH (0.1 mmol·L-1)and ClO- (0.8 mmol·L-1)in aqueous solution of PBS (0.01 mol·L-1,pH 2.0~12.0)


Fig.S8 HRMS of intermediate 1The peak (m/z)at 160.111 89 corresponds to [M+H]+ ion (Calcd:159.10)

Fig.S9 HRMS of 2-hydroxybenzoic acidThe peak (m/z)at 139.038 97 corresponds to [M+H]+ ion (Calcd:138.03)

Fig.S10 HRMS of intermediate 2The peak (m/z)at 282.115 84 corresponds to [M+H]+ ion (Calcd:281.11)
On titration of hypochlorite under acidic conditions,HRMS data manifested the peaks atm/z=160.111 89 and 139.038 97,corresponding to the intermediates 1 and 2-hydroxybenzoic acid;while under basic conditions,HRMS gives result form/z=123.044 06 and 282.115 84 consistent with intermediate 2 and salicylaldehyde,respectively.
2.6 Detection of ClO- in 84 disinfectant and tap water samples
Hypochlorite is widely used in our daily life.Thus,the probe PAH had been applied to analyse the content of ClO-in real samples (such as 84 disinfectant and tap water).The real sample was filtered for the removal of insoluble species.In a PBS (0.01 mol·L-1,pH 5.0)solution of 3 mL containing the probe PAH (0.1 mmol·L-1),the sample of 84 disinfectants was added with different volumes of 2.0,4.0,6.0 μL,respectively.The calculated concentration of ClO-of this 84 disinfectant sample is (151±6.68)mmol·L-1(Table 1),According to the linear regression equation of UV-Vis titration (y=1.586 78-0.524 51x,R2=0.998 52).Further,the content of ClO-in tap water and purified water were also measured under the same conditions,and the calculated concentration of ClO-is 7.96 μmol·L-1for the tap water sample (Table 1).Except for one sample,the percentage recoveries were in the range of 93%~103%.These results indicated that the probe PAH could potentially be used for the quantitative detection of ClO-concentrations in real water samples.

Table 1 Determination of ClO- concentrations in real samples
3 Conclusions
In conclusion,based on the oxidative activity of ClO-,the PAH can be utilized as a highly selective colorimetric naked-eye probe for ClO-detection via oxidative cleavage of itself in absolute aqueous solution,which leads to a tunable color process from yellow to purple then to colorless.The specificity of probe PAH can work in water environments,and coexisting ions only show a slight effect on ClO-detection.More importantly,acidic solid conditions accelerate the response time.Moreover,PAH exhibited a fast response time and naked eye detection toward ClO-at various pH values with highly-sensitive detection at pH 5.0.We have also successfully applied the probe to detect ClO-in real water samples.This research will open up new avenues for developing novel “naked-eye”probes for the detection of environmental pollutants in an absolute aqueous system.
