A highly stable terbium metal-organic framework for efficient detection of picric acid in water
2021-12-29ZiYingLiZhoQunYoRuiFengMingHuSunXioTinShnZiHoSuWeiLiXinHeBu
Zi-Ying Li,Zho-Qun Yo,Rui Feng,Ming-Hu Sun,Xio-Tin Shn,Zi-Ho Su,Wei Li,*,Xin-He Bu,,*
a TKL of Metal and Molecule Based Material Chemistry &School of Materials Science and Engineering,Tianjin 300350,China
b State Key Laboratory of Elemento-Organic Chemistry,College of Chemistry,Tianjin 300071,China
Keywords:Metal-organic framework Detection Nitro aromatics Picric acid Fluorescent
ABSTRACT A highly stable fluorescent terbium MOF (Tb4(paip)6•1.2H2 O,paip=5-(1H-pyrazole-4-yl)isophthalate)showing a sharp green emission (545 nm) and a quantum yield of 21.0% was successfully synthesized.This compound is shown to be a recyclable sensor for detecting picric acid in aqueous solution with both high sensitivity and selectivity,attributed to the electron transfer quenching mechanism.
As the most commonly used ingredients of explosives and intermediates of the industrial commodity,nitro aromatics have brought a multitude of security issues to our daily life[1,2].Among these nitro compounds,picric acid (PA) has received particular attention in national security,military fields and environmental pollution due to its high explosive power and toxicity [3-5].To date,the detection methods of picric acid include infrared and Raman spectroscopies [6],X-ray imaging techniques [7],fluorescence sensing [8-16],etc.Notably,fluorescence sensing,owing to its advantages of simplicity,high sensitivity,and rapid detection response,has been developed as a very promising technique.
To achieve efficient detection,luminescent probing materials with high sensitivity and selectivity to PA are highly thought after.Luminescent metal-organic frameworks (LMOFs) have great potential in sensing nitro aromatics due to their tuneable compositions,diverse structures,and high porosity [17-22].A number of MOFs have been applied to detect PA[23-26],however,most of them are unable to function in water.In addition,PA shows strong acidity and may cause severe structural collapse,which places higher requirements on the stability and detection capability of MOF probes.In this regard,developing PA detecting MOFs with high water and pH stability remains a challenge.Lanthanide MOFs possess high stability and environmental resistance by virtue of large coordination numbers and high metal valence states [27-29].Therefore,they have advantages as PA probes in aqueous solution.In addition,they have virtues of narrow emission bands,large storks shifts,and long lifetimes which are beneficial for luminescent probing[30-32].Based on the above consideration,we herein report a new terbium MOF Tb4(paip)6•2H2O (NKU-130),which can efficiently sense PA in water with high sensitivity,excellent selectivity as well as remarkable recyclability.More importantly,the efficient detection of PA can be realized in the presence of other nitro explosive interferences.
NKU-130was synthesized by reacting Tb(NO3)3•6H2O and electron rich π-conjugated H2L (5-(1H-pyrazole-4-yl)isophthalic acid) in the hydrothermal conditions.It displays a threedimensional network with one-dimensional triangular channels and its connectivity can be described as stp topology.It is worth noting thatNKU-130possesses high stabilities in boiling water for one week and even in acidic/alkaline solutions for over 300 days.Meanwhile,NKU-130emits characteristic narrow peaks at 489,545,585 and 624 nm under 365 nm excitation due to the antenna effect of the ligand.
Single crystal X-ray diffraction results revealNKU-130crystalizes in the P3c1 space group and powder X-ray diffraction(PXRD)of synthesized crystals affirmed the phase purity (Fig.S6 in Supporting information).The crystallographic data were summarized in Tables S1 and S2(Supporting information).The minimum asymmetric unit contains two thirds crystallographic independent Tb atoms and one ligand.Both Tb1 and Tb2 are 9-coordinated by O atoms,and the neighbouring Tb1 or Tb2 atoms are connected by three bridging μ2-O atoms from carboxylate groups to form rodlike[Tb(COO)3]SBUs(Fig.1a).The free program SHAPE 2.0 suggests that the proximate ideal geometry of the nine-coordinated Tb1 and Tb2 is spherical tricapped trigonal prism(STTP),and their τ values have been listed in Table S3 (Supporting information).The rod SBUs of Tb1 and Tb2 are alternatively arranged and these atoms exhibit different spiral directions.The connecting m-phthalate groups between rod SBUs exhibit a two-fold disorder respectively towards inner and outer orientations along the c-axis(Fig.1b).The pending electron-rich pyrazolyl groups of ligands are towards the inside of the aperture,which leads to a pore shape alteration from original hexagonal to triangular and an aperture reduction to 4.0 Å(Fig.1c).The space-filling model of the trigonal channel is shown in Fig.1d.From the topological point of view,when simplifying the ligand as a 4-connected node,the single 3D framework ofNKU-130can then be viewed as a 4,6-connected stp net with the point symbol of {4.62}3{46.63.86} (Fig.S7 in Supporting information).
Considering the requirements for thermal stability of MOFs in fluorescent detection,thermogravimetric analysis (TGA) was carried out.The bulk crystals ofNKU-130were soaked into DMF and EtOH separately for three days,dried in vacuum at 100°C and TGA was performed subsequently under Ar atmosphere.The TGA curve(Fig.S10 in Supporting information)indicates a slight weight loss under 150°C,which demonstrated the removal of guest solvents in minuscule channels.Meanwhile,the curve shows a platform before 540°C,manifesting a remarkably high thermal stability.Variable-temperature powder XRD measurements demonstrated that the framework ofNKU-130could remain stable up to 400°C (Fig.S11 in Supporting information).
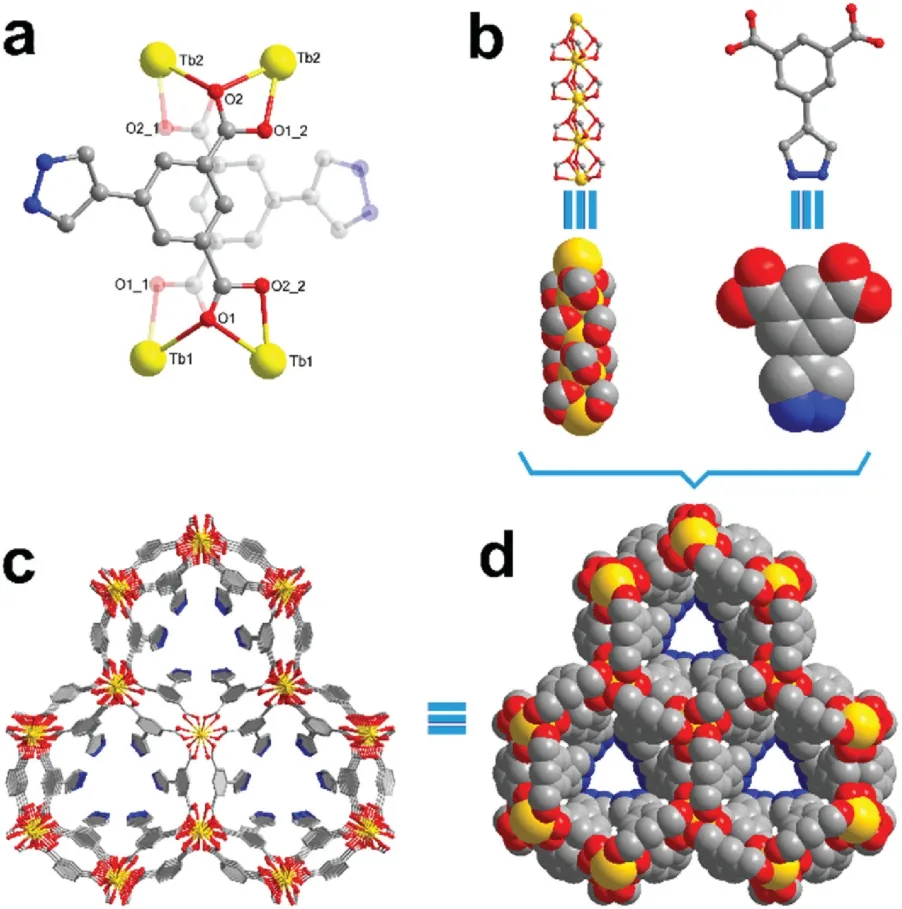
Fig.1.(a)The asymmetric unit of NKU-130,A=-x+y,y,1/2+z;B=1-x+y,y,-1/2+z.The numbers after underline in atom labels represent for different sets of disorder structures.Yellow:Tb,red:O,blue:N,grey:C.(b) The rod SBU of [Tb(COO)3]and ligand.(c) View of the 3D framework structure of NKU-130 along the c-axis.(d)Space-filling model of NKU-130.
The solvent and pH stability ofNKU-130was verified by PXRD measurements.As shown in Fig.S12(Supporting information),the as-synthesized crystals were immersed in several common solvents (including DMF,DMAc,ethyl acetate,acetone,dichloromethane,methanol,tetrahydrofuran and water) for 7 days.The PXRD patterns are consistent well with the simulated one,indicating excellent resistance ability to multiple solvents.The stabilities in aqueous solution with different pH values were also performed,andNKU-130was proved to maintain its crystallinity between pH 3-12 even after 300 days (Fig.2).Benefit from its thermal and pH stabilities,NKU-130can be a candidate material for fluorescent detection in the liquid phase under harsh conditions.
The photoluminescence(PL)spectra and fluorescence quantum yield ofNKU-130in the solid state were measured at room temperature(Fig.S13 in Supporting information).The sharp peaks in PL spectra observed at 489,545,585 and 624 nm can be assigned to the5D0→7F1,5D0→7F2,5D0→7F3and5D0→7F4transitions,respectively,which are attributed to the antenna effect and characteristic f-f emission bands [33-35].Fluorescence quantum yield ofNKU-130in the solid state was calculated to be 21.0%.In addition,the PL spectra ofNKU-130dispersed in H2O and DMF also possess strong emission at 545 nm when excited at 327 nm.
To characterize the trace-quantity sensing ability towards nitro explosives,fluorescence titrations were performed with the incremental addition of analytes toNKU-130dispersed in water(Fig.3).After incremental addition of PA solution(1×10-3mol/L),rapid and high fluorescence quenching was observed with the naked eyes.As shown in Fig.3a,the bright green emission ofNKU-130sharply weakens with gradually adding PA solution.For illustrating the selectivity of detecting PA,fluorescence quenching efficiencies were also performed with other nitro aromatics,such as 1,4-dinitrobenzene (1,4-DNB),2,6-dinitrotoluene (2,6-DNT),1,3-dinitrobenzene(1,3-DNB)and nitrobenzene(NB).As shown in Fig.S14 (Supporting information),with the dropping of diverse nitro explosives at the concentration of 2.5×10-3mol/L,the fluorescence ofNKU-130could still be observed.The remaining nitro explosives have almost no quenching effect,indicating thatNKU-130has a high PA selectivity (Fig.S15 in Supporting information).
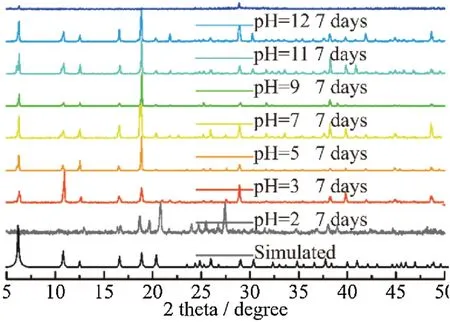
Fig.2.PXRD patterns for NKU-130 immersed in aqueous solutions(pH 2-13)and exposed in air.
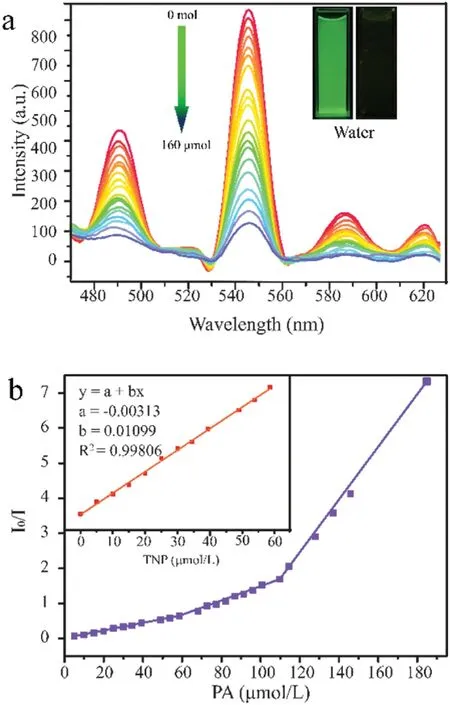
Fig.3.(a)Emission spectra of NKU-130 suspension in water treated with different concentrations of PA.(b) Stern-Volmer plot of I0/I vs.the concentration of PA in aqueous solution.
Affected by the selective quenching effect,we also performed luminescence quenching titration with low concentration PA,thus obtaining the detection limit of PA.The fluorescence quenching efficiency was analyzed by the Stern-Volmer (SV)equation,(I0/I)=Ksv[A]+1,where I0and I are the initial fluorescence intensity before and after the analyte is added,respectively.[A]is the molar concentration of the analyte,and Ksvis the quenching constant(L/mol)[36-38].At low concentrations,the SV plot of PA is almost linear.The quenching constant for PA was calculated to be 2.012×104L/mol from the direct fitting of the plot,which demonstrates the high quenching capability ofNKU-130toward PA.The calculated detection limit is 1.3×10-5mol/L(3 ppm).Besides,we also carried out the titration experiments to investigate the recyclability and immediate responsiveness ofNKU-130(Figs.S14 and S16 in Supporting information).Even after twenty cycles,it remains good quenching strength at a concentration of 10-3mol/L.The PXRD spectrum shows it maintains crystalline after the sensing experiments (Fig.S17 in Supporting information).Similar fluorescence experiments were also taken for the suspensions in DMF and the results show that the sensitivity and recyclability in DMF ofNKU-130are reminiscent of those in water(Fig.S18 in Supporting information).Furthermore,in order to verify the practical use ofNKU-130as a sensor for PA detection,the luminescent paper-based visual sensors were prepared.The filter papers were prepared by filtering the suspension ofNKU-130(10 mg) in water (10 mL) [39].It showed that the fluorescence intensity of the test paper changes significantly before and after the addition of PA,demonstrating it could be applied conveniently(Fig.S19 in Supporting information).
To fully understand the quenching behavior ofNKU-130in the presence of PA,the quenching mechanism needs to be well corroborated.Common fluorescence quenching mechanisms include structural collapse,energy resonance theory (RET) and photo-induced electron transfer (PET) [31,40,41].Firstly,PXRD pattern of the powder sample after recycling test showed that the framework structure was intact during the detection process,which excludes the decomposition induced fluorescence quenching mechanism[42].Secondly,the fluorescence resonance energy transferred from the fluorophore to the analyte might be one reason for the quenching process [43].However,the UV-vis adsorption of nitro explosives andNKU-130shows negligible overlap,which rules out this possibility (Fig.S20 in Supporting information).The observed overlap between the absorption band of the nitro explosives and the excitation band of the sensingMOF-130indicates there is a competitive adsorption mechanism(Fig.S21 in Supporting information).With an excitation wavelength of 327 nm,PA has a much stronger absorption over other explosives.This electronic characteristic of PA results in lower absorbance of the ligand and lower energy transfer from the ligand to Tb3+ion,hence ultimately leading to the fluorescence quenching.Thirdly,photo-induced electron transfer (PET) can also contribute to the fluorescence quenching process.The lower the lowest unoccupied orbit(LUMO),the more likely the process of photogenic electron transfer will occur.Since the LUMOs of PA are lower compared to those from other nitro compounds (PA<1,4-DNB<1,3-DNB<2,6-DNT In conclusion,we have successfully synthesized a highly thermal and water stable terbium MOF (NKU-130) which emits strong green light with a high quantum yield of 21.0%.Notably,NKU-130can be utilized for quick and sensitive PA detection through fluorescence quenching and the mechanism is attributed to the cooperative electron transfer and competitive adsorption.The high stability and recyclability ofNKU-130make it a potential material candidate for sensing PA in harsh conditions. Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Fig.4.(a) HOMO and LUMO energies for calculated nitro aromatics by using the B3LYP/6-31 G(d,p)level of theory.(b)Schematic representation of the PET process.(c) The quenching mechanism for detecting PA by NKU-130. Acknowledgments This work was supported by the National Natural Science Foundation of China (NSFC,Nos.22035003,91856124,22001132 and 19JCZDJC37200) and China Postdoctoral Science Foundation(No.2019M651011). Appendix A.Supplementary data Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2021.03.008.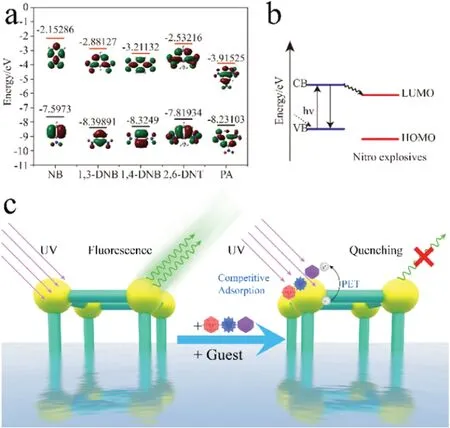
杂志排行
Chinese Chemical Letters的其它文章
- Colorimetric recognition of melamine in milk using novel pincer zinc complex stabilized gold nanoparticles
- Reviving chloroquine for anti-SARS-CoV-2 treatment with cucurbit[7]uril-based supramolecular formulation
- Mechanism and selectivity of nickel-catalyzed [3+2]cycloaddition of cyclopropenones and α,β-unsaturated ketones:A computational study
- Full synthesis and bioactivity evaluation of Tn-RC-529 derivative conjugates as self-adjuvanting cancer vaccines
- Deep insight into the charge transfer interactions in 1,2,4,5-tetracyanobenzene-phenazine cocrystal
- Recent progress in carbon-dots-based nanozymes for chemosensing and biomedical applications
