Regulatory role of long non coding RNAs (lncRNAs) in neurological disorders:From novel biomarkers to promising therapeutic strategies
2021-12-17
Department of Life Sciences, Presidency University, Kolkata, India
Keywords: Non coding rnas Alzheimer’s disease Huntington’s disease Parkinson’s disease Schizophrenia Regulatory
ABSTRACT Long non coding RNAs (lncRNAs) are non-protein or low-protein coding transcripts that contain more than 200 nucleotides.They representing a large share of the cell’s transcriptional output,demonstrate functional attributes viz. tissue-specific expression,determination of cell fate,controlled expression,RNA processing and editing,dosage compensation,genomic imprinting,conserved evolutionary traits etc.These long non coding variants are well associated with pathogenicity of various diseases including the neurological disorders like Alzheimer’s disease,schizophrenia,Huntington’s disease,Parkinson’s disease etc.Neurological disorders are widespread and there knowing the underlying mechanisms become crucial.The lncRNAs take part in the pathogenesis by a plethora of mechanisms like decoy,scaffold,mi-RNA sequestrator,histone modifiers and in transcriptional interference.Detailed knowledge of the role of lncRNAs can help to use them further as novel biomarkers for therapeutic aspects.Here,in this review we discuss regulation and functional roles of lncRNAs in eight neurological diseases and psychiatric disorders,and the mechanisms by which they act.With these,we try to establish their roles as potential markers and viable diagnostic tools in these disorders.
1.Introduction
It has now been shown that almost 90% of human genome is transcribed into RNA molecules [1],and only 1.2% of these transcripts are translated into protein molecules [2].Earlier,these non-coding transcripts were thought to be the degraded products of RNA processing machinery [3].However,the ENCODE,consortia have re-established that (mostly non-coding) transcripts cover 62%–75% of human genome [4,5].Once the human genome project was completed,exploration of the biology of these huge amounts of non-coding RNAs (ncRNAs) began and it led to the fact that they serve as important regulators of multiple physiological and cellular functions.Based on their length,ncRNAs are classified into small non coding transcripts like miRNA,snRNA,piwi RNA and long non-coding RNA (lncRNAs) (transcripts longer than 200 nucleotides) [6]. Numerous studies have demonstrated the involvement of small ncRNAs like microRNAs (miRNAs) in various complex diseases [1]. Simultaneously,the importance of lncRNAs as crucial regulators in development,progression and manifestation of metabolic diseases has started to unravel.LncRNAs are classified into different categories based on transcript length,association with annotated protein-coding genes,association with other DNA elements of known function,protein-coding RNA resemblance,association with repeats,association with a biochemical pathway or stability,sequence and structure conservation,expression in different biological states,association with subcellular structures,genome location and context,functioning and targeting mechanism [7,8]. Some of the salient features of lncRNAs include poor sequence conservation across hierarchy and sequences with fewer exons.LncRNAs may or may not be poly-adenylated and these molecules are mostly dependent on their secondary structure for their function and the expression patterns of lncRNAs are tissue specific [9]. Similar to mRNAs,lncRNAs are transcribed by RNA polymerase II,capped at 5end,spliced and have promoter regions.Most of them are also polyadenylated at the 3end [10]. The functional roles of these lncRNAs can be broadly categorized as-decoy,scaffold,mi-RNA sequestrator,histone modifiers and in transcriptional interference [11,12]. They can be eithercis-
ortrans-
a cting based on their silencing or gene expression activation on same or different chromosome [9]. LncRNAs are very heterogeneous and exhibit multifaceted biological functions and interact with a variety of other proteins [11].Depending on their subcellular localization in the nucleus or cytoplasm,lncRNAs can interfere with many transcriptional and post-transcriptional gene regulations by recruiting or inhibiting transcription factors [13,14],alternative splicing 15],as well as mRNA translation [5,11,16]. Nuclear transcripts,for example,can mediate epigenetic gene modifications [17,18]or transcriptional activation and silencing,whereas cytoplasmic lncRNAs often interact with miRNAs to post-transcriptionally regulate gene expression or act as molecular scaffolds for RNA-protein complexes [15,19,20]. Various ways of functioning of lncRNAs are shown in Fig.1. In past decade,a large number of functional studies have been established and now these transcripts are shown to have regulatory role in fine-tuning of various biological processes.The worldwide prevalence of neurodegenerative disorders makes to of utmost importance.Alzheimer’s disease (AD) contribute to over 60% of total 50 million dementia patients worldwide [21],while over ten million people are living with Parkinson’s disease (PD) [22]. The worldwide occurrence of Huntington’s disease (HD) was estimated to be 2.71 per 100 000 (95% CI:1.55–4.72) based on a meta-analysis of 13 studies [23]. Motor neuronal disease like amyotrophic lateral sclerosis (ALS) has an incidence rate of 2.2 per 100 000 person-years (py) in European population as estimated by European registry consortium named EURALS,0.89 per 100 000 py in East Asia and 0.79 per 100 000 py in South Asia [24].According to WHO,worldwide one in 160 children suffers autism spectrum disorder (ASD) [25]while over 264 million people across all ages suffer from depression globally [26]. LncRNAs are involved in neurological disorders as well.Here we summarize involvement of lncRNAs in eight neurological disorders and psychiatric disorders,namely AD,schizophrenia,HD,PD,ASD,ALS,major depressive disorder,cerebral injury and neuroimmunological disorder.
2.Role of lncRNAs in neurological disorders
2.1.Role of lncRNAs in AD
AD is primarily characterized by accumulation of amyloid beta (Aβ
) plaques in the brain tissue and confers subtle contribution to the pathogenesis of the diseases that results in dementia [27]. A membrane bound aspartic protease b-site APP cleaving enzyme 1 (BACE1) is responsible for catalysing the cleavage of amyloid precursor protein (APP) and production of Aβ
plaques.A conserved antisense transcript of BACE1,b-site APP cleaving enzyme 1 antisense strand (BACE1-AS) has been found to be up-regulated in brains of Alzheimer’s patients [28,29]. BACE1-AS binds with the BACE1 transcripts and stabilizes it,thereby increasing the synthesis of BACE1 enzyme,and consecutively Aβ
plaques [28].A microRNA miR-485–5p has been reported to inhibit BACE1 expression by competitively binding withBACE1-AS
[30].The lncRNA antisense to brain-derived neurotrophic factor (BDNF-AS) is antisense transcript to BDNF,and negatively regulates BDNF level bothin
vivo
andin
vitro
[31],that further down regulates an immediate-early gene,involved in synaptogenesis and synaptic plasticity called activity-regulated cytoskeleton-associated protein (ARC) [32]. Treatment with Aβ
in PC12 cells decreases BDNF concentration but increases BDNF-AS level.Repressing BDNF-AS increases BDNF levels,that promotes cell viability [33]. Early B cell factor 3 (EBF3) (also known as olf),a DNA binding transcription factor,is expressed in the olfactory receptor neurons and their precursors [34]and is involved in neurogenesis,cell cycle arrest and apoptosis [35,36]. The level of EBF3 is found to be elevated in the hippocampus of AD mice.The lncRNA EBF3-AS is transcribed from the opposite strand of EBF3 and is up-regulated in the hippocampus of APP/PS1 mice.In human SH-SY5Y cell,EBF3-AS deficiency decreases EBF3 levels inhibits okadaic acid (OA) or Aβ
induced apoptosis,showing its relevance as a biomarker and therapeutic targets of AD [37].The lncRNA-long nucleolar non-coding RNA (LoNA) binds to nucleolin and decreases its activity,thereby regulates rRNA transcription.It also interacts with fibrillarin and regulates rRNA methylation.Protein translation taking place at neuronal soma has crucial role in synaptic development and plasticity.At the level of translation,LoNA has regulatory activity by modulation of ribosomal components and its assembly [38–40]. LoNA concentration is significantly up-regulated in the hippocampus of AD mice along with reduced rDNA levels.rDNA silencing has shown to be responsible for AD-related ribosomal deficiency and suppressed rRNA 28 S/18 S ratio [41]. Knocking down LoNA has shown restoration of rRNA levels and improvement of cognitive deficits in AD mice [42]. The mouse lncRNA lincRNA-Cox2 has diverse function of both inducing and repressing immune genes,as it interacts with heterogeneous nuclear ribonucleoprotein A/B and A2/B1 which is required for target gene inhibition [43].The other mouse lncRNA antisense UchL1 remains partially overlapped with UchL1 mRNA and activates polysomes for its translation [44].Two other mouse lncRNA MIAT and Pnky are involved in neurogenic commitment and neurogenesis regulation of embryonic and postnatal neural stem cell populations.Dysregulated MIAT causes defective splicing of Wnt7b and has pleiotropic effects on brain development [45],while Pnky mediated neurogenesis regulation of embryonic and postnatal neural stem cell populations takes place by its interaction with splicing factor PTBP1 [46].The lncRNA PVT1 mediates autophagy protects hippocampal neurons from impaired synaptic plasticity [47],while the lncRNA Evf2 controls expression of Dlx5,Dlx6 and Gad1 by recruiting transcription factors DLX and MECP2 in Dlx5/6 intergenic region [48].
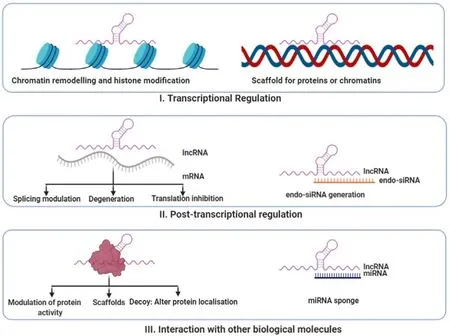
Fig.1–Various ways of functioning of lncRNAs.I.LncRNAs can regulate transcriptional processes by either acting as chromatin remodeler or by modifying histone proteins.It can also act as scaffold for proteins or chromatins.II.LncRNAs can also have post transcriptional regulatory functions.It can module splicing,help in degeneration of mRNA or can inhibit translation.Some lncRNAs can also generate endo siRNA.III.At the level of translation,it can act as modulator of protein activity,scaffold,decoy of as a miRNA sponge.
Another lncRNA brain cytoplasmic (BC) −200 RNA (BCYRN1
) has been found to be involved in pathogenesis of AD by binding with poly(A)-binding protein 1 (PABP1),a regulator of translation initiation,after being transported as ribonucleoprotein particles to the dendritic processes.Thus,by regulation of the translation process,it modulates the gene expression [49].It has also been found to be associated with abnormal protein localization by interacting with RNA-binding proteins [50].Synaptic or dendritic degeneration can take place by over expression of BC-200,as it assumes clustered perikaryal localization in a stress compensation mechanism mediated by dendritic sprouting and remodeling [50].BC-200 level has also been found higher in AD-affected brain region Brodmann area 9 in Alzheimer’s patients compared to the healthy individuals [50].Further detailed study may provide insights into the role of BC-200 in pathogenesis of AD [51].The homolog of BC-200 in mouse,called BC1 binds with fragile X syndrome protein (FMRP) and induces translation of APP [52].Aggregation of Aβ
plaque is inhibited in Alzheimer’s mice on depleting BC1 or BC1-FMRP complex.It also improves learning and memory in mice [52].The lncRNA-17A causes excess of Aβ
production when overexpressed.It also alternatively splices GABA receptor B (GABAB) and produces an isoform variant of it by directing G-protein coupled receptor 51 (GPR51).The GABA receptor isoform A cannot bind with this isoform variant and cannot produce functional heterodimeric receptor [53].Another lncRNA,SNHG1 (small nucleolar RNA host gene 1) mediates miR-137 sponge that causes attenuation of the Aβ
mediated effect by selectively targeting the untranslated region of an intrinsic pro-apoptotic transmembrane receptor kringle containing transmembrane protein 1 (KREMEN1).Aβ
treatment induces expression of SNHG1 while repression of it in Aβ
treated cells reduces effectst of Aβ
on mitochondrial membrane potential and cell viability [54–56].In SH-SY5Y and human primary neuron cells,this takes place by SNHG1 mediated miR-137 sponge that selectively targets the untranslated region of a transmembrane receptor with intrinsic pro-apoptotic activity,called targeting KREMEN1 [56].SNHG1 also interacts with its protein partners MATR3,Ezh2 [56].The lncRNA NAT-Rad18 is up-regulated in Alzheimer’s and post transcriptionally regulates Rad-18 protein,involved in proliferating cell nuclear antigen (PCNA) ubiquitination,DNA repair and nerve injury and increases the susceptibility to neuronal apoptosis and cell death [57].Similarly,lncRNA 51A,produced from intron 1 of sorting protein-related receptor 1 (SORL1) gene,helps in accumulation of Aβ
42 by altering the spliced form of SORL1 mRNA [58].An lncRNA–GDNFOS (antisense of glial-cell-line-derived neurotrophic factor) overlaps with 5–UTR of GDNF (glial-cell-line-derived neurotrophic factor) and negatively regulates the expression of GDNF and promote pathogenesis of AD.In mature temporal gyrus of AD patients,the GDNF peptide is down-regulated showing a halting of GDNF mediated neuroprotective effect [59,60].The lncRNA LRP1-AS decreases LRP1 expression at both protein and RNA levels;LRP1-AS decreases transcription of LRP1 transcription by reducing LRP1 promoter activity induced by a transcriptional complex consisting of transcription factor Srebp1,which regulates LRP1 transcription and its interacting partner Hmgb2 [61].In the cerebral cortex of the developing mouse brain,Sox2OT is binds with proteins FUS and YY1 and promotes neurogenesis and neuronal differentiation by repressing Sox2 [62].Sox2OT is also differentially expressed in early and late disease stages the AD model mouse that suggests its potential role as a biomarker in AD [63].
Neuroblastoma differentiation marker 29 (NDM29),transcribed by RNA polymerase III,leads to inducing Ab secretion,APP synthesis in AD [64].The lncRNA H19 promotes HDAC1-dependent M1 microglial polarization and causes neuroinflammation [65].Lethe,a lncRNA in mouse has shown to regulate inflammatory signaling.Lethe-RelA (NF-B subunit RelA) interaction inhibits binding of RelA with DNA and consequently hinders target gene expressions [66].
The lncRNA Dali is involved in neural differentiation regulation by regulation DNA methylation of CpG island associated promoters by the interaction of DNMT1 DNA methyltransferase in trans [67].Another lncRNA RMST is needed for the binding of promoter regions of neurogenic transcription factors to Sox2 and is involved in neural stem cell fate regulation [68].The lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1),binds with NONO,SFPQ,PSF,Ezh2 and relocates SFPQ from IL8 promoter to the paraspeckles,that results in transcriptional activation of antiviral cytokines like IL8 [69–73].The lncRNA MALAT1 is involved both in Immune response and synaptic density regulation.It promotes Regulation of glucose mediated up-regulation of inflammatory cytokines IL-6 and TNF-alpha by activation of SAA3 expression [74]and regulates synaptic density by modulating the recruitment of serine/arginine-rich (SR) family pre-mRNA-splicing factors (SRSF1,SFPQ) at the transcription site [75–77].Polymorphism in lncRNA TCONS_00021856/linc-SLITRK5–11 gene at rs7990916 (T
>
C
) is differentially present in Alzheimer’s patients compared to heathy individual [78].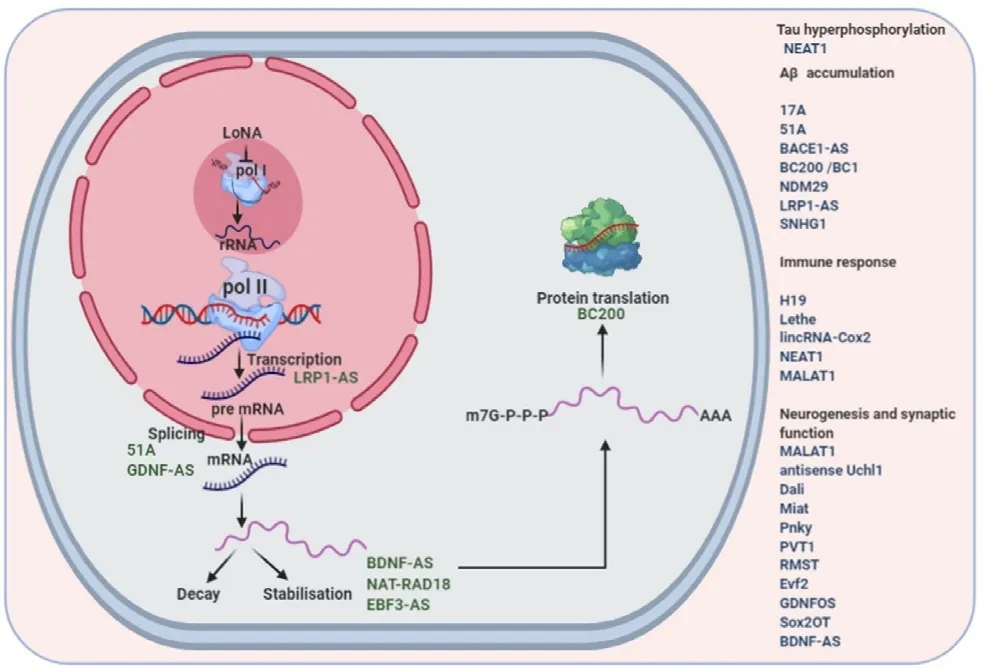
Fig.2–Various roles of lncRNAs in Alzheimer’s disease.
Zhou et al.have found majorly intergenic 84 down-regulated and 24 up-regulated lncRNAs in AD patients,one of these down-regulated lncRNAs,n341006 shows association with protein ubiquitination pathway while another up-regulated lncRNA,n336934,is associated with cholesterol homeostasis after gene set enrichment analysis (GSEA) [79].Zhang et al.have discovered 114 significantly down-regulated and 97 significantly up-regulated lncRNA transcripts from the SAMP8 (senescence-accelerated mouse prone 8) and SAMR1 (senescence-accelerated mouse resistant 1) model.These transcripts are involved in mitogen activated protein kinase signaling pathway,nerve growth factor term and the AD pathway [80].Table 1 and Fig.2 summarises various regulatory mechanisms off lncRNAs in AD.
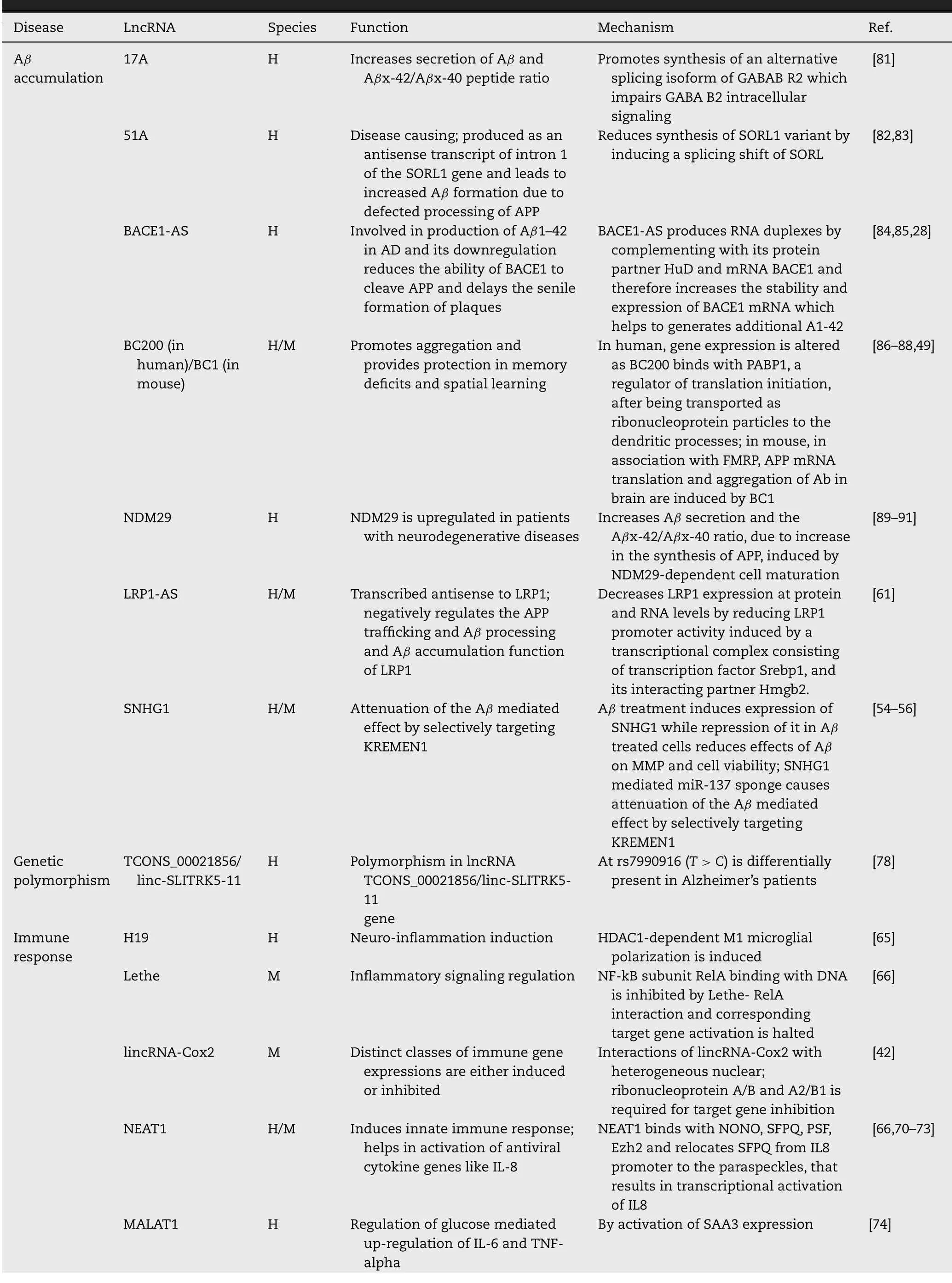
Table 1–Role of lncRNAs in Alzheimer’s disease.
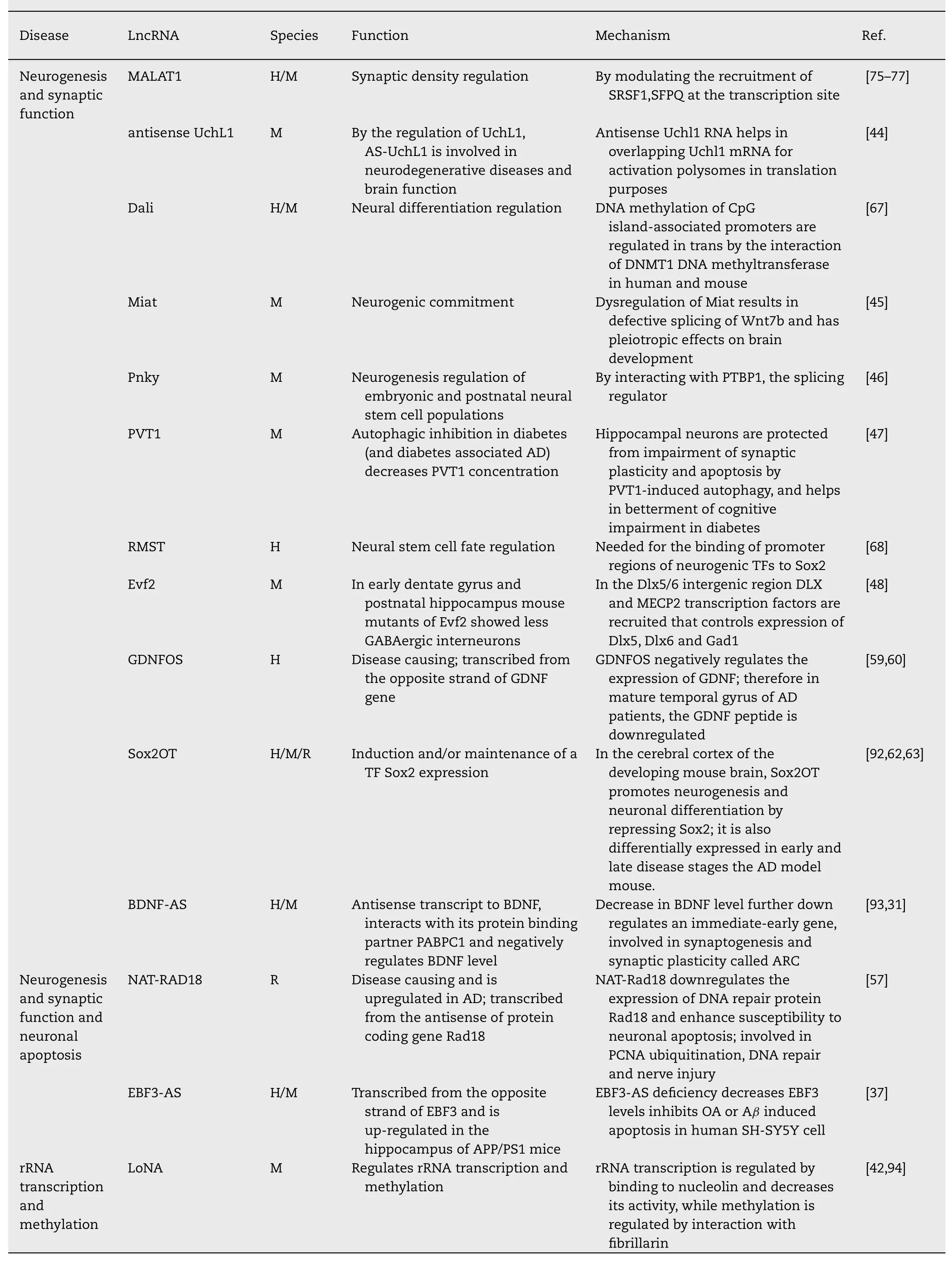
Table 1 (continued)
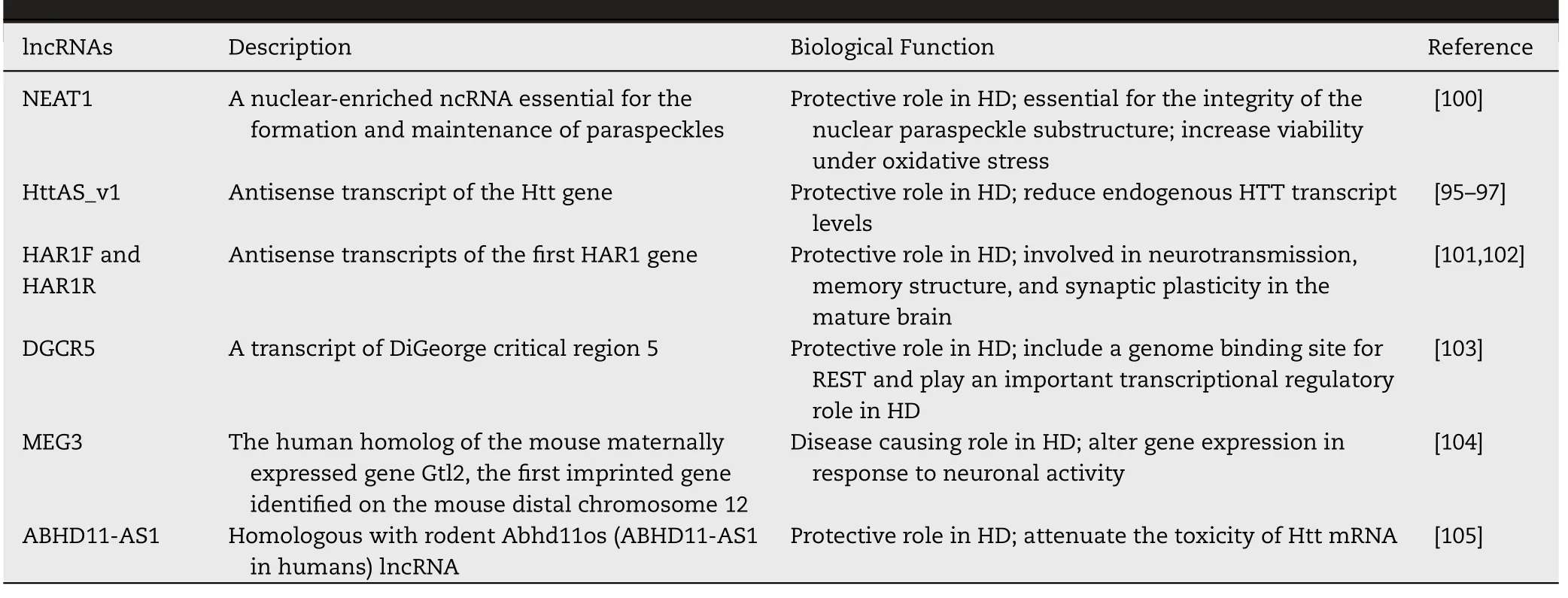
Table 2–Role of lncRNAs in Huntington’s disease.
2.2.Role of lncRNAs in HD
HD is a hereditary neurodegenerative disorder characterized by psychiatric disturbances,progressive dyskinesias,chorea and dementia,and is caused by an abnormal expansion of CAG trinucleotide in the first exon of the huntingtin gene.The antisense transcript of the Htt gene called as lncRNA HttAS_v1 has lower expression level in the frontal cortex of HD patients,that results in higher expression of Htt mRNA,HD pathogenesis [95].Htt functions as a modulator of nuclear translocation of the transcriptional repressor RE1 silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF).A mutation in Htt results in abnormal nuclear-cytoplasmic transport of REST/NRSF,leading to abnormal expression of REST target genes [96,97].
Another lncRNA antisense to brain-derived neurotrophic factor (BDNF-OS),upregulates BDNF concentration and has a protective role on neurons and thus it improves the Huntington disease phenotype [98].The concentration of NEAT1 has been found higher in R6/2 mice and HD patients [99].It is also essential for the production and maintenance of subnuclear bodies found in mammalian cells called paraspeckles [100].
The lncRNAs HAR1F and HAR1R,antisense to HAR1 (human accelerated region 1) gene have been found to be involved in synaptic plasticity,memory structure,neurotransmission in the mature brain,and are down-regulated in striatum of human HD brain as reported [101].In striatum of HD,excessive REST nuclear-cytoplasmic exchange has been found to effectively repress HAR1 transcriptionally [102].Another lncRNA DGCR5 (DiGeorge critical region 5) contains a genome binding site for REST and are down-regulated in HD,thus playing a crucial role in pathophysiology of HD [103].REST has also been found to inhibit downregulation of the lncRNA MEG3 (maternally expressed gene 3),that is otherwise down-regulated in HD brain tissue [104].In recent studies it has been found that knocking out the lncRNA Abhd11os (ABHD11-AS1 in humans) gene in the HD mouse model produces neurotoxicity,but overexpression of Abhd11os has a neuroprotective effect and neutralises the toxicity of Htt mRNA in murine models of HD [105].Another lncRNA TUG1,that is up-regulated in HD interacts with PRC2 after being activated by p53 and regulates the downstream genes [104,106].The lncRNA TUNA is highly expressed in the thalamus and striatum.Deregulation of hTUNA in the caudate nucleus may have involvement in the pathophysiology of HD [107].Table 2 and Fig.3 displays roles of lncRNAs in Huntington disease.
2.3.Role of lncRNAs in PD
PD is a neurodegenerative disorder caused by depletion of dopamine-secreting neurons,that results in impairments of motor abilities.LncRNAs have crucial role and altered expression profile in PD pathogenesis [108].The lncRNA antisense ubiquitin carboxy-terminal hydrolase L1 (AS-UchL1) has been found to increase the expression of UchL1 protein,that is closely related to brain function and neurodegenerative diseases,at post-transcriptional level depending on a 5overlapping sequence and an embedded inverted SINEB2 sequence [67].As a component of Nurr-1 dependent gene network,down-regulated AS-Uch1 causes decreased translation of UchL1 protein in neurochemical models of PD.This leads to inhibition of ubiquitin-proteasome system [109](Fig.5).Impaired motor function or abnormal dopamine release is associated with abnormality in the expression of PTEN-induced kinase 1 (PINK1) [110].A human specific non-coding RNA NaPINK1 has been found to stabilize PINK1,thereby increasing its expression [111].The lncRNA metastasis associated lung adenocarcinoma transcript 1 (MALAT1) (also called as NEAT2) is highly expressed in neurons and upregulatesα
-synuclein production when overexpressed [75,98].Targeting MALAT1 withβ
-asarone reduces its level and therefore can serve as a potential therapeutic target for PD [112].Another commonly known 2.2-kb-long lncRNA HOTAIR (Hox transcript antisense intergenic RNA),is up-regulated in mice Parkinson’s model upon intraperitoneal injection of MPTP and stabilises Leucine-rich repeat kinase 2 (LRRK-2) involved in initiation and development of PD [113].It further induces neuronal apoptosis [114].Few lncRNAs H19 upstream conserved 1 and 2 (Huc1 and Huc2),lincRNA-p21,MALAT1,SNHG1,and TncRNA have been found to be differentially expressed in PD suggesting their involvement in disease pathogenesis,that is yet to be discovered [115].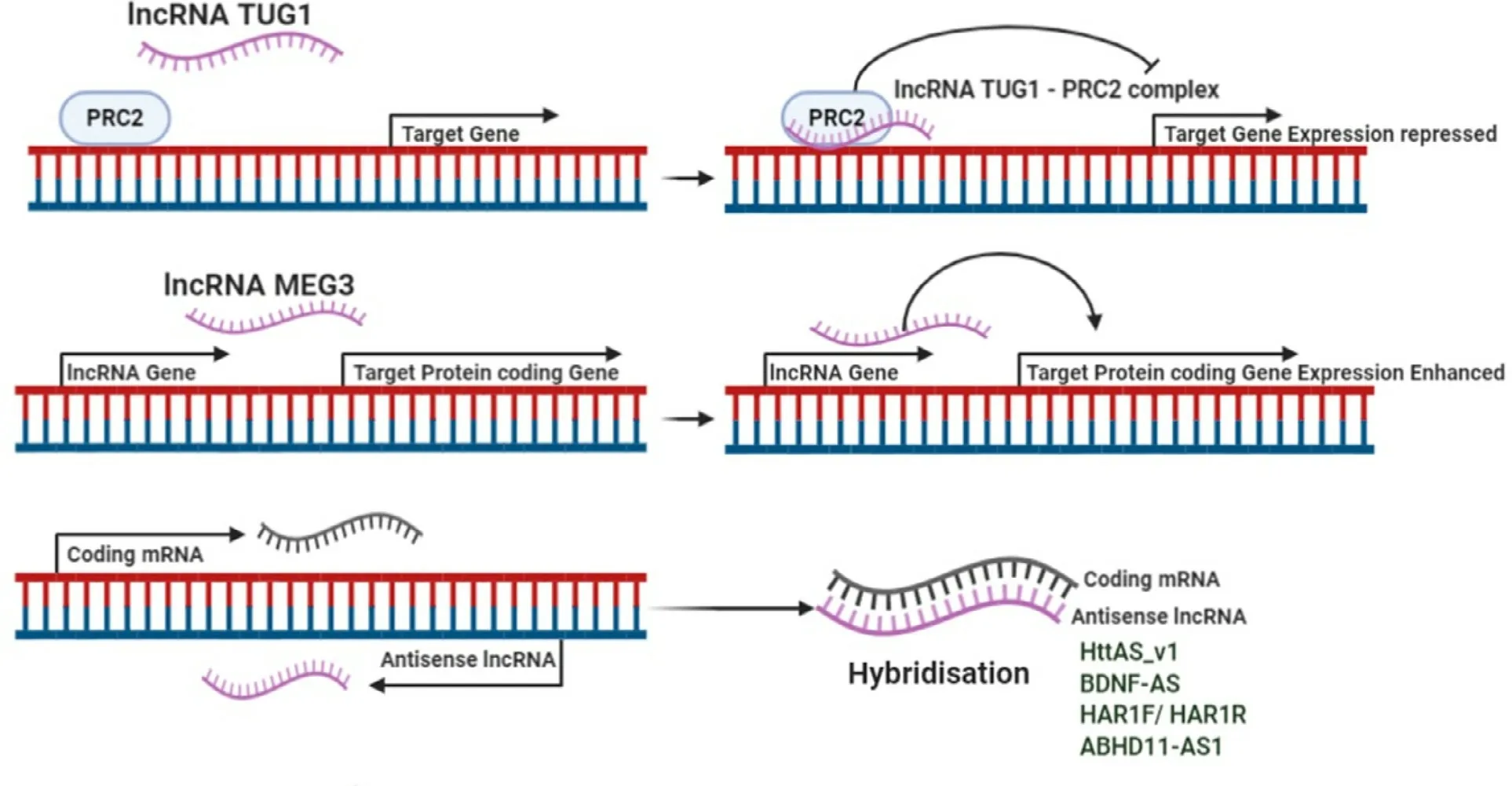
Fig.3–Regulatory mechanisms of lncRNAs in HD.
Recent studies have shown that in neuronal SH-SY5Y cells lncRNAs AL049437 and SNGH1 contribute to MPP cytotoxicity [116–118].The lncRNA MAPT-AS1 (microtubule-associated protein tau antisense 1) is down-regulated in brains of PD patients,and acts as an epigenetic regulator of MAPT expression that has pathogenic role in PD [119].In SH-SY5Y cells treated with MPP,and in the substantia nigra of PD patients NEAT1 is significantly up-regulated.It and promotes autophagy and has protective role against oxidative stress and neuronal injury [120–122].In MPP-induced SH-SY5Y cells LncRNA-p21 has been found to regulate the neuronal injury via the miR-626-TRMP2 axis [123].The lncRNA BACE1-AS reduces nitric oxide synthase and prevents oxidative stress by upregulating microRNA-34b-5p in PD rat model [124].LncRNA HAGLROS is up-regulated in SH-SY5Y cells and PD mouse model and is associated with inhibition of apoptosis and autophagy by the activation of PI3K/Akt/mTOR pathway and regulation of miR-100/ATG10 axis [125].In mice PD models,the lncRNA H19 that was earlier reported in multiple cancers and heart diseases has been found to show protective role against apoptosis and dopaminergic neuronal loss by regulating miR-301b-3p and miR-585–3p [126,127].Again,in mice model of PD,the lncRNA GAS5 has been found to promote microglial inflammatory via regulating NLRP3 pathway by sponging miR-223–3p [128].In MPP treated PD disease model SH-SY5Y cells,NORAD has been found down-regulated.It has protective roles against MPP induced cytotoxicity [129].The lncRNA UCA1 upregulates SNCA and promotes PD development [130].The lncRNA LINC-PINT has been found have increased expression in substantia nigra of PD patients.RNAi mediated depletion of this lncRNA shows increased death of cultured N2A and SH-SY5Y cells under oxidative stress,thereby suggesting a neuro protective function of LINC-PINT in PD pathophysiology [131].knockdown of AK021630 resulted in decreased mitochondrial mass,mitochondrial transmembrane potential (Δψ
m),cell viability,and tyrosine hydroxylase (TyrH) secretion in human neuroblastoma SH-SY5Y cell line suggesting protective role of AK021630 in PD[109,133],and the lncRNA NR_030777 has shown a protective role in Paraquat-induced neurotoxicity via regulating Zfp326 and Cpne5 [133].In substantia nigra of paraquat and MPTP induced mouse model Nrf2-related lncRNAs have been found to be involved in oxidative stress [134].In anti-NGF AD11 transgenic mouse the lncRNA Sox2OT is involved in regulating co-transcribed Sox2 gene expression to down neurogenesis [135].The lncRNAs UchL1-AS,PINK1-AS,HAR1A,Sox2OT,BCYRN1,ANRIL,are reported in PD patients in the Hungarian population.They are involved in interfering with the binding affinity of transcription factors like HNF4A,potentially resulting in abnormal expression of target genes,such as BCYRN1 [136].The regulatory mechanisms of lncRNA involved in PD are listed in Table 3 and Fig.4 .
Table 3–Role of lncRNAs in Parkinson’s disease.
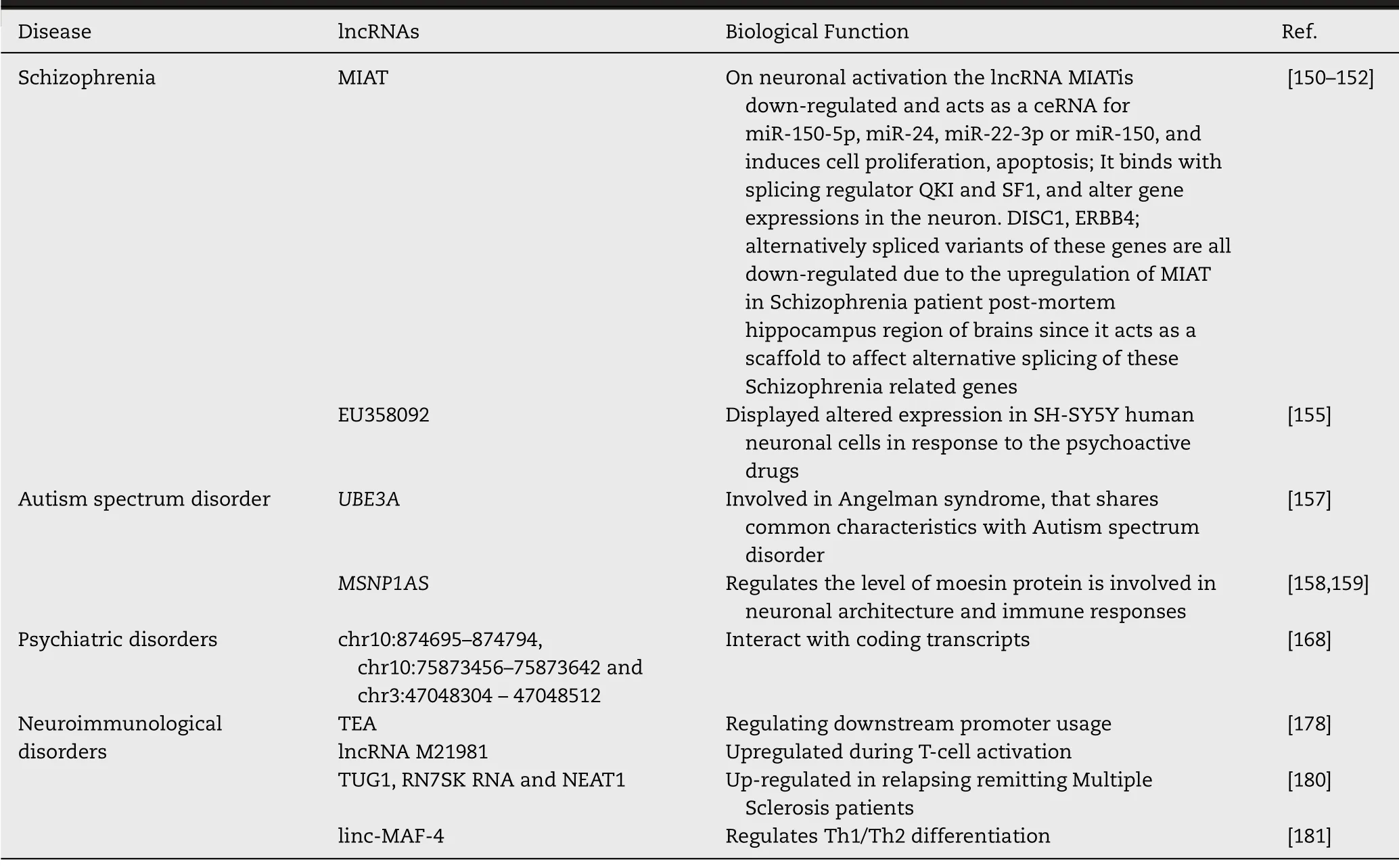
Table 4– Role of lncRNAs in Schizophrenia,Autism spectrum disorder,psychiatric disorders and other neuro-immunological disorders.
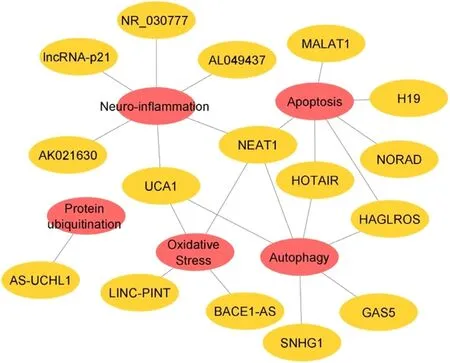
Fig.4–Network view of lncRNAs in PD and their
2.4.Role of lncRNAs in schizophrenia
Schizophrenia is a mental disease characterized with neurocognitive impairments.Pathophysiology of schizophrenia is caused by both genetic and environmental factors including lncRNAs [137–139].A number of lncRNAs have altered expression in both the periphery and the CNS of patients with schizophrenia [138,140–142].Studies have shown the lncRNA MIAT (residing on chromosome 22q12.1,near to the schizophrenia candidate region,chromosome 22q11.2),is down-regulated in schizophrenia patients [143].The G to T polymorphism at the MIAT SNP rs18944720 has also been connected to susceptibility to paranoid schizophrenia [144].MIAT regulates alternative splicing in schizophrenia by binding to the splicing factors,SF1,QKI,SRSF1 and CELF [143,145,146]and is expressed in neuronal populations in CNS,where mature transcripts are nucleus localised [147,148].Upon neuronal activation the lncRNA MIAT,(also called as Gomafu [143]or RNCR2),is down-regulated in schizophrenia [149]and acts as a competitive endogenous RNA (ceRNA) for miR-150–5p,miR-24,miR-22–3p or miR-150,thereby induces cell proliferation,apoptosis,MIAT can also bind with splicing regulator quaking homolog (QKI) and SF1,and can alter gene expressions in the neuron (Fig.6).DISC1 (disrupted in schizophrenia 1),ERBB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4) and alternatively spliced variants of them are all down-regulated because of the upregulation of MIAT in schizophrenia patient postmortem hippocampus region of brains [150–152]as it acts as a scaffold to affect alternative splicing of these schizophrenia related genes as described earlier [153,154,149].A novel lncRNA,EU358092 on chromosome 1p21.3,expressed in CNS has been found to be associated with schizophrenia by bioinformatics analysis and GWAS [155].EU358092 also showed altered expression in SH-SY5Y human neuronal cells in response to the psychoactive drugs [155],thereby showing potential relationships with schizophrenia pathology.
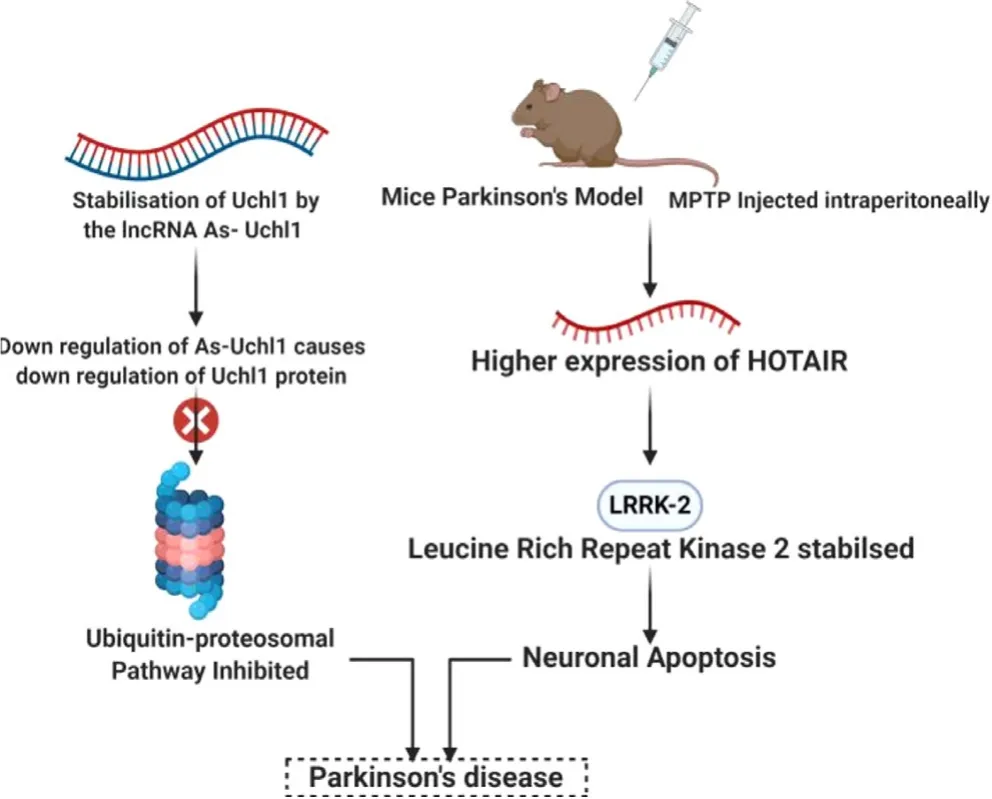
Fig.5–Regulatory role of HOTAIR and As-UchL1 in PD.

Fig.6–Regulatory role of MIAT in schizophrenia.
2.5.Role of lncRNAs in ASD
A group of heterogeneous neuro developmental disorders,characterized by impaired reciprocal social interactions,communication and repetitive stereotyped behaviors,is defined as ASD [156].A total of 222 differentially expressed lncRNAs have been identified in ASD.It has been shown that number of differentially expressed lncRNAs are higher with control individuals as compared to Autistic samples [157].Many of the differentially expressed lncRNAs are associated with neurodevelopmental and psychiatric diseases.As for exampleUBE3A
(ubiquitin protein ligase E3A) is involved in Angelman syndrome,that shares common characteristics with ASD.A 3.9 kb lncRNA MSNP1AS,encoded by the antisense strand of the moesin pseudogene 1 (MSNP1
),has been identified in genome wide association studies (GWAS) of ASD.It regulates the level of moesin protein is involved in neuronal architecture and immune responses.In postmortem ASD temporal cortex,MSNP1AS is significantly up-regulated [158,159].2.6.Role of lncRNAs in ALS
The neurodegenerative disease ALS is characterised by the progressive paralysis of the limbs and muscles and degeneration of spontaneous motor neurons that causes difficulty in speech swallowing,and respiration.The first identified causative mutation in ALS and frontal temporal dementia was the repeated amplification of a six-nucleotide motif (GGGGCC) in the protein-coding gene C9ORF72 (chromosome 9 ORF 72) [160,161].The bidirectional transcription at C9ORF72 locus that produces both sense and antisense RNAs,[162]are localised in nucleus [163]and both are elevated in ALS patients and the antisense lncRNA can inhibit C9ORF72 mRNA expression.Although it has been found that the corrected disease related gene in fibroblast cannot cure the disease [163].Two nucleus localised RNA-binding proteins,namely TDP43 (TAR DNA-binding domain protein 43) and FUS/TLS (fused in sarcoma/translated in liposarcoma) are accumulated abnormally in cytosol and lead to misfolding of wtSOD1 (wild-type Cu/Zn superoxide dismutase) in SALS (sporadic ALS) and non-SOD1 FALS (familial ALS),thereby contributing to pathophysiology of ALS [164].LncRNAs have been found to recruit FUS/TLS to cyclin D1 genomic locus to repress transcription of cyclin D1 [165,166].(Fig.7)
2.7.Role of lncRNA in psychiatric disorders
A common psychiatric disorder,major depressive disorder (MDD) is associated with significantly higher level of morbidity,disability and mortality [167].Three lncRNAs at positions chr10:874,695–874,794,chr10:75,873,456–75,873,642 and chr3:47,048,304–47,048,512 are identified to interact with coding transcripts and are involved in Major depressive disorder [168].Cui et al.,has shown six lncRNAs (TCONS_00019174,ENST00000566208,NONHSAG045500,ENST00000517573,NONHSAT034045 and NONHSAT142707) as down-regulated in MDD patients [193].These lncRNAs also showed lowered expression in generalized anxiety disorder (GAD) [194].In another research,Li et al.showed 9 lncRNAs (TCONS_L2_00001212,NONHSAT102891,TCONS_00019174,ENST00000566208,NONHSAG045500,ENST00000591189,ENST00000517573,NONHSAT034045,NONHSAT142707 (P
<
0.05) are significantly down-regulated in PBMC of MDD patients [195].Using microarray genome-wide expression analysis and lncRNA-mRNA co-expression network analysis,Liu et al.have shown that the lncRNAs located at chr10:874,695–874,794,chr10:75,873,456–75,873,642 and chr3:47,048,304–47,048,512 can be crucial in regulating the expression of mRNAs in MDD [196].2.8.Role of lncRNAs in cerebral injury
Stroke is the second most common cause of death in the world and is caused by haemorrhagic damages or cerebral ischemic in brain [169,170].Specific temporal and spatial expression pattern of lncRNAs have been found in cerebral ischemia injury as well as in brain damages caused by hypoxic ischemic [171–175].Post-ischemic pathophysiology may be modulated by chromatin-modifying proteins (CMPs) activities of lncRNAs.After focal ischemia lncRNAs were found to be dysregulated in rats by middle cerebral artery occlusion [171].These lncRNAs were homologous to protein coding genes [171].It was further shown that after brain ischemia,177 of the 2497 lncRNAs expressed in rat cerebral cortex displayed strong binding to either paired amphipathic helix protein Sin3A (Sin3A) or corepressors of the RE-1 silencing transcription factor (coREST) [172].It has been recently found that in anin-vitro
model of ischemic-reperfusion injury,miR-377 along with lncRNAS can modulate Ncam1 and Negr1 mRNAs maintain neuronal structure and function during neuronal development [173].In hypoxic-ischemic brains of rats a total of 322 lncRNAs that includes lncRNA BC088414 (related with genes involved in apoptosis) have been found to be differentially expressed [175].Other than these,after ischemic stroke endothelial-selective lncRNAs has been found to function as a class of novel master regulators in cerebrovascular endothelial pathologies [174].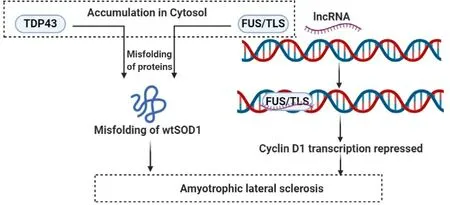
Fig.7–Regulatory role of lncRNAs in ALS.
2.9.Role of lncRNAs in neuroimmunological disorders
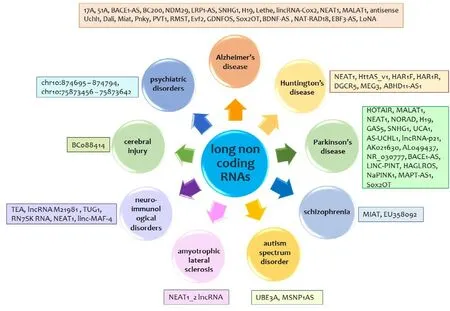
Fig.8–Regulatory role of various lncRNAs against neurological and psychiatric disorders.
LncRNAs are associated with neuroimmunological disorders as well [176,177].A lncRNA obtained from mouse T earlyα
(TEA) promoter has been found in regulating downstream promoter usage [178].A good number of lncRNAs are to be dynamically expressed in the process of differentiation and the activation of T-cell [177].Out of many lncRNAs that are nested in introns of IL2RA gene,the lncRNA M21981 is significantly up-regulated during T-cell activation that suggests partly its regulatory role in pathogenesis of neuroimmunological disorders.LncRNAs have shown significant regulatory relationship in multiple sclerosis,a complex autoimmune disorder.In the peripheral blood mononuclear cells of patients with multiple sclerosis,a total of 2353 up-regulated lncRNAs,389 down-regulated lncRNAs have been identified [179].Three lncRNAs namely 7SK small nuclear (RN7SK RNA),taurine up-regulated 1 (TUG1) and NEAT1 have been found to be up-regulated in relapsing remitting Multiple Sclerosis patients compared to healthy controls [180].The lncRNA linc-MAF-4 that regulates Th1/Th2 differentiation has been found in the pathogenesis of Multiple Sclerosis via in a process of targeting MAF [181].Table 4 summarises role of lncRNAs in four neurological diseases namely,schizophrenia,ASD,psychiatric disorders and neuroimmunological disorders.3.Potential clinical and therapeutic aspects
LncRNAs are appearing as novel targets for diagnosis and treatment of a range of human diseases in recent days [197–200]especially against an array of neurological disorders (Fig.8).Levels of lncRNA transcripts and their post transcriptional modifications can be determined using PCR,RNA sequencing,microarray and the single cell analyzing techniques,like scRNA sequencing.Intracellular trafficking of lncRNA can be measured by the contents of micro-vesicles in blood and cerebrospinal fluid [201].Oligonucleotide molecular beacons and quantum dot nanoparticles that serves as novel molecular imaging probes are being used in visualizing lncRNAs with a potential to be used further in real timein
vivo
imaging.This can be utilized in clinical approach by using lncRNAs as molecular markers.As for example Kam et al.reported FIT-PTA molecular beacons for detection of a lncRNA CCAT1 in both living cells as well as human adenocarcinoma colon tissue samples [202].As a therapeutic strategy,recombinant zinc-finger nuclease (ZFN) with a property of introducing RNA destabilizing elements has shown promising results in silencing the lncRNA NEAT2 [203].Initialin
vitro
strategies like using ZFN based therapies for neurological disorder which includes a T cell-oriented strategy for glioblastoma (NCT01082926),shows way to further promising therapeutic potentials.Targeting epigenetic enzymes since these enzymes have regulatory roles in disease context,has shown clear evidence of altered expression of lncRNAs [204].In summary there had been evidences of using lncRNAs as potentially therapeutic targets,which are to be further investigated in future.4.Conclusion
Metabolic abnormalities are diversely complex and are determined by intricate networks and cross-talks between several cellular and tissue-level entities.LncRNAs play roles in fine-tuning of cellular metabolism.Their discovery has given new paradigm shift in understanding fine-tuning of cellular processes.Ease of availability and advent in methodologies to identify lncRNA with very low copy numbers has given new opportunities to establish them as markers.lncRNAs also have multifaceted intracellular regulatory functions and capabilities to alter intercellular communication and interactions [182].Half-lives of these RNA molecules are relatively shorter than protein-coding transcripts.But their association with RNA-binding proteins and folding into secondary structure provide them enhanced stability and resistance against degradation by RNases.By virtue of its secondary structure and poly-A tail,lncRNAs are able to survive in body fluids [183].It has been shown that lncRNAs can be detected in wide range of extracellular body fluids such as whole blood,plasma,serum,urine,saliva,gastric juice and display a dynamic alteration upon diseases [11,184–186].LncRNAs can also enter the blood stream encapsulated in exosomes [187]and extracellular vesicles or can be released from the apoptotic bodies [188].Therefore,with these properties,lncRNAs are transcripts of particular interest to serve as a novel class of non-invasive prognostic and diagnostic marker/biomarker [184,189,190]and they have been well established in various neurological disorder [191,192].Here we have tried to look into various aspects of lncRNAs and their roles in regulations of various neurological diseases including neurodegenerative disorders.Here in this review we tried to look into the potential of various lncRNAs for being used as therapeutic targets and diagnostic markers in broad range of various neurological and neurodegenerative diseases.
Conflicts of interests
None.
Acknowledgement
We acknowledge Wajid Waheed Bhat (Michigan State University) for his kind support in drawing the figures using Biorender.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Commentary of the mRNA vaccines COVID-19
- Relationship and improvement strategies between drug nanocarrier characteristics and hemocompatibility:What can we learn from the literature
- Use of graphene-based materials as carriers of bioactive agents
- Role of the cation-chloride-cotransporters in the circadian system
- Anti-EpCAM functionalized graphene oxide vector for tumor targeted siRNA delivery and cancer therapy
- Enhanced delivery efficiency and sustained release of biopharmaceuticals by complexation-based gel encapsulated coated microneedles:rhIFN α-1b example
