A novel temporary keratoprosthesis technique for vitreoretinal surgery
2021-11-30ChristosSkevasEileenBigdonAlexanderSteinhorstToamKatzPhilippSchindlerRobertKromerMartinStephanSpitzer
Christos Skevas, Eileen Bigdon, Alexander Steinhorst, Toam Katz, Philipp Schindler, Robert Kromer,Martin Stephan Spitzer
Department of Ophthalmology, University Medical Center Hamburg-Eppendorf, Hamburg 20246, Germany
Abstract
INTRODUCTION
The combination of vitreoretinal surgery with penetrating keratoplasty (PKP) is a useful method for treating patients with vitreoretinal disorders complicated by corneal opacifications. In patients with corneal and vitreoretinal pathology often corneal and retinal pathology need to be addressed simultaneously. The etiology of corneal pathology and opacification can be trauma, corneal scar or ulcer. A temporary keratoprosthesis (TKP) is used as an intraoperative bridge between anterior and posterior segment procedures. It is placed at the beginning of the procedure to allow visualization of the posterior segment during vitreoretinal surgery[1]. Once posterior segment surgery is concluded, the TKP is removed,and a corneal allograft is placed. Visual potential in eyes with such complex pathology is variable and often depends on the extent of posterior segment damage and the status of the retina[2-3].
Two commercially available TKP devices are the Eckardt®and Landers Foulks models[4-5]. Positive outcomes have been reported by various authors[1,6]. TKP facilitates visualization of intraocular structures in eyes that might otherwise be inoperable due to corneal opacities and allow implementation of vitreoretinal procedures which might otherwise be delayed while waiting for a corneal graft to clear. Moreover, it also avoids endangering the survival of the corneal graft as it cannot get damaged during vitreoretinal surgery.
The primary goal of this paper is to present an alternative surgical treatment option for the management of complex cases with corneal opacification and vitreoretinal diseases that require timely surgical intervention.
SUBJECTS AND METHODS
Ethical ApprovalThe Ethics Committee of Hamburg was consulted and deemed that no approval for this study was required and no informed consent from the patients was required (WF-137/20) .
We report three cases, two male and one female patient. All three eyes had significant corneal opacification precluding adequate visualization of the posterior segment so B scan ultrasonography had to be performed in order to confirm the retinal pathology that necessitated surgery.
Case 1An 80-year-old male patient with corneal opacification due to neurodermitis and secondary glaucoma due to herpes simplex infection presented with symptoms of retinal detachment. The affected left eye had a history of multiple perforated corneal ulcera, three corneal graft rejections and was pseudophakic. The last PKP was 4mo prior to the retinal detachment. The intraocular pressure (IOP) was controlled with pressure-lowering eye drops and the corneal graft showed signs of renewed rejection with opacification and vascularization. Ultrasonography (Figure 1C) showed clear signs of retinal detachment. Preoperative best corrected visual acuity (BCVA) was hand movements (HM). The fellow right eye was enucleated 10 years ago due to secondary glaucoma.As this was his last eye the patient decided to undergo surgery to preserve some vision even if the chances for preserving function and the globe clearly were limited given the extremely complicated situation.
The procedure was performed, under general anesthesia, as follows: standard sclerotomies were made for a 23-gauge vitrectomy. A Flieringa Ring®(Storz Ophthalmics, Heidelberg,Germany) was secured to the sclera (Ethicon, Somerville,NJ, USA). The corneal button was excised using a 6.0 mm trephine (Kai industries Co., Ltd., Seki City, Japan). A larger trephination was not possible due to the pronounced vascularization of the peripheral cornea. The insertion of an Eckardt®keratoprosthesis (Dutch Ophthalmic Research Center, Dutch Ophthalmic USA, Exeter, NH, USA) was attempted but due to the multiple operations, the too large size of the Eckardt®keratoprosthesis for a trephination diameter of 6 mm and the thin recipient bed we were not able to stabilize the Eckardt®keratoprosthesis (Figure 1A). Next, we decided to use a soft bandage contact lens (Bausch and Lomb-Pure Vision Lens) that was not sutured to the corneal trephination edges but on the globe as a substitute for the Eckardt®keratoprosthesis(Figure 1B). We secured the bandage contact lens with eight 7-0 Vicryl to minimize leakage (Figure 1B) and commenced with the three port 23G pars plana vitrectomy (PPV). The visualization of the central and the peripheral retinal was excellent throughout the procedure and allowed core and a peripheral vitrectomy without complications (Figure 1D). The peeling of proliferative vitreoretinopathy (PVR) membranes was done in a safe manner. The retina was re-attached using perfluorocarbon liquid (PFCL), the breaks were identified,treated with laser and at the end a PFCL 5000 cSt silicone oil exchange war performed. The sclerotomies were closed with 7-0 Vicryl sutures. The soft bandage contact lens provided clear media that allowed posterior segment surgery and retinal reattachment.
After the vitreoretinal surgery, the soft contact lens TKP was removed, and the donor cornea was secured with 10-0 nylon interrupted sutures (Ethicon). To support the open globe in the short time span between soft contact lens removal and fixation of the allograft, viscoelasticum was injected into the anterior chamber to prevent leakage of silicone oil. After completion of the PKP, no supplement of silicon oil was needed.
Results of Case 1At all postoperative follow up visits the retina was completely re-attached. There were no signs of silicone oil in the anterior chamber and no signs of endophthalmitis. The BCVA was HM. An amnion membrane transplantation had to be performed 1mo after vitreoretinal surgery due to recurrent corneal erosions. We decided for a permanent silicone oil filling. During follow-up no silicone oil migrated to the anterior chamber. Thus, the corneal endothelium of the graft was not endangered at any time point after surgery.
Case 2A 73-year-old woman with diffuse corneal opacification after PKP (Figure 2B) presented with luxation of an intraocular lens (IOL) and severe non-clearing vitreous hemorrhage. The primary diagnosis was iridocorneal endothelial syndrome which led to IOP decompensations,corneal ulceration, and scarring. The past medical history was remarkable for 2 corneal graft rejections and the last PKP was 12mo prior to presentation. The IOP was compensated under topical medication. The preoperative BCVA was HM. An ultrasonography was performed (Figure 2A).
The procedure was performed under general anesthesia,as follows: Standard sclerotomies were made for a 23G vitrectomy. A Flieringa Ring®(Storz Ophthalmics, Heidelberg,Germany) was secured to the sclera (Ethicon, Somerville,NJ, USA). The corneal button was excised using a 7.0 mm trephine (Kai industries Co., Ltd., Seki City, Japan). A soft bandage contact lens was placed as a TKP (Bausch and Lomb-Pure Vision Lens) on the globe. We secured the bandage contact lens with six 7-0 Vicryl to minimize leakage (Figure 2C, 2D). We commenced with three port 23G PPV. The visualization of the central and the peripheral retinal made a core and a peripheral vitrectomy possible. The IOL was secured with an alligator forceps and removed under the soft bandage contact lens (Figure 2C, 2D). There was no hypotony during vitrectomy and during removal of the IOL under the soft contact lens. The retina was attached at all times. A laser treatment was performed due to lattice degeneration and peripheral breaks. At the conclusion of the vitrectomy a fluid air exchange was performed. The TKP provided clear media that allowed posterior segment surgery.
After the vitreoretinal surgery, the soft contact lens keratoprosthesis was removed, and the allograft cornea was secured in place with 10-0 nylon interrupted sutures (Ethicon).
Results of Case 2All postoperative follow ups showed a completely attached retina, with compensated IOP under the same therapy as pre surgery and no signs of endophthalmitis.BCVA was HM.
Case 3A 65 years old male with corneal decompensation after corneal ulcer. This patient had a PKP two years ago and the corneal transplant had to be replaced one month prior, due to decompensation (Figure 3A). The ultrasonography prior to surgery was deemed necessary and there were signs of highly reflective membranes, and a retinal detachment could not be excluded. The patient hat multiple glaucoma surgeries and was tested and verified with positive QuantiFERON®test.The IOP was compensated under topical medication and the preoperative BCVA was HM.

Figure 1 Surgical steps and echography findings A: Insertion of Eckardt® keratoprosthesis is not possible; B: Soft contact lens is stabilized on the globe with 7-0 Vicryl sutures; C: Ultrasonography depicting a retinal detachment; D: Intraoperative visualization of the retina.
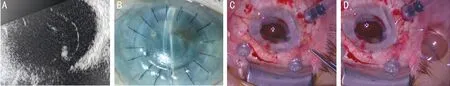
Figure 2 Surgical steps and echography findings A: Ultrasonography depicting the luxated IOL; B: Diffuse corneal opacification; C: IOL extraction under the soft contact lens; D: IOL removed.

Figure 3 Surgical steps A: Corneal decompensation after PKP; B: Corneal transplant removed; C: Soft contact lens is stabilized on the globe with 10-0 Nylon sutures; D: Intraoperative visualization of the retina.
The procedure was performed under general anesthesia,as follows: standard sclerotomies were made for a 23G vitrectomy. The corneal transplant was removed after removing the old corneal sutures. A soft bandage contact lens was placed as a TKP (Bausch and Lomb-Pure Vision Lens)on the globe (Figure 3B, 3C). We secured the bandage contact lens with 10-0 Nylon to minimize leakage. We commenced with three port 23G PPV. The visualization of the central and the peripheral retinal made a core and a peripheral vitrectomy possible (Figure 3D). There was no retinal detachment but hyaloid opacification, peripheral traction, and multiple lattice degeneration on the peripheral retina. The retina was attached at all times. A laser treatment (360°) was performed due to lattice degeneration. At the conclusion of the vitrectomy a fluid air exchange was performed. The TKP provided clear media that allowed posterior segment surgery.
After the vitreoretinal surgery, the soft contact lens TKP was removed, and a new allograft cornea was secured in place with 10-0 Nylon interrupted sutures (Ethicon).
Results of Case 3All post-operative follow-ups showed a completely attached retina, with compensated IOP under the same therapy as pre surgery and no signs of endophthalmitis.BCVA was HM.
RESULTS
There were no intraoperative or postoperative complications(expulsive hemorrhage, choroidal detachment, endophthalmitis,phthisis bulbi). The central, peripheral vitrectomy and the laser treatment was safely performed due to the good visualization of the retinaviathe contact lens. The tight fixation of the contact lens allowed an uncomplicated silicone oil PFCL exchange in the first case and fluid air exchange in the second case (Figure 4). No eyes were enucleated during the period of follow-up and no secondary glaucoma cases were observed.Resolution of the vitreoretinal pathologies with removal of the luxated IOL and retinal reattachment were achieved in all eyes.

Figure 4 The soft contact lens is placed on the globe and is fixated with sutures to minimize leakage. The visualization of the central and peripheral retina allows a safe central and peripheral vitrectomy.
DISCUSSION
When treating patients with coexisting corneal opacification and vitreoretinal disorders, the triple procedure(PPV+TKP+PKP) has been commonly employed[1,4,7]. The concept and application of TKP for ocular surgery was first published by Landers in 1981[8]. Before their development of the Landers Foulks keratoprosthesis, eyes with corneal opacities requiring vitreoretinal surgery were managed with either open sky vitrectomy or corneal transplantation followed by vitreoretinal surgery as a delayed second procedure.Although the Landers Foulks keratoprosthesis was a great advancement, it had several limitations including a long optical cylinder limiting visualization of the pars plana and vitreous base, a long cylindrical axis limiting its use in aphakic eyes and damage to the corneal rim of the host as the keratoprosthesis is screwed into place. Some of these shortcomings were eliminated with the development of the Eckardt®prosthesis[1,4].There is a number of permanent keratoprosthesis designs that have been developed throughout the world. The Boston type 1 and 2, the AlphaC or artificial cornea, the Legeais BioKpro III and the osteo-odonto keratoprosthesis represent the commonest forms[9-12].
However, they are usually not feasible as a TKP for vitreoretinal surgery. Surgical outcomes have improved with the introduction of silicone oil to stabilize the retina and reduce the risk of failure from PVR by providing a long-term tamponade. The use of silicone oil, however, can result in complications and can harm the clarity of corneal grafts owing to the silicone oil in contact with the corneal endothelium or to an increase of IOP[13-14].
Khouriet al[7]reported a retinal reattachment rate of 92% in a case series of 23 patients who underwent combined surgery using a temporary Eckardt®keratoprosthesis. No complications like secondary glaucoma, migration of silicone oil in the anterior chamber were reported[10]. Corneal graft rejection after combined surgery, however, is a complication that numerous authors have reported. Khouriet al[7]reported graft rejection in 21% of the cases and Leeet al[15]at a corneal graft rejection of 73% even though the success of vitreoretinal surgery reached 81.8%. One of the hypotheses could be the presence of active inflammation in the cornea at the time of surgery[10].
Helsenet al[13]reported on the use of the Eckardt®keratoprosthesis as an emergency temporary tectonic seal for a full-thickness,large decentered corneal perforation. The TKP was well tolerated during the 3-week period while awaiting final repair with a corneal donor button[1,9]. Currently, we do not know whether the same could be achieved with our technique of simply suturing a soft contact lens over the corneal opening.
We present a novel, quick and low-cost technique that may serve as an alternative to current TKP for vitreoretinal surgery.In all three cases vitrectomy was possible without significant intraoperative difficulties such as leakage or hypotony. We achieved good visualization of the central and peripheral retina. The major advantage of our technique is that the TKP is a simple soft contact lens, represents a one size fits all eyes approach and easily available throughout the world (Figure 4). The size of the trephination of the corneal host, which is important if Eckardt®or Landers Foulk TKP are to be used, is irrelevant with this technique. This is a very important factor to be taken into consideration because an Eckardt®keratoprosthesis might not be available at all times and everywhere[1,10].
In our study, 23G vitrectomy with valved trocars was used in all cases. We believe that the use of valved trocars is advantageous, given the complexity of the cases and the higher risk for intraoperative choroidal detachment/hemorrhage predisposed by intraoperative IOP fluctuations.
This surgical technique offers the advantage of not being permanent (e.g., with Boston keratoprosthesis). The soft contact lens can be easily removed and replaced with an allograft at the end of surgery. The contact lens is sterile and removed after the vitreoretinal part of the procedure has been completed and therefore we believe that the risk of an endophthalmitis will be lowered.
Although using TKP for combined corneal and vitreoretinal surgery is not without complications, it is a valuable tool in the management of complicated ocular cases. Even though the case series is limited to three patients, we believe the presented novel technique is a safe, easy, and low-cost method to deal with complex corneal and vitreoretinal cases.
ACKNOWLEDGEMENTS
Authors’ contributions:Manuscript preparation, data acquisition, internal review (Skevas C, Spitzer MS); data acquisition and manuscript preparation (Bigdon E, Steinhorst A, Katz T, Kromer R).
Conflicts of Interest: Skevas C,None;Bigdon E,None;Steinhorst A,None;Katz T,None;Schindler P,None;Kromer R,None;Spitzer MS,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Toric implantable collamer lens for the management of pseudophakic anisometropia and astigmatism
- Angle-closure glaucoma with attenuated mucopolysaccharidosis type l in a Chinese family
- Novel biallelic compound heterozygous mutations in FDXR cause optic atrophy in a young female patient: a case report
- Human umbilical cord-derived mesenchymal stem cells treatment for refractory uveitis: a case series
- lntroduction of longstanding complicated sulcus intraocular lens into the intact capsular bag
- Applications of dynamic visual acuity test in clinical ophthalmology
