Evaluation of gases’ molecular abundance ratio by fiber-optic laser-induced breakdown spectroscopy with a metal-assisted method
2022-01-10YuZHU朱瑜PingweiZHOU周平伟andShengfuLI李生福
Yu ZHU (朱瑜), Pingwei ZHOU (周平伟) and Shengfu LI (李生福)
Institute of Fluid Physics, China Academy of Engineering Physics, Mianyang 621900, People’s Republic of China
Abstract A metal-assisted method is proposed for the evaluation of gases’ molecular abundance ratio in fiber-optic laser-induced breakdown spectroscopy(FO-LIBS).This method can reduce the laser ablation energy and make gas composition identification possible.The principle comes from the collision between the detected gases and the plasma produced by the laser ablation of the metal substrate.The interparticle collision in the plasma plume leads to gas molecules dissociating and sparking, which can be used to determine the gas composition.The quantitative relationship between spectral line intensity and molecular abundance ratio was developed over a large molecular abundance ratio range.The influence of laser ablation energy and substrate material on gas quantitative calibration measurement is also analyzed.The proposed metal-assisted method makes the measurement of gases’molecular abundance ratios possible with an FO-LIBS system.
Keywords: gases’ molecular abundance ratio, collision of plasma, fiber optical laser induced breakdown spectroscopy, metal assisted method
1.Introduction
Laser-induced breakdown spectroscopy (LIBS) refers to focusing a pulsed laser onto the surface of target to create a high-temperature plasma.The emission spectrum of plasma can provide information about the elemental composition of the target[1–3].LIBS has been used for element analysis in a wide variety of fields, such as material analysis [4, 5],environmental monitoring[6],biological identification[7,8],forensics [9], and security, due to the advantages of online,in situ, and simultaneous multi-element analysis [10].It is a potential method for atomic spectrum analysis for species diagnosis.Several books and review articles have provided a complete description of its theoretical foundations and recent applications in industrial and analytical situations [11–13].
The measurement of gases’ molecular abundance ratios has garnered great attention in combustion and energy-related research.The optical methods are of great importance due to their effectiveness in many situations where intrusive measurements are inconvenient.The real-time,in situnature of LIBS techniques makes them suitable to many applications requiring online data acquisition [14–16].The LIBS technique has been used to investigate gases’molecular abundance ratio in gas mixtures such as the in-air detection of Cl[17],F[18],and pollutant concentrations [19], as well as the deuterium/hydrogen ratio in gas reaction products[20].Of particular note,it has also been used for equivalence ratio measurement in a sparkignited engines and methane–air premixed flames,which arises from the desire to quantify real-time, localized combustion performance [21–24].Although LIBS has been considered a promising method for elemental analysis in gaseous detection,the conventional LIBS system (without using optical fibers to deliver the laser pulses) is limited when the environment is too hazardous or access space is restricted [25].Fiber-optic LIBS(FO-LIBS)systems,in which the laser beam is transferred to the target by a transmission fiber, provide a solution to the above problem.Researchers have developed many fiber-coupled laser-induced breakdown spectroscopy instruments for analysis of target elements[26–28].However,compared with traditional LIBS technology,the laser energy delivered to the target surface is limited by the aperture and the damage threshold of the fiber,which is lower than the breakdown threshold of the gas[29,30].In FO-LIBS, it is impossible to detect the gas by directly focusing the laser pulse delivered by the optical fiber on the gas,while increasing the incident laser energy would lead to inevitable optical fiber damage [31].Using optical fiber LIBS to realize gas composition identification is very important for harsh or narrow environment gas identification; however, to our knowledge,there has been no report on the measurement of gas composition using FO-LIBS.
It is worth mentioning that surface-enhanced LIBS,based on a metal substrate,which was proposed by M A Aguirre to improve the detection limit of LIBS with the assistance of a metal substrate, exhibits superior spectral enhancement and repeatability [32, 33].The strong collision between the plasma and the detected particles in the plasma plume results in enhanced luminescence of the detected particles [34, 35].This gives us inspiration that the gas composition detection could be realized below the gas breakdown threshold in FOLIBS with the assistance of a metal substrate.The excitation radiation induced by the collision between metal particles in plasma and surrounding gas molecules could provide information about the elemental composition of the surrounding gaseous sample.
In the current work, we propose a metal-assisted FOLIBS method to determine gas composition, and we take a methane mixture as an example to analyze the molecular abundance ratio over a wide range of mixture fractions.Atomic emissions from laser-induced plasma were observed and the ratios of the elemental line intensity were used to infer the molecular abundance ratio in mixture fractions.The advantage of this method is that gas detection could be realized using FO-LIBS as the laser energy transmitted by the fiber is lower than the breakdown threshold of the gas, while the fiber is not damaged.Compared to the traditional gas detection LIBS method,the metal substrate-assisted FO-LIBS is effective when the environment is too hazardous or the access space is restricted.Meanwhile,metal substrate-assisted FO-LIBS expands the field of applications of FO-LIBS from solid detection to gas detection [26, 31].
2.Experiment
For gas detection, a sample chamber was designed as shown in figure 1(a).Before filling, the sample chamber was evacuated to 0.01 Pa by a two-stage rotary vane vacuum pump and the gas pressure was measured by a capacitance pressure gauge.We first inflated a single gas component N2in the sample chamber at a pressureP1, and refilled the chamber with the second gas component CH4to the last immobile gas pressureP2=90 kPa; the final gas pressure was slightly below atmospheric pressure in order to prevent mingling of atmosphere.The gas flow of all experiments was set at 10 l min−1, and the purity of N2and CH4used in the experiment was more than 99.99%.Follow-up spectral testing will not be carried out until the two gases are mixed uniformly, and the waiting time is about 1 min.
The schematic diagram of the metal-assisted FO-LIBS system for measurement of gases’ molecular abundance ratios is shown in figure 1(a)and the principle schematic diagram of the metal-assisted FO-LIBS method was shown in figure 1(b).A thin aluminum plate was fixed located near the chamber window as the metal substrate.The metal substrate was ablated using a Q-switch Nd:YAG laser (pulse width: 9 ns; repetition rate: 10 Hz; wavelength: 1064 nm).The laser beams were coupled into a 3 m long optical fiber using a single lens with a focal length of 100 mm.A fused silica fiber is used.The core diameter of the optical fiber is 1 mm.At the distal end of the fiber, the output beam from the fiber is focused on the metal substrate surface by a probe head.The probe consists of two lenses with a focal length of 100 mm and 50 mm respectively,which is used to focus the laser beam on the surface of metal substrate through the 10 mm thick chamber window.The probe head is mounted outside the window.The distance between the probe and the metal substrate is about 50 mm.
As shown in table 1,the delivery efficiency of the fiber as the coupled laser energy ranges from 20 mJ to 44 mJ is about 86%.The maximum output energy at the distal end of the fiber probe is approximate 40 mJ,which was measured at the output end of the 50 mm focal length lens.To protect the fiber from damage,the largest energy used in this work was 30 mJ;no fiber damage was observed after more than 5 min of laser transmission.The emission from the plasma was collected in the optical fiber by the probe head, reflected by a dichroic mirror to the spectrograph slit, and then analyzed by the intensified charge-coupled device (ICCD) detector.The spectrometer equipped with a home built 1024×1024-pixel intensified charge-coupled device (ICCD) detector analyzed the plasma spectroscopy.The grating used here was 150 grooves/mm and the spectrometer has a focus length of 750 mm.The detection system has a spectral range of approximately 100 nm with a resolution of 0.35 nm.The experiment timing includes a laser Q-switch,and ICCD delay time control was performed through a TTL signal generator.The ICCD detector started signal integration with a delay time 400 ns relative to the ablation of aluminum sample, and the gate time was set to 1000 ns.A spectrum was recorded for each laser shot and 10 spectrum data were recorded to analyze the relative standard deviation (RSD).
3.Results and discussion
3.1.Metal-assisted FO-LIBS used for gas detection
We first compare the gas detection capability of the direct focusing and metal substrate-assisted methods.The fractional molecular equilibrium ratios of nitrogen and methane are 12.1%and 87.9%respectively,while the concentrations error is about 1%.The laser energy for both was 30 mJ at the front of the probe head.The spectrum of the laser direct focusing method is shown as the solid line in figure 2(a),which just is a straight line and does not exhibit any spectral peak signal.The gases at the focus are not excited by the laser or transformed into plasma because the laser energy is lower than the gas breakdown threshold,while increasing the laser energy would lead to the fiber damage.The dashed solid line in figure 2(a)presents the spectrum of the gas sample with metal substrateassisted FO-LIBS.The spectrum ranging from 780 nm to 860 nm contains the line spectral of N 818.8 nm,N 821.6 nm,N 824.2 nm, C 833.5 nm, O 846 nm, N 856.7 nm, N 859.4 nm, and N 862.9 nm.The substrate used in the experiment is a thin aluminum bulk and the surface of the aluminum bulk was simply polished by sandpaper following cleaning by alcohol and deionized water.Compared with the direct focusing method, the metal-assisted method can obtain a clear gas composition spectrum at the same laser energy.It is obvious that metal-assisted method makes gas detection possible with the FO-LIBS system.
The ablation trace of the metal substrate is measured as the size of the laser focus spot,which is 400 μm.The laser energy under the focusing lens is 30 mJ, corresponded to an average power 2.6 GW cm−2.While the ablated threshold of aluminum is about 1–5 J cm−2for a spot size of approximately 160 μm,the corresponding power is 0.1–0.5 GW cm−2[36] and the effective breakdown threshold of gas reaches approximately 100–1000 GW cm−2[37].The laser pulse energy used in this experiment is larger than the ablated threshold of aluminum but lower than the gas breakdown threshold.We were able to observe the atom line spectrum, shown as solid arrows in figure 2(a),when undertaking metal-assisted FO-LIBS with an aluminum substrate.However, we could not observe an atom line spectrum, even the continuous spectral, during direct focusing.The difference between direct focusing and metal substrate-assisted methods may be explained as follows.The laser energy is not enough to induce the gas breakdown into the plasma state in direct gas ablation,whereas the plasma particles produced by the ablation of the aluminum bulk substrate collided with the surrounding detected gas molecules and induced the gas breakdown.The collision between the detected gases particles and fast particles, include ions and electrons in the aluminum plasma, could lead to gas molecules dissociating to excited atoms and entering an ion state, as the schematic diagram in figure 1(b)shows.As the plasma cools,excited atoms and ions relax back into their ground states,emitting light at the characteristic atomic frequencies.Therefore, gas component detection could be achieved as the laser energy is lower than the breakdown threshold in the metal-assisted FO-LIBS system.
Comparisons between the different mixing ratio gases were useful for molecular abundance ratio detection.The spectral comparison is shown in figure 2(b).The molecular abundance ratios of CH4are 0, 12.1%, and 22.7% respectively, and the total pressure was the same for all situations.When the CH4molecular abundance ratios rise from 0% to 22.7%, a significant increase of the C 833 nm spectral line intensity was noticed as the internal magnificent plate showed, while the N emission decreased as the CH4molecular abundance ratios rise.In all mixed sample tests,the laser energy and other parameters are the same, so the plasma temperature and electron concentration should be equal.The number of total particles that collide with plasma particles(include atom, ion and electron) is constant.The number of excited C atoms increases as the density of the CH4molecule increases, enhancing the collision probability between CH4molecule and aluminum particles when the CH4molecular abundance ratios rise.Finally, the C atom spectral line intensity increases and the N atom spectral line intensity decreases as the CH4molecular abundance ratios rises.The quantitative molecular abundance ratio measurement could be achieved based on the above process.
3.2.Quantitative relationships between spectral line intensity and molecular abundance ratio
The potential contamination presented on the metal substrate surface and the gas filling progress is inevitable.To eliminate the interference of carbon and nitrogen atoms sticking to the metal substrate surface and mixing in the gas filling progress,we firstly addressed the contamination quantity of carbon and nitrogen atoms before the analysis of quantitative relationships between spectral line intensity and molecular abundance ratio.The spectrum of the metal substrate in pure N2and CH4surroundings was shown in figure 3.The solid line was the metal substrate spectrum of pure CH4,where the spectral line intensity of N was used for background elimination of nitrogen atoms.The dashed line was the metal substrate spectrum of pure N2, where the spectral line intensity of C was used for background elimination of carbon atoms.To obtain the atom line spectral intensity of the detected gases,the nearby spectrum peak C 833 nm and N 818 nm at different CH4molecular abundance ratio were fitted by the single peak and multi-peak Lorentz functions; then the spectral peak intensities of C 833 nm and N 818 nm were extracted from the fitting parameter.Finally, the spectral peak intensity that represented the surrounding gases was obtained from subtracting the above fitting spectral peak intensity from the background C and N spectral intensity.
To study the quantitative relationship between spectral line intensity and molecular abundance ratio, the mean values and standard deviations of the spectral peak intensity ratios were calculated and given in the calibration curves as shown in figure 4(a),where CH4molecular abundance ratios range from 0%to 22.7%.A function y =b+a·b·exp (-s·x) ,wherexdenotes the relatively molecular abundance ratio of CH4/N2,was fitted to the data points in the curve of figure 4(a).This fitting leads to the C/N calibration curve versus the molecular abundance ratio of CH4/N2, approximately corresponding toIC833/N818=0.005 +0.3·x·exp (1.25x).Meanwhile, for the low CH4abundance ratio range, the molecular abundance ratio of CH4and N2could be fitted to the linear functiony=0.00446+0.368xas the CH4abundance ratios range from 1%to 6%,and the fitting result was shown in figure 4(b).Just like most trace element detection, there exist lower concentration limits,which are known as the linear dynamic range[38].The calibration curves at high concentrations deviated from linearity,and the most likely reason is the self-absorption phenomenon,which comes from the fact that the emissions of the central hotter regions are absorbed by the colder atoms surrounding the plasma [39].
In order to clearly understand the influence of the gas concentration on quantitative concentration measurements,the C 833 nm and N 818 nm spectral line intensities were shown in figures 4(c) and (d) as the molecular abundance ratios increasing at three laser pulse energy levels.It is clear that the evolution trend is different between the C 833 nm and N 818 nm spectral line intensities as the fractional molecular abundance ratio increases.The C 833 nm spectral line intensity showed a saturation phenomenon, which becomes more and more obvious with the decrease of the incident laser pulse energy,which is evidently self-absorption.While the N 818 nm spectral line intensity showed an exponential-like function increase as the N2molecular abundance ratio increased, this seems to be an avalanche mechanism.The collision between the plasma particles produced by aluminum ablation and the N2molecule induced the latter to the excited N atom and ion state.Excited atoms and ions get enough energy that they could even re-collide and re-ionize other ground state N2molecules.Finally,more CH4molecules lead to the increment ofIC833stagnating due to the self-absorption phenomenon, while more N2molecules lead to an increasingIN818avalanche due to secondary particle collision between N atoms and ions.The calibration curves deviate from a linear function at larger abundance ratios,which is the joint result of the C self-absorption phenomenon and the N avalanche mechanism.
3.3.The incident laser pulse energy
The incident laser pulse energy is a key factor in quantitative concentration measurements in the experimental calibration of LIBS.Figure 5 illustrates three calibration curves of CH4and N2mixture for incremental laser pulse energy from 20 mJ to 30 mJ with an aluminum plate substrate.The spectral line intensity ratio between C 833 nm and N 818 nm is extracted for analysis of the molecular abundance ratios in the CH4and N2gas mixture.Three calibration curves show a similar trend:the peak valueIC833/IN818positive correlation with the fractional molecular abundance ratio of CH4/N2.In a similar manner,we fitted the C/N calibration curve with the functionIC833/N818=b+a·x·exp (s·x),wherebdenotes the background of plasma luminescence,arepresents the linear relation between intensityIC833/N818and the fractional molecular abundance ratio of CH4/N2, andsrepresents the degree of the deviation from linearity.The fitted parametersis 2.25,1.93,and 1.51 as the laser energy is 20 mJ,25 mJ,and 30 mJ, respectively.The fitting curve presents a lager curvature as the incident laser pulse decreases, which indicates that the degree of the deviation from linearity is weakening along with the increase of the incident laser pulse energy.If the linear relationship between the spectral line intensity and molecular immunity ratio is satisfied,the molecular immunity ratio of gas can be inferred directly from spectral line intensity without considering the influence of self-absorption or other effects.To acquire suitable linear quantitative concentration measurements of gases in metal-assisted FO-LIBS, the incident laser pulse energy should be increased to produce more plasma particles at higher plasma temperatures.

Figure 1.(a) Schematic diagram of metal-assisted FO-LIBS for measurement of gases’ molecular abundance ratios.The sample chamber connects with the CH4 and N2 mixing gas tank.The laser pulse output from the Nd:YAG lasers was transferred to the target by a fused silica fiber and focused by a probe head.(b) Principle schematic diagram of a metal-assisted FO-LIBS system for measurement of gases’ molecular abundance ratios.The plasma particle (PP) produced by the laser ablation of aluminum plate (AP)collision with gases’ molecule (GM) leads to the gas molecule excitation.

Figure 2.(a)The compared spectra of gases with the same composition between directly focusing on the gas and the metal-assisted method with FO-LIBS.(b)The compared spectra between the different gases’molecular abundance ratios.The inset shows a detailed amplification of line spectral C 833 nm.

Figure 3.The background spectral line intensity assessment using the method of spectroscopy measurement of subtraction in a pure CH4 and N2 surrounding.The backgrounds of the C, N, O atom spectral lines were marked corresponding.

Figure 4.The quantitative relationship between spectral line intensity and the molecular abundance ratio as the molecular abundance ratio of CH4/N2 (a) ranges from 0 to 30% and (b) ranges from 0 to 6%.(c) The intensity of the C 833 nm line spectral as the fractional atomic abundance of CH4 ranges from 0 to 0.2 for laser ablation at three energy levels.(d)The intensity of the N 818 line spectral as the fractional atomic abundance of N2 ranges from 0.7 to 1.0 during laser ablation at three energy levels.
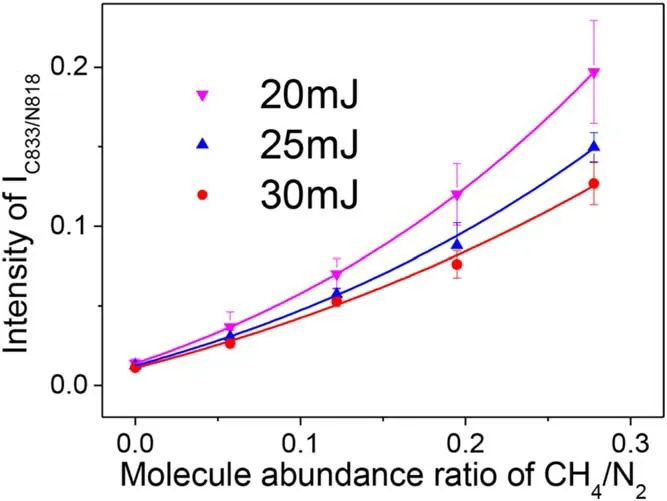
Figure 5.The influence of incident laser energy on the quantitative calibration of molecular abundance ratio.

Figure 6.The quantitative concentration measurements of the CH4/N2 mixture over large range of molecular abundance ratios.
3.4.Quantitative concentration measurements at wide abundance ratio range
Figure 6 gives the quantitative concentration measurement results when the molecular abundance ratios of CH4/N2range from 0 to 14 and the experimental data were fitted with a linear function.It is clear that the values for all experiment points ofIC833/N818are larger than the fitted data as the molecular abundance ratio of CH4/N2is greater than 5.Additionally,the intensity ofIC833/N818decreases with the increase of the incident laser energy at the same molecular absorption ratio,which is consistent with the range of the molecular abundance ratio from 0% to 22.7%.Several factors combined lead the quantitative curve to deviate from linear function, such as the selfabsorption and collision avalanche of gas molecules.Even though the experiment points of the intensity ofIC833/N818deviate from a linear relation at a large CH4, the linear calibration analysis is relatively effective for the molecular abundance ratio of CH4/N2over wide range as the coefficient of the calibration curveR2of all fitted curves is larger than 0.99.Regardless, the proposed metal-assisted FO-LIBS is an efficient method for the measurement of gases’ molecular abundance ratios over a wide abundance ratio range.

Figure 7.Comparison of molecular abundance ratio measurement using aluminum and copper as metal substrates.
3.5.The influence of different metal material substrates
To further study the effects of different metal substrates on the analysis of the component abundance ratios in a mixing gas with metal-assisted FO-LIBS, quantitative analyses of molecular abundance ratio of CH4with different metal substrates were carried out.The quantitative curve is shown in figure 7.Obviously, when Al is used as the metal substrate, the quantitative curve between the spectral line intensity and molecular immunity ratio is more linear.Comparing the two cases where Cu and Al are used as metal substrates, the total pressure of mixing gas in the two cases is the same as that of the molecular abundance ratio,and the laser energy is enough to induce metal substrate ablation; thus, the primary reason that causes the difference between the quantitative curve in the two cases should be the difference in matrix elements of the substrate.The boiling points of Cu and Al are 3200 K and 2792 K.The ablation threshold of the target was positively correlated with the boiling point of the target and the ablation amount of the target was negatively correlated with the boiling point[34,40].As a result,the lower the boiling point of the metal substrate,the higher the temperature and electron concentration of the plasma produced by laser ablation under the same conditions,which may have caused the more-intense particle collisions in the plasma.Eventually, the low boiling point of metal subtracts results in a more linear quantitative curve of the quantitative analyses of the gases.It is favorable that the boiling point of the metal substrate is lower in metalassisted FO-LIBS for gas detection.
4.Conclusions
To perform gas detection with the FO-LIBS system, a metalassisted laser-induced breakdown spectroscopy method was proposed for the measurement of gases’molecular abundance ratios as the laser energy is delivered by optical fiber.The quantitative relationship between the spectral line intensity and gases’molecular abundance ratio was studied over a large range.The influences of ablation laser energy and the substrate material on the quantitative calibration were also analyzed, which demonstrate that producing more plasma particles at higher plasma temperatures and electron densities is beneficial for undertaking the linear quantitative calibration of gas measurement.The proposed metal-assisted method makes the measurement of gases’molecular abundance ratios possible with the FO-LIBS system, which is a practical strategy for gas detection when the environment is too hazardous or the access space is restricted.
Acknowledgments
This work was supported by the National Key R&D Program of China (No.2017YFC1200400), the Development Fund of Institute of Fluid Physics, China Academy of Engineering Physics (No.SFZ20150302).
杂志排行
Plasma Science and Technology的其它文章
- Numerical simulation of nanosecond laser ablation and plasma characteristics considering a real gas equation of state
- The propagation dynamics and stability of an intense laser beam in a radial power-law plasma channel
- Effect of edge magnetic island on carbon screening in the J-TEXT tokamak
- Relativistic effect on synergy of electron cyclotron and lower hybrid waves on EAST
- Influence of the X-point location on edge plasma transport in the J-TEXT tokamak with a high-field-side single-null divertor
- A simulation study of protons heated by left/right-handed Alfvén waves generated by electromagnetic proton–proton instability
