Robust Waterborne Polyurethane/Wool Keratin/Silk Sericin Freeze-Drying Composite Membrane for Heavy Metal Ions Adsorption
2021-12-21CHENXiZHANGMingyueZENGYiyangLIUHeLIUHongling
CHEN XiZHANG MingyueZENG YiyangLIU HeLIU Hongling
1 Key Laboratory of Textile Science and Technology, Ministry of Education, Donghua University, Shanghai 201620, China2 College of Textiles, Donghua University, Shanghai 201620, China
Abstract: Wool keratin(WK) and silk sericin(SS) have the ability to interact with metal ions. In order to take advantage of this potential, a novel environmentally friendly waterborne polyurethane (WPU)/WK/SS membrane named as WPU & WK & SS membrane with crosslinked structure was constructed by freeze-drying via self-assembly style. Surface morphology and chemical structure characterization were investigated by scanning electron microscopy(SEM) and Fourier transform infrared spectroscopy(FT-IR). In addition, the adsorption experiments of Cu2+ and Cr6+ were performed to evaluate the adsorption of WPU & WK & SS membrane, including adsorption kinetics, adsorption isotherm models and various factors affecting adsorption. Further investigation indicates that the maximum adsorption capacity of Cu2+ and Cr6+ can reach 54.21 mg·g-1and 85.21 mg·g-1, respectively, which are higher than most of the reported adsorbents. Through adsorption kinetics and thermodynamic analysis, it is find that the pseudo-second-order kinetic equation and the Langmuir adsorption isotherm model are more suitable for the static adsorption of Cu2+ and Cr6+ by WPU & WK & SS membrane.
Key words: adsorption; waterborne polyurethane(WPU); heavy metal ion; wool keratin(WK); silk sericin(SS)
Introduction
The pollution of heavy metal ions, such as Cu2+, Cr6+, and Pb2+, is a great threat to the environment, water ecosystem and human health[1-3]. Owing to the low concentrations of heavy metal ions in wastewater solution, they are very difficult to remove by the wastewater treatment system[4]. Moreover, these toxic heavy metal ions will eventually gather in organisms through the food chain[5], which has a harmful effect on human health. For example, excessive amounts of copper ions (Cu2+) can damage the nervous and digestive systems[6]. Chromium ions (Cr6+) are easily absorbed by human body, inducing autophagy of artificial blood stem cells and seriously harming the respiratory system[7]. Therefore, reducing the harm of heavy metal ions has become an urgent and challenging issue.
The heavy metal ions are generally removed by ion exchange[8], chemical precipitation[9], reverse osmosis[10]and solvent extraction. Although the treatment methods are proved to be efficient, but there are some disadvantages, such as secondary pollution and high cost. Recently the use of biosorbents to remove metal ions has been discussed widely. Compared with other methods, it has the advantages of low cost and environment friendliness. Since 1960, biosorption materials such as keratin[11-12]and chitosan[13]have been used to remove Cr6+, but they are still used as adsorbents in various forms.
The dissolved wool keratin[14](WK), is composed ofα-helix protein and various amino acids, especially containing many hydrophilic amino acids which have high affinity with ionic species such as metal ions[15]. Silk sericin[16](SS), contained with random coil,β-sheet and many hydrophilic amino acids such as carboxyl (—COOH) and amino (—NH2). Additionally, these functional groups can combine with the bonds between heavy metal ions, in which the heavy metal ions are removed by crosslinking. Nikiforovaetal.[15]studied the adsorption properties of WK for Cu2+, Cd2+, Ni2+and Zn2+, indicating that the chelating structure of metal complex was obtained at the acidic adsorption site. Jinetal.[4]studied the adsorption capacity of different proportions of WK/polyester (PET) blend membranes. The study on the adsorption of heavy metals by protein based polymer blends shows that WK and SS contain a large number of polar and ionizable groups in their side chains, which can combine with charged metal ions in water, so they can effectively adsorb heavy metal ions[17]. However, if pure WK and SS are made into membrane materials respectively, their common disadvantages are easy to crack, and cannot be put into actual production. Hence, it is necessary to modify them to prepare membrane materials with certain mechanical strength. However, there are few studies on the preparation and adsorption properties of protein blend membranes, mainly in the biomedical field[18-20]. In order to increase the stability of membrane materials[21], waterborne polyurethane (WPU) was introduced.
WPU is a flexible linear polymer composed of hard segment and soft segment[22]. This is mainly due to the strong polar group in the hard segment, which is easy to form hydrogen bonds, so that the intermolecular force is enhanced, and it is difficult to slip between the molecular chains. The results show that there are hydrogen bonds between carbamate group and hydroxyl group of WPU, which can reduce the interfacial tension between different components and make it more compatible[23]. In order to improve the brittleness of WPU & WK &SS membrane, a new type of high-performance metal ion adsorption membrane is developed by blending sericin, WK solution and WPU, forming hydrogen bonds or other force between the added WPU and protein polypeptide chain, and constructing macromolecular cross-linking network structure through the synergistic effect of microstructure. Recently, there are few studies on the combination of WPU and protein for adsorption. Manrique-jurezetal.[24]studied the adsorption properties of polyurethane and keratin membranes for Cr6+, indicating that the removal efficiency of Cr (VI) reached 58%. However, this method might cause secondary pollution in the operation process.
In this work, we choose WK and SS solutions extracted from wools and silk as suitable biosorbents to remove and Cu2+and Cr6+. Especially, owing to the synergistic effect of protein material and WPU, the properties of WPU & WK & SS membrane, constructed by freeze-drying via self-assembly style, are improved. At the same time, chemical structure and surface morphology of WPU & WK & SS membrane are investigated by Fourier transform infrared spectroscopy(FT-IR) and scanning electron microscopy(SEM) , respectively. The effects of pH value, adsorption time and initial concentration of metal ions on the adsorption properties of Cu2+and Cr6+are investigated in detail. Furthermore, the kinetic model, the adsorption isotherm model and the adsorption mechanism are studied.
1 Experiments
1.1 Materials
Wool and cocoons were supplied by Nanshan Group Co., Ltd., Yantai, China). All chemicals were analytically pure and without further purification. Acetone, ethanol, sodium sulfide, sodium dodecyl sulfonate, copper sulfate pentahydrate (CuSO4·5H2O), lead nitrate (Pb(NO3)2), dilute hydrochloric acid (HCl), disodium ethylenediamine tetraacetate (EDTA), and other drugs were provided by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Phenylcarbazide (chromogenic agent), phosphoric acid, sulfuric acid, and potassium dichromate were purchased from Titan chemical reagent company, Shanghai, China.
1.2 Extraction of WK and SS solutions
First, the prepared wool was sheared and dissolved in a mixed solution containing a certain amount of sodium dodecyl sulfate, urea, and sodium sulfide. At 60 ℃, the bath ratio was 1∶20. During the experiment, frequent stirring is required to make the wool fully dissolved. Next, the WK mixed solution was put into a centrifuge tube and centrifuged for 20 min, and the upper liquid was taken to remove the undissolved wool fiber to obtain a pale-yellow WK solution. After filtering and dialysis for 72 h, the WK solution with a mass fraction of 2% was obtained.
The cocoons were washed with deionized water, dried at 65 ℃ in the oven, and then cut into pieces of 1 cm × 1 cm in size. After boiling for about 3 h in a high-temperature sterilization pot, the solution was filtered and evaporated to obtain a sericin solution with mass fraction of 2%, and stored at -4 ℃ for refrigeration.
1.3 Preparation of composite membrane
First, WPU was mixed with WK solution (mass fraction of 2%) and SS solution (mass fraction of 2%) according to the certain proportion, and then a certain amount of glycerol was added to plasticize. Next, the glass rod was used to stir them evenly, and poured the mixed solution into a 4 cm × 4 cm demoulding box. Finally, after 36 h of freeze-drying, the membrane was taken out.
1.4 Characterizations
The morphology and the pore size distribution of the samples were determined with SEM (DXS-10ACKT, USA), operating at 25 kV. The functional chemical groups of different adsorbent membranes were detected by an FT-IR spectrum analyzer (Nicolet-TM 5700, USA). For each measurement, all analyses were performed with the range of 4 000-400 cm-1.
1.5 Adsorption methods
Batch experiments were carried out by mixing blend membrane with 100 mg·L-1copper ion solution and chromium ion solution, respectively. In the pH studies, the initial pH was adjusted with 0.1 mol·L-1buffer solution in the range from 2.0 to 7.0. In the equilibrium time measurement experiment, the ion concentration in the solution after adsorption was measured every 1 h. In the kinetic experiment, WPU & WK & SS membrane was added into the heavy metal ion solution, and the water bath was oscillated at a constant temperature for 4 h, so that the adsorption reached equilibrium. Meanwhile, the change of adsorption rate with time were measured.
Using a UV-VIS-NIR spectrophotometer (U-4100, Japan) to determine the relative metal ions concentration. The adsorption capacityQof Cu2+and Cr6+is calculated as
(1)
whereC0(mg·L-1) andCt(mg·L-1) are the initial andt-moment concentration of Cu2+with Cr6+, respectively,V(L) is the volume of the solution , andm(g) is the mass of WPU & WK &SS membrane.
2 Results and Discussion
2.1 Surface morphology of WPU & WK & SS membrane
Figure 1 shows the surface images of freeze-dried samples with porous structure distribution, which is conducive to the adsorption of heavy metal ions[25]. Compared with the pure SS freeze-dried membrane [shown in Fig. 1(a)], the uniform and clear distribution and loose structure of the membrane decreased and the structure became loose after adding WK and WPU [Fig. 1(b)]. Additionally, the color of WPU & WK & SS membrane became lighter gradually, from yellow to white. The addition of WK and WPU can form network structure with sericin. Owing to the open porous arrangements, the metal ions easily interacted with immobilized active sites of WPU & WK &SS membrane[24]. In addition, the pore size of WPU & WK &SS membrane is determined by the size of ice crystals formed during freezing, and the smaller pore size of SS membrane can be attributed to the smaller ice crystal structure. In other words, the higher the content of WK is, the easier the tangle among WK, SS and hard segment chain of WPU is, resulting in the folding of protein peptide chain. The appearance of smaller and irregular pores can be attributed to the decrease of ice crystal space.
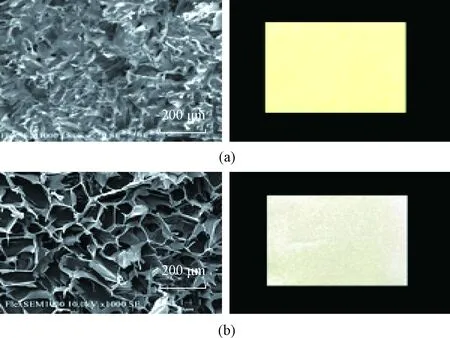
Fig. 1 SEM images: (a) pure SS; (b)WPU & WK &SS (1∶2∶2) membrane
2.2 Surface chemical properties of WPU & WK & SS membrane
FT-IR was used to determine the chemical structure changes of WPU & WK &SS membrane. As shown in Fig. 2, at 3 300-3 600 cm-1, there is an N—H bond stretching band absorption peak[26]. The absorption peak of pure WPU membrane is located at 3 366 cm-1, where the absorption intensity is the highest. And the absorption peak of pure WK membrane and SS membrane are located at 3 313 cm-1and 3 301 cm-1, respectively. After introducing WK and SS, the absorption peak at 3 366 cm-1shifted to 3 320 cm-1, and the absorption intensity decreased, which might be due to the chemical reaction among them. According to Refs.[27-28], the spectra band range of 1 665-1 650 cm-1showsα-helix structure in WK, and SS changes from random curling toβ-sheet structure under the action of glycerol, with the corresponding absorption peaks of 1 635-1 616 cm-1. Compared with Fig. 2(a), Fig. 2(b) shows the characteristic absorption peak in amide Ⅰ band changed from 1 653 cm-1to 1 619 cm-1, demonstrating the decrease of the content ofβ-sheet and the increase of the content ofα-helix. The absorption peak of carbonyl group in WPU appears at 1 738 cm-1. According to Coleman's research[22], the maximum absorption peak of crystalline carbonyl group is at 1 724 cm-1, and that of amorphous carbonyl group is at 1 737 cm-1. In addition, in the pure WPU membrane, the absorption peak of carbonyl group is between 1 724 cm-1and 1 737 cm-1, indicating that there are both crystalline and amorphous carbonyl groups. Interestingly, some characteristic bands associated with H-bonded urethane carbonyl group stretching and C—N stretching/N—H bending of WPU & WK &SS membrane are found to shift to lower wavenumbers by the introduction of WK and SS in the WPU membrane. The changes of positions and intensities of those characteristic bands of WPU & WK &SS membranes as a function of the protein content, demonstrating the presence of specific interactions between the hydroxyl (—OH) groups of amino acid macromolecule and the urethane/urea functional groups in the hard segments of WPU via multiple hydrogen bonds at the molecular level[26]. In other words, owing to the addition of protein, intermolecular hydrogen bonding force is formed between the side chain of amino acid macromolecule with strong polarity and WPU, which induces the hard segments to be arranged in order, thus further promoting the microphase separation of WPU.

Fig. 2 FT-IR spectrogram: (a) WPU; (b) WPU & WK &SS membrane; (c) WK; (d) SS
2.3 Effects of pH values on ion adsorption
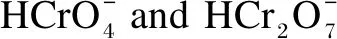
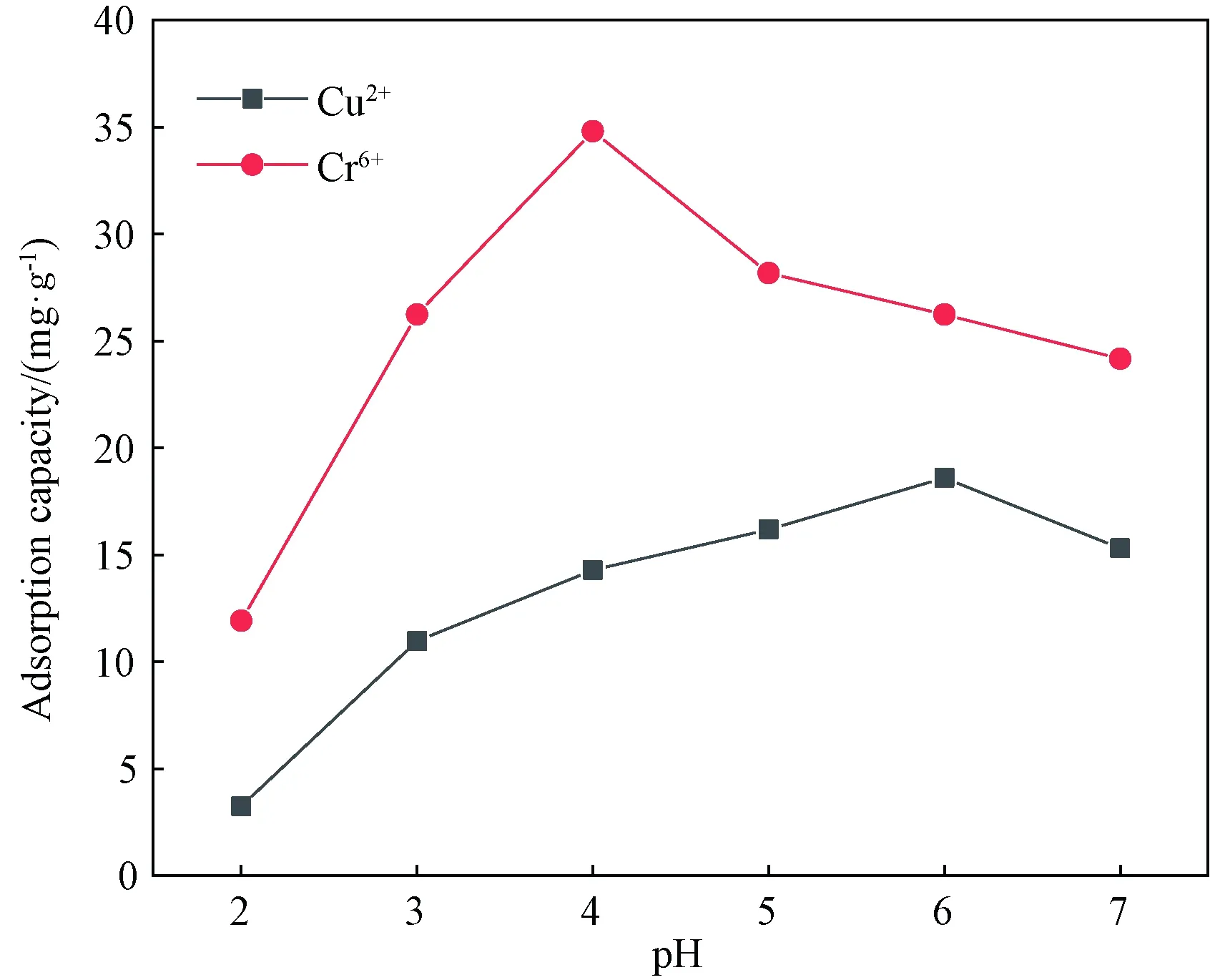
Fig. 3 Effect of pH values on Cu2+ and Cr6+ adsorption capacity of WPU & WK &SS membrane
2.4 Effect of concentration on ion adsorption
The effect of concentration of Cu2+and Cr6+on the adsorption capacity of WPU & WK & SS membrane is shown in Fig. 4. The higher absorptive capacity was achieved at higher concentration and then tended to be stable with the increase of the concentration of the metal ions. This might be due to the phenomenon that more metal ions entered the inner space of WPU & WK & SS membrane and crosslinked with more polar groups such as amino group and carboxyl group, which made the adsorption capacity increase. At the same time, with the increase of metal ions concentration, these metal ions will produce "molecular pressure" in the solution, which will also increase the adsorption capacity[13]. However, when the initial concentration exceeds a certain amount, the adsorption capacity tends to be stable and will not increase any more. The result shows that the active sites of adsorbed metal ions have been occupied, and the voids and polar groups of WPU & WK &SS membrane are fully combined with Cu2 +and Cr6+.
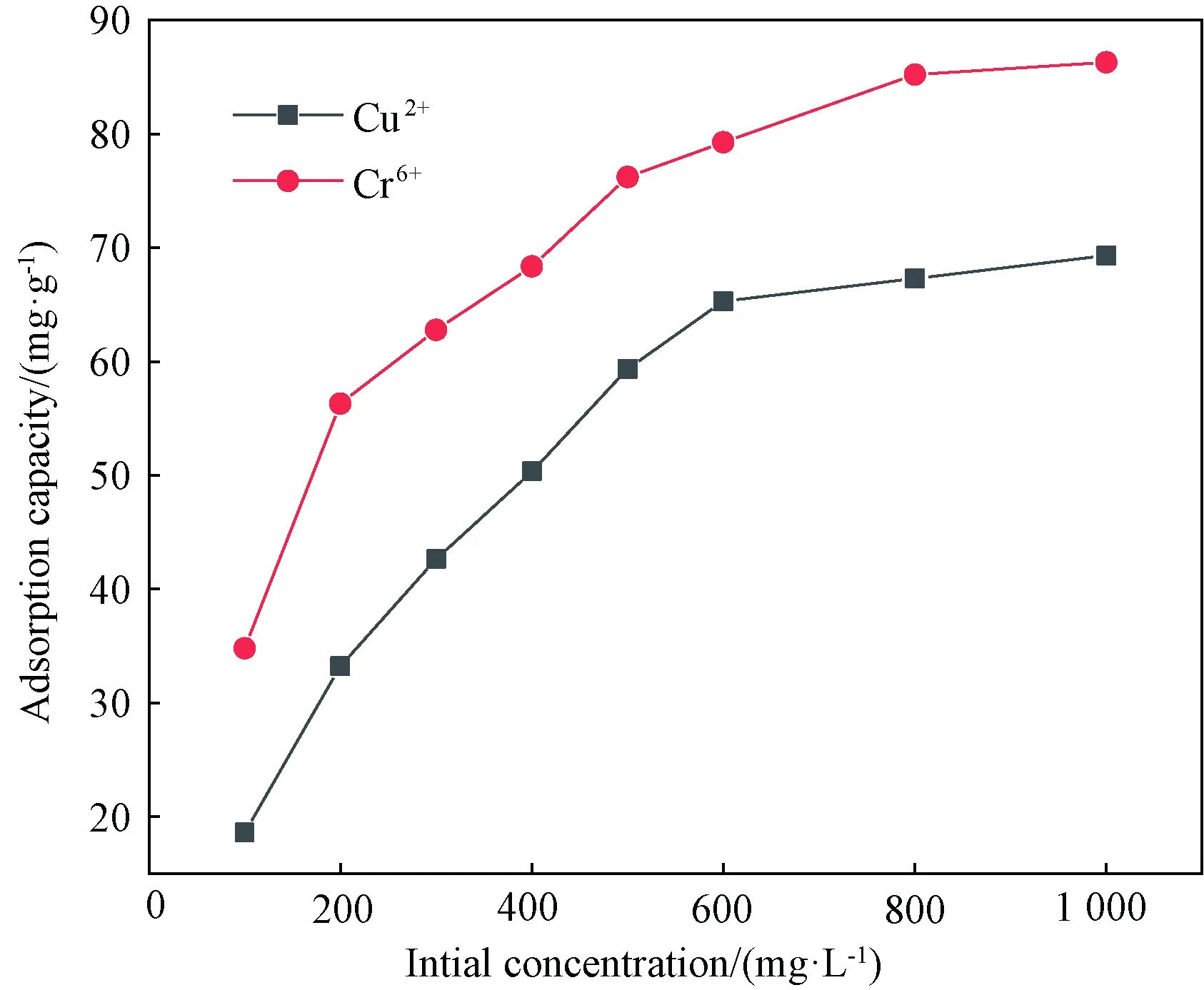
Fig. 4 Effect of initial Cu2+and Cr6+ concentration on adsorption capacity of WPU & WK &SS membrane
2.5 Effect of time on ion adsorption
Figure 5 shows the effect of adsorption time on Cu2+and Cr6+adsorption on WPU & WK &SS membrane. For Cu2+ions, the adsorption capacity of WPU & WK &SS membrane increased rapidly within 30 min, whereas a slight increase from 15.60 mg·g-1to 19.28 mg·g-1was noticed from 60 min to 120 min, meaning that the adsorption reached nearly equilibrium. For Cr6+, the adsorption capacity of WPU & WK & SS membrane increased rapidly in the first 60 min, whereas the adsorption of Cu2+decreased from 60 min to 90 min, which was slow adsorption. When the adsorption time was 90 min, the adsorption of Cu2+reached saturation of 34.60 mg·g-1. The reasons may be the existence of functional groups such as amide bond, amino group and carboxyl group on the surface of WPU & WK & SS membrane, which can coordinate with metal ions. In the slow adsorption stage, a large number of metal ions have been adsorbed on the surface of WPU & WK & SS membrane, the polar groups on the surface are occupied, so the adsorption rate becomes slow until the adsorption equilibrium is reached.
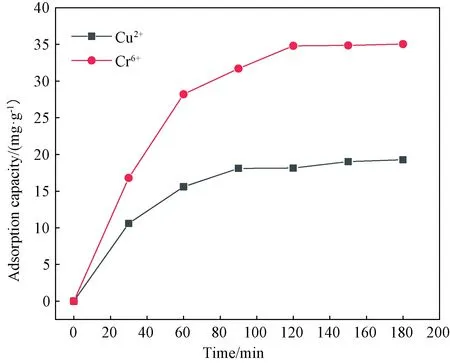
Fig. 5 Effect of adsorption time on Cu2+ and Cr6+ adsorption capacity of WPU & WK &SS membrane
2.6 Adsorption kinetics of copper and chromium ions
The kinetic models were used to describe the adsorption behavior of heavy metals including pseudo-first-order kinetic model[29]and pseudo-second-order kinetic model[30], which are used to evaluate the adsorption process given by
ln(qe-qt)=lnqe-k1t,
(2)
(3)
wherek1(min-1) is the pseudo-first-order adsorption rate constant,k2(g·mg-1·min-1) is the pseudo-second-order adsorption rate constant,qe(mg·g-1) is the adsorption amount of metal ions at adsorption equilibrium, andqt(mg·g-1) is the adsorption amount of metal ions on WPU & WK & SS membrane att-moment.
Based on the above two models, the data obtained from Cu2+and Cr6+adsorption experiments were fitted by the pseudo-first-order and the pseudo-second-order kinetic models as shown in Fig. 6. Compared with the pseudo-first-order kinetic model, the pseudo-second-order kinetic model has higher correlationR2(shown in Table 1) for the adsorption of Cu2+and Cr6+with WPU & WK & SS membrane. Additionally, theqevalues of Cu2+and Cr6+are 19.56 mg·g-1and 35.89 mg·g-1, respectively, which are closer to the experimental date. Therefore, it can be assumed that the adsorption of heavy metal ions on WPU & WK & SS membrane is mainly chemical adsorption[31], based on the exchange between WPU & WK & SS membrane and metal ions. In addition, the more active sites in WPU & WK & SS membrane, the stronger the adsorption capacity is[6].
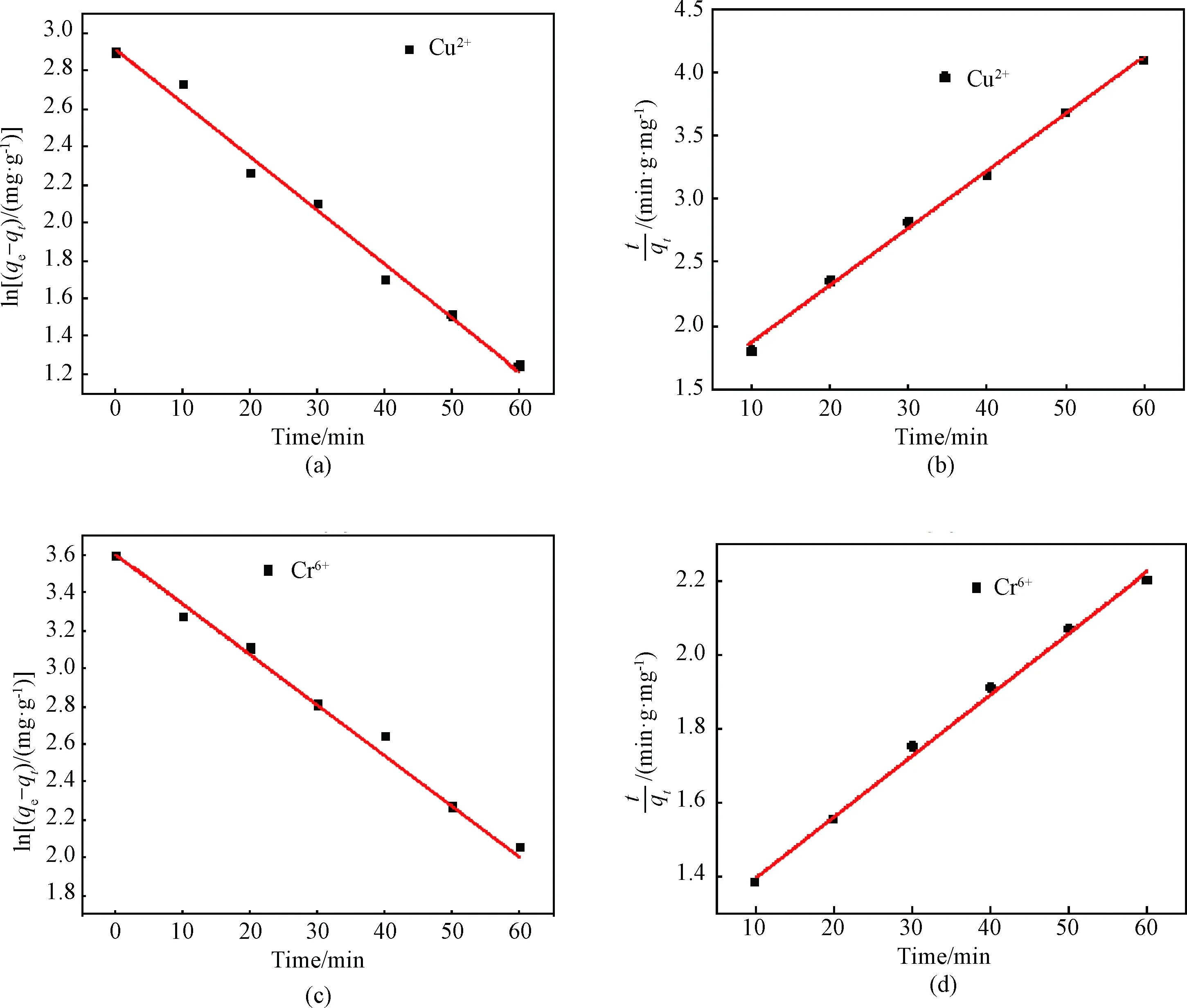
Fig. 6 Kinetics curves: (a) pseudo-first-order kinetics curve of Cu2+; (b) pseudo-second-order kinetics curve of Cu2+; (c) pseudo-first-order kinetics curve of Cr6+; (d) pseudo-second-order kinetics curve of Cr6+

Table 1 Kinetic parameters for adsorption of metal ions by WPU & WK & SS membrane
2.7 Adsorption isotherms of copper and chromium ions
In order to further investigate the adsorption characteristics of the metal ions, two isotherm models have been used including Langmuir and Freundlich models. Figure 7 shows the adsorption equilibrium isotherms for Cu2+and Cr6+by WPU & WK & SS membrane. The linear equation of the Langmuir adsorption isotherm model[32]as
(4)
whereCe(mg·L-1) is the concentration of metal ions in equilibrium solution,qmax(mg·g-1) is the maximum adsorption capacity, andkL(L·mg-1) is the Langmuir adsorption constant .
The Freundlich adsorption isothermal model describes the adsorption process on heterogeneous surfaces with a linear expression of
(5)
wherekF(mg·g-1) andnare the Freundlich adsorption constants which are related to adsorption capacity and intensity.

Fig. 7 Adsorption isotherms: (a) Langmuir for Cu2+; (b) Freundlich for Cu2+; (c) Langmuir for Cr6+; (d) Freundlich for Cr6+
The experimental data after Cu2+and Cr6+adsorption equilibrium was treated by the Langmuir and the Freundlich equations as shown in Table 2. It shows that the experimental data was consistent with both isotherm models. In addition, the Langmuir model showed more important coefficients (R2>0.998) than in case of the Freundlich adsorption isotherm model. This indicates that the adsorption capacity of each part of the surface of WPU & WK & SS membrane is the same, and one active point can only adsorb one metal ion. When the active center is occupied, the adsorption will reach saturation. The maximum adsorption values were 71.47 mg·g-1and 83.33 mg·g-1for Cu2+and Cr6+with WPU & WK & SS membrane.

Table 2 Fitting parameters of adsorption isotherms for WPU & WK & SS membrane
2.8 Adsorption mechanism
According to the above research, the special crosslinked network of WPU & WK & SS membrane is formed by intermolecular hydrogen bonding between the side chain of amino acid macromolecule with strong polarity and WPU, which can be attributed to the synergy betweenα-helix,β-sheet structure and hard-soft chain segments. Therefore, the possible adsorption mechanism of WPU & WK & SS membrane for Cu2+and Cr6+came from two parts (shown in Fig. 8): electrostatic attraction, complexation and ion exchange. Cu2+adsorption is mainly ascribed to the chelation of Cu2+with the carboxyl group (—COOH) on WPU & WK & SS membrane. The adsorption of Cr6+is mainly due to the protonation of amino group to NH3+under acidic conditions, which produces electrostatic adsorption with hexavalent chromium. At the same time, a part of Cr6+can be reduced to Cr3+, and the reduced Cr3+can coordinate with the amino group.
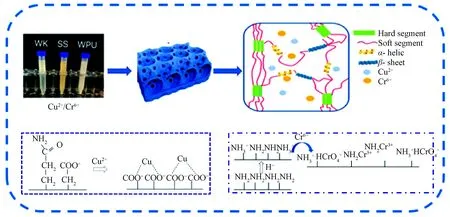
Fig. 8 Schematic diagram of adsorption mechanism of WPU & WK & SS membrane
3 Conclusions
In summary, the adsorption properties of WPU & WK & SS membrane for heavy metal ions were studied by using Cu2+and Cr6+as adsorption models. SEM showed that there was a pore structure in WPU & WK & SS membrane, which was conducive to the adsorption of heavy metal ions. And FT-IR indicated that the crosslinked network structure of WPU & WK & SS membrane was constructed by multiple hydrogen bonds produced by the synergistic action between the WPU and the side chain of amino acid macromolecule with strong polarity. Additionally, the effects of pH values, initial concentration of heavy metals and adsorption time on the adsorption were studied, and the adsorption model was preliminarily discussed. After processing and fitting the experimental data, the results demonstrated that the adsorption processes of Cu2+and Cr6+were more accurate to the pseudo-second-order kinetic model and the Langmuir adsorption isotherm mode, the theoretical maximum adsorption capacities of Cu2+and Cr6+could reach 71.47 mg·g-1and 83.33 mg·g-1, respectively.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Effect of Surface Energy of Electrospun Fibrous Mat on Dynamic Filtration Performance for Oil Particles
- Seam Damage Control and Image Analysis for Cuprammonium Fabrics
- Textile-Based Capacitive Pressure Distribution Measurement System for Human Sitting Posture Monitoring
- Enhancing Accuracy of Flexible Piezoresistive Pressure Sensors by Suppressing Seebeck Effect
- Reduced Switching-Frequency State of Charge Balancing Strategy for Battery Integrated Modular Multilevel Converter
- Data Augmentation Based Event Detection
