海洋藻类中甘氨酸甜菜碱的浓度特征与影响因素综述
2021-11-26宋若晗陆长坤曲克明崔正国赵婉玉胡清静毕相东
宋若晗 陆长坤 曲克明 崔正国 赵婉玉 胡清静 毕相东
摘要 甘氨酸甜菜碱(GBT)是海洋藻类中广泛存在的一种含氮渗透调节物质,其被降解后产生的有机胺可通过海气交换进入大气中。近年研究表明,大气中有机胺可以促进新粒子生成及增长,具有潜在重要的气候效应,因此,海洋环境中有机胺的形成机制越来越受到关注。概述了海洋藻类中GBT合成及其降解为有机胺的途径,归纳了不同藻类体内GBT的浓度分布特征,探讨了影响藻类体内GBT浓度的因素,剖析了该领域待解决的科学问题,并对今后的研究工作进行了展望,以期为提高对海洋环境中有机胺来源的认识提供科学参考。
关键词 甘氨酸甜菜碱;有机胺;海洋藻类;浓度特征;影响因素
中图分类号 X 173 文献标识码 A
文章编号 0517-6611(2021)21-0019-08
doi:10.3969/j.issn.0517-6611.2021.21.006
开放科学(资源服务)标识码(OSID):
Summary of Concentration Characteristics and Influencing Factors of Glycine Betaine in Marine Algae
SONG Ruo-han LU Chang-kun QU Ke-ming 3 et al
(1.College of Fishery,Tianjin Agricultural University,Tianjin 300392; 2.Yellow Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Key Laboratory of Sustainable Development of Marine Fisheries,Ministry of Agriculture and Rural Affairs,Qingdao,Shandong 266071; 3.Laboratory for Marine Fisheries Science and Food Production Processes,Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao,Shandong 266071)
Abstract Glycine betaine (GBT) is a nitrogen containing osmotic adjustment substance widely found in marine algae.The organic amines produced after degradation can enter the atmosphere through sea air exchange.Recent studies have shown that organic amines in the atmosphere can promote the generation and growth of new particles,and have potentially important climate effects.Therefore,the formation mechanism of organic amines in the marine environment has attracted more and more attention.This article outlined the pathways of GBT synthesis and degradation into organic amines in marine algae,summarized the distribution characteristics of GBT in different algae,discussed the factors that affect the concentration of GBT in algae,and analyzed the scientific problems to be solved in this field.The future research work is prospected,hoping to provide a scientific reference for improving the understanding of the sources of organic amines in the marine environment.
Key words Glycine betaine;Organic amines;Marine algae;Concentration characteristics; Influence factors
基金项目 国家重点研发计划(2019YFD090140 2019YFD0900500);国家自然科学基金青年基金项目(41606097);中国水产科学研究院黄海水产研究所基本科研业务费(20603022020006,2020TD12)。
作者简介 宋若晗(1996—),女,河北沧州人,硕士研究生,研究方向:海洋氮循环方面。*通信作者:胡清静,助理研究员,博士,硕士生导师,从事海洋氮循环方面研究;毕相东,教授,博士,硕士生导师,从事养殖水域生态学研究。
收稿日期 2021-06-23;修回日期 2021-07-30
经典CLAW[1]假说认为海洋浮游植物合成的二甲巯基丙酸内盐(DMSP)会被降解为二甲基硫(DMS),其在大气中继续被氧化为甲基磺酸(MSA)及硫酸等,MSA和硫酸进而核化产生新粒子或促进新粒子的增长,对全球气候变化产生重大影响。但是,近年来CLAW假说受到了科学家的质疑[2-4],因为除了DMSP外,浮游植物还会选择甘氨酸甜菜碱(GBT)等季胺类化合物作为渗透调节物质[3,5-6]。GBT被细菌降解后会产生三甲胺(TMA),其再进一步被降解为二甲胺(DMA)和一甲胺(MMA)[3,7-8]。有机胺通过海气交换进入大气中(其约占全球大气中有机胺的28%[9]),也能促进大气中新粒子的生成及颗粒物增长,具有潜在重要的气候效应[10-12]。尽管大部分研究显示大气颗粒物中有机胺盐的浓度低于甲基磺酸盐(MSA-)的浓度[13-14],但是,Hu等[15]研究发现在我国近海大气颗粒物中三甲胺盐(TMAH+)和二甲胺鹽(DMAH+)浓度是同航次检测的大气颗粒物中MSA-浓度的10~60倍[16],因此推测有机胺可能具有更重要的气候效应。受检测技术限制,现阶段海洋环境中有机胺的形成机制尚不明确。GBT作为有机胺的重要前体物,认识其在海洋藻类体内的浓度特征及影响因素,对认识海洋环境中有机胺的形成机制及其气候效应具有重要意义。
1 GBT的重要作用
对海洋藻类来说,GBT最重要和最广泛的作用是渗透调节功能。海洋环境中的藻类常常面临盐度、温度等因素的浮动,GBT的存在可以帮助藻类应对这些胁迫。例如,随着环境中盐度的升高,铜绿紫球藻(Porphyridium aerugineum)细胞内的GBT浓度呈线性增加趋势[17]。这可能是因为在高盐环境中,GBT可以通过保护并增强细胞内酶活性来保护藻体[18]。在对5种海洋底栖硅藻进行低温处理时发现细胞内的GBT浓度会明显升高,其可以作为冷冻保护剂使藻类免受低温侵害[19]。Mao等[20]观察到GBT在干燥的条斑紫菜(Porphyra yezoensis)中会快速积累,这一结论证实了GBT在保护藻类应对干旱胁迫方面的作用。在光保护方面,GBT可以通过保护光反应中的第一个蛋白质复合物Photosystem Ⅱ 来增强植物对光胁迫的耐受性[21]。GBT还具有潜在的浮力作用,海洋浮游植物可以通过改变有机渗透物质的浓度和碳水化合物的储备来改变自身密度。Boyd等[22]提出硅藻可以通过100 mol/m3的季铵化合物以维持其保持位置所需的浮力。在一些植物的生存发育中GBT也发挥作用,GBT在高等植物滨藜(Atriplex halimus L.)中似乎直接参与了叶绿体的保护[23],藻类中低浓度的GBT可以促进作物中叶绿素的生成[24],因而在海藻液体肥料中GBT是重要的化合物,会对植物的生长起到促进作用。因此,在许多海洋藻类中,GBT被认为具有渗透保护、冷冻保护、光保护和潜在的浮力作用,以及保护与促进生长等生物功能[25-27]。
2 海洋藻类中GBT的合成与降解
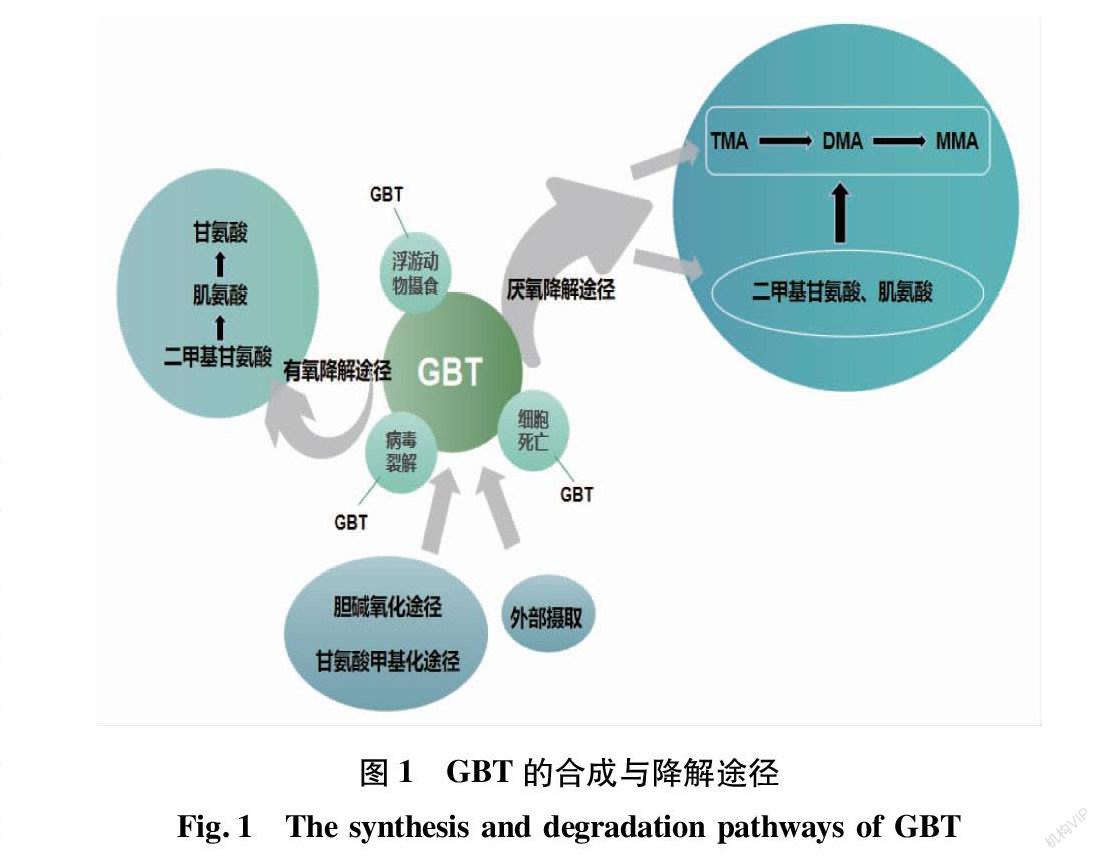
2.1 GBT的生物合成 藻类中的GBT可以通过自身进行合成,主要分为2种途径:胆碱氧化途径和甘氨酸甲基化途径(图1)。在胆碱氧化途径中,胆碱在胆碱脱氢酶(CDH)的催化下氧化为甜菜碱醛,而后在甜菜碱醛脱氢酶(BADH)的作用下氧化为甜菜碱;在甘氨酸甲基化途径中,甘氨酸在甘氨酸肌醇甲基转移酶(GSMT)和肌氨酸二甲基甘氨酸甲基转移酶(SDMT)的催化下,经过3次N-甲基化,分别合成肌氨酸、二甲基甘氨酸和甜菜碱。Mao等[20]研究发现红藻中的条斑紫菜(Pyropia yezoensis)利用胆碱氧化途径合成GBT,且该途径也是嗜盐蓝藻中GBT合成的主要方式[28]。另外,嗜盐蓝藻中的Aphanothece halophytica也可以通过甘氨酸甲基化途径合成GBT[29]。Kageyama等[30]研究发现硅藻中的伪矮海链藻(Thalassiosira pseudonana)也存在以上2种GBT生物合成途径。
藻类中的GBT也可以通过外部来积累。在嗜盐蓝藻中的Synechocystis DUN52这一物种中存在一种主动运输系统来积累外源GBT,这一部分的GBT不参与代谢,而是积累到细胞内的GBT池中作为一种内源性渗透调节物质,这可能是嗜盐蓝藻对高盐胁迫的一种适应机制[31]。
2.2 GBT的降解 藻类中的GBT主要通过被浮游动物摄食、细胞死亡和病毒裂解等方式被释放[32],并通过细菌被降解,主要包括玫瑰杆菌、SAR11等细菌[8,33]。在GBT的碳元素标记试验中,发现在短时间内80%的14C-GBT没有被转化,推测是海洋细菌快速降解GBT的能力不足和需要保留GBT来维持渗透平衡,而在4~5 h后,大部分GBT就会被海洋细菌所降解,且降解速率受到温度、盐度、微生物数量和降解酶浓度等因素的影响[34]。
GBT的降解普遍存在于海洋环境中,包括厌氧降解和有氧降解[35-36](图1)。由于海水中的氧气溶解度会随着海水升温、高盐度、高压力等因素降低,因而海洋中GBT的厌氧降解较为普遍,主要分为2种途径:①由于海洋沉积物具有低溶氧的特性,海洋沉积物中的GBT可在海洋玫瑰杆菌的作用下厌氧发酵,产生TMA并伴有乙酸盐的生成,再进一步降解为DMA和MMA[5,8,36]。King[37]研究发现潮间带沉积物中的GBT被发酵为TMA和乙酸盐,其中TMA再通过活性硫酸盐的还原,TMA迅速转化为甲烷。在厌氧条件下,TMA和其他甲基化胺可以被产甲烷菌降解[38]。因而在海洋沉积物中甲烷的重要来源可能与GBT的分解代谢有关[5]。②GBT在同型乙酸菌与还原剂硫酸盐的去甲基化作用下,产生二甲基甘氨酸和肌氨酸,再进一步产生TMA等有机胺[8,36,39-40]。
Charlotte[8]认为海洋环境中具有有氧降解GBT能力的细菌较少。而Diaz等[41]研究表明在有氧条件下,海洋细菌MD 14-50对GBT进行连续的去甲基化作用,逐步产生二甲基甘氨酸、肌氨酸,并最终降解为甘氨酸。这可能是由于这些细菌中存在二甲基甘氨酸脱氢酶和肌氨酸氧化酶,但是该途径无法产生TMA、DMA和MMA[41-43]。
3 海洋藻类中GBT的浓度分布特征
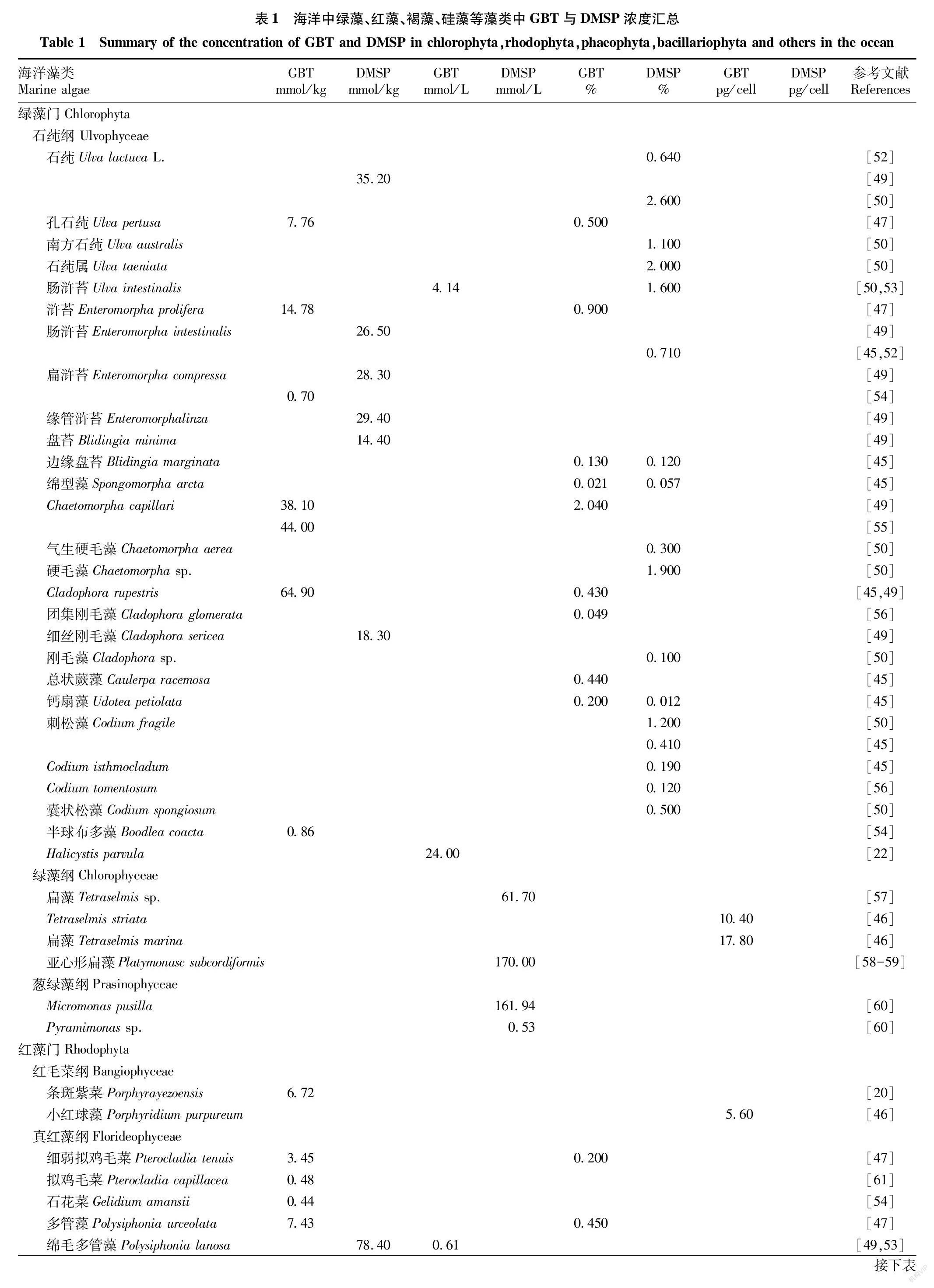
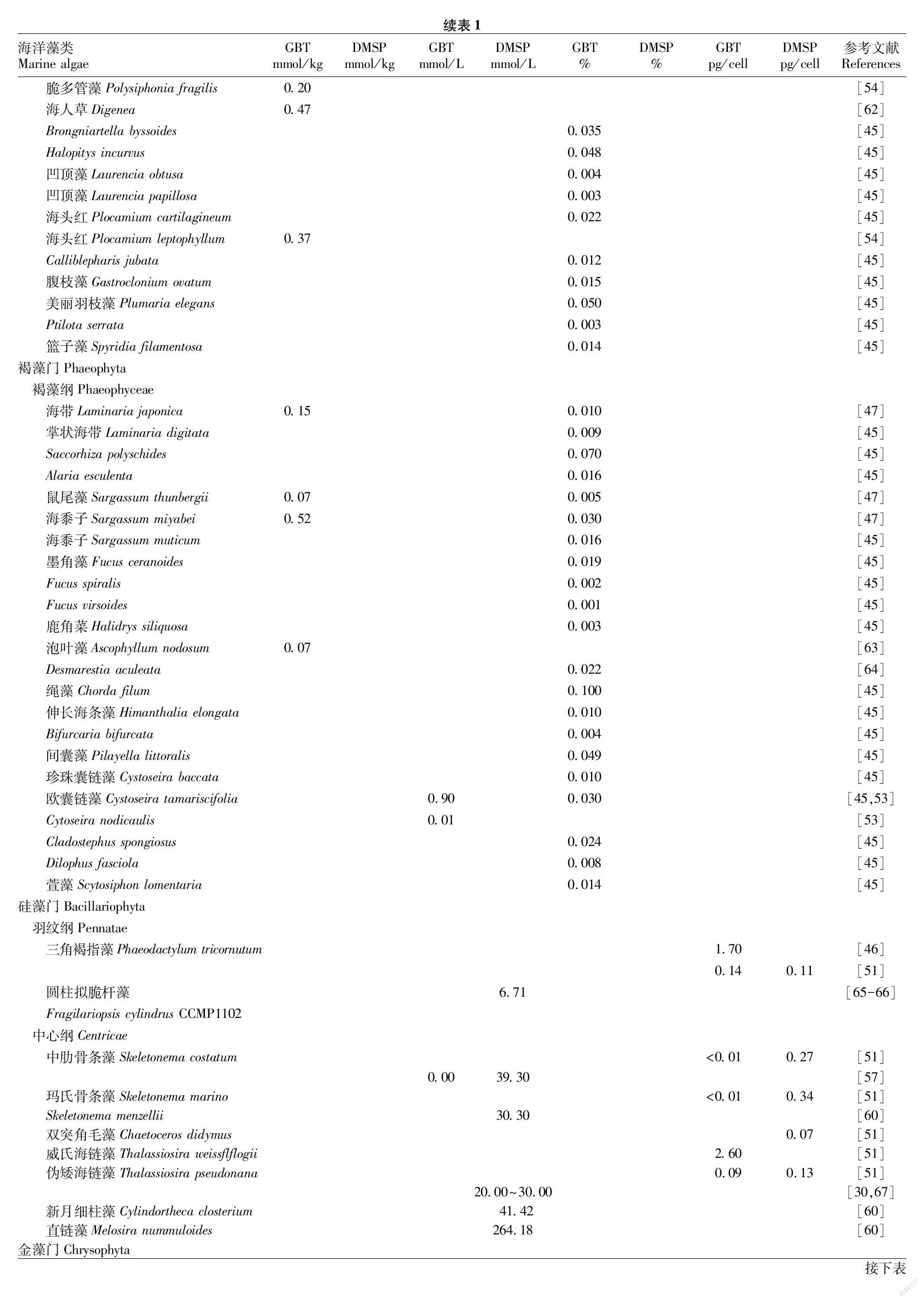
表1中对绿藻、红藻、褐藻和硅藻等109种藻类中的GBT和DMSP浓度进行了汇总,通过对比发现,蓝藻与绿藻中GBT含量在所有门类的藻类中往往是最高的,红藻与硅藻中GBT含量次之,褐藻中GBT含量则最低。在Mohammad等[44]的检测中,嗜盐蓝藻中以GBT为主要成分的季胺化合物浓度高达2 430 mmol/L。Blunden等[45]对62种海藻中的GBT和含硫化合物进行了检测,其中绿藻中GBT含量相对较高,占干重的0.021%~2.040%,绿藻中刚毛藻的主要成分是GBT,而褐藻中的GBT含量较低,仅占干重的0.001%~0100%,在部分绿藻中GBT的含量高褐藻2个数量级。Al-Amoudi等[46]对几类藻体中GBT浓度进行检测,在绿藻扁藻中的Tetraselmis marina和Tetraselmis stirata中檢测到最高浓度的GBT(分别为17.8和10.4 pg/cell),且硅藻中的三角褐指藻(Phaeodactylum tricornutum)的GBT含量约是绿藻T.marina中的1/10~1/6。在对我国青岛附近海域藻类的检测中,绿藻和红藻的GBT水平较高,其中绿藻中鲜浒苔(Enteromorpha prolifera)GBT含量为14.78 mmol/kg,占干重的09%,红藻中多管藻(Polysiphonia urceolata)GBT含量为743 mmol/kg,褐藻中GBT的水平较低,浒苔GBT的含量是褐藻中海带(Laminaria japonica)GBT含量的90倍[47]。但是,赵鹏等[48]的检测中浒苔中GBT的浓度仅是海带的1.2倍,2份报告中结果的差异可能与检测的海藻来源不同等因素相关。
通过对表1中绿藻和硅藻的DMSP与GBT含量进行对比,发现绿藻中的部分种类如硬毛藻属、刚毛藻属、钙扇藻属中的GBT含量超过了DMSP[45,49-50],但在石莼属、浒苔属等种类中似乎DMSP浓度更高[47,49-50]。在硅藻中,GBT浓度为<0.01~2.60 pg/cell,DMSP浓度为0.07~0.34 pg/cell,GBT平均浓度约为DMSP平均浓度的4倍,因而推测GBT也是部分硅藻中主要的渗透调节物质[46,51]。
4 影响藻细胞中GBT合成的因素
4.1 盐度
GBT和DMSP作为海洋藻类重要的渗透调节物质对盐度响应敏感[17]。室内培养研究中发现,红藻中的紫球藻(Porphyridium aerngineum)和硅藻中的三角褐指藻(Phaeodactylum tricornutum)、隐秘小环藻(Cyclotella cryptica)、梅尼小环藻(Cyclotella meneghiniana)的细胞内盐度由150 mol/cm3增加至1 000 mol/cm3时,GBT浓度会增加一个数量级[17]。同样,蓝藻中的GBT也对盐度响应敏感。嗜盐蓝藻中的Synechocystis DUN52在海水盐度增加8倍的情況下,以GBT为最主要成分的季胺化合物的增加量为1 200 mmol/dm3[71]。Incharoensakdi等[72]的室内培养中,嗜盐蓝藻中的另一物种Aphanothece halophytica在盐胁迫下生长6 d,GBT的积累增加了约20倍。因此,许多研究认为嗜盐蓝藻使用GBT来平衡细胞质与外部盐度[73],体内GBT的积累可能是它们在高盐环境中存活的重要原因[44,7 74]。在现场观测中,Hu等[75]研究发现渤海及北黄海的大气颗粒物中TMAH+、DMAH+粒子的浓度与海水盐度存在极显著的正相关。该海域的浮游植物主要以硅藻和蓝藻为主[76]。因而推测盐度的升高会导致该海域中硅藻与蓝藻产生的GBT含量增加,GBT降解进而导致大气中有机胺浓度的升高。
但是,并非所有海洋藻类对盐度响应敏感,Mulholland等[77]在对高等植物Spartina anglica的培养中,GBT对盐度变化响应不明显。盐度对藻类中GBT含量的影响也被认为与处理时间相关。绿藻中的Chaetomorpha capillaris在24 h内GBT含量随盐度无明显变化[49]。Reed[55]的室内培养中同样提到藻类中的GBT在短期(24 h)内对盐度不敏感,GBT合成持续时间较长,只有当藻类长期(30 d)处在高盐环境中时,GBT才会积累并起到调节渗透压的作用。这说明不同海洋藻类或藻类的培养时间对盐度的敏感程度有区别。
4.2 氮营养盐
虽然GBT与DMSP都是海洋藻类重要的渗透调节物质,但两者区别在于:GBT是含氮渗透调节物,DMSP是含硫渗透调节物。在海洋生态系统中,氮营养盐是藻类生长发育所必需的营养元素,也是藻类生长最常见的限制因子之一[78]。Andreae[79]提出,由于海水中硫酸盐的浓度(约28 mmol/L)远大于氮营养盐的浓度(1~10 μmol/L),因而在氮营养盐限制条件下,氮仅用来满足藻类的生长,选择合成更多的含硫化合物DMSP起到渗透调节作用[8,80],并且观察到GBT与DMSP竞争相同的运输系统[8,34],因此氮营养盐浓度的变化会对海洋藻类体内GBT和DMSP的浓度产生影响。室内培养研究中发现,在氮营养盐充足的条件下,处于指数生长期的金色藻(Chrysochromulina sp.Lackey)体内GBT浓度大于DMSP,而当到达氮营养盐限制的稳定生长期,GBT浓度大幅下降,但DMSP浓度变化幅度不大[57]。Keller等[81]研究发现,氮营养盐限制培养条件下,NO3-的增加导致培养的3种藻类伪矮海链藻(Thalassiosira pseudonana)、赫氏颗石藻(Emiliania huxleyi)、卡特前沟藻(Amphidinium carterae)中GBT含量增加,其中伪矮海链藻、卡特前沟藻体内的氮营养盐与DMSP呈负相关。在对中肋骨条藻(Skeletonema costatum)的室内培养中发现,高氮营养盐有利于该硅藻的生长,而细胞中DMSP的水平随氮营养盐含量的增加而降低[82]。Gibb等[83]观测到在以硅藻为优势种的阿拉伯海上升流海域中有机胺浓度较高,其推测该海域上升流携带的大量NO3-、NH4+等无机氮会被硅藻用于GBT的合成,GBT的降解会导致该海域有机胺的浓度升高。在白令海的现场观测中,氮营养盐与DMSP呈负相关[84]。在我国的东海现场观测发现,当海水中硝酸盐浓度小于1.0 μmol/L时,海水中的颗粒态DMSP与氮营养盐呈显著正相关,当海水中硝酸盐浓度大于1.0 μmol/L时,颗粒态DMSP与氮营养盐呈负相关[85]。说明高氮营养盐可能会促进海洋藻中GBT的生成、而抑制DMSP的合成。Hu等[15]研究发现我国近海大气颗粒物中有机胺浓度比世界其他海域高1~3个数量级,这可能与我国近海富营养化有关,但还需要现场观测进一步确认。
有些藻类也不受氮营养盐的影响。在Van Alstyne等[86]的研究结果中,氮营养盐对石莼中DMSP的合成没有显著影响。也有现场观测发现浮游植物中DMSP和GBT的增加或减少与氮营养盐的添加并不呈现显著的线性关系[87]。推测不同藻类体内GBT对氮营养盐的响应存在差异性,这可能是与藻类物种和生理状态的不同相关。
5 展望
综上所述,虽然目前国内外对海洋藻类体内GBT的合成与降解途径、浓度特征及影响因素开展了一定的研究,但还有许多问题需要解决,具体体现在以下3点:
(1)提升对多种海洋藻类体内GBT合成途径的认识。目前国内外仅有少数研究报道了蓝藻、硅藻等藻类体内GBT的合成途径。未来需要通过提高检测技术,进一步加强对多种海洋藻类体内GBT合成途径的认识。
(2)探究海洋藻類体内GBT对大气中有机胺的贡献。多数研究认为海洋藻类体内的GBT在水体或沉积物中被细菌降解为有机胺,它们再通过海气交换进入大气中。但是,少数研究在海洋大气颗粒物中检测出GBT[88],因而需要现场及室内模拟试验进一步探究大气颗粒物中GBT如何被降解以及是否对大气中的有机胺具有贡献。
(3)揭示富营养化海域大气中有机胺的潜在气候效应。尽管国际上多数研究发现大气颗粒物中MSA-的浓度高于有机胺盐,但是在富营养化的近海(如我国近海海域),有机胺的浓度可能会超越MSA-,具有更重要的气候效应。因此,未来需要加强认识在氮营养盐充足的海域,多种海洋藻类体内GBT的变化特征及其对水体及大气中有机胺的影响,从而揭示富营养化海域有机胺的潜在气候效应。
参考文献
[1]
CHARLSON R J,LOVELOCK J E,ANDREAE M O,et al.Oceanic phytoplankton,atmospheric sulphur,cloud albedo and climate[J].Nature,1987,326(6114):655-661.
[2] QUINN P K,BATES T S.The case against climate regulation via oceanic phytoplankton sulphur emissions[J].Nature,201 480(7375):51-56.
[3] GREEN T K,HATTON A D.The claw hypothesis:A new perspective on the role of biogenic sulphur in the regulation of global climate[J].Oceanography and marine biology,2014,52:315-336.
[4] SELLEGRI K,PEY J,ROSE C,et al.Evidence of atmospheric nanoparticle formation from emissions of marine microorganisms[J].Geophysical research letters,2016,43(12):6596-6603.
[5] WELSH D T.Ecological significance of compatible solute accumulation by micro-organisms:From single cells to global climate[J].FEMS Microbiology Reviews,2000,24(3):263-290.
[6] TORSTENSSON A,YOUNG J N,CARLSON L T,et al.Use of exogenous glycine betaine and its precursor choline as osmoprotectants in Antarctic sea-ice diatoms[J].Journal of phycology,2019,55(3):663-675.
[7] CARPENTER L J,ARCHER S D,BEALE R.Ocean-atmosphere trace gas exchange[J].Chemical society reviews,201 41(19):6473-6506.
[8] CHARLOTTE C.Distributions of glycine betaine and the methylamines in coastal waters:Analytical developments and a seasonal study[D].Plymouth,UK:University of Plymouth,2015.
[9] GE X L,WEXLER A S,CLEGG S L.Atmospheric amines-Part I.A review[J].Atmospheric environment,201 45(3):524-546.
[10] ALMEIDA J,SCHOBESBERGER S,KRTEN A,et al.Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere[J].Nature,2013,502(7471):359-363.
[11] CHEN H H,VARNER M E,GERBER R B,et al.Reactions of methanesulfonic acid with amines and ammonia as a source of new particles in air[J].The journal of physical chemistry B,2016,120(8):1526-1536.
[12] YAO L,GARMASH O,BIANCHI F,et al.Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity[J].Science,2018,361(6399):278-281.
[13] FACCHINI M C,DECESARI S,RINALDI M,et al.Important source of marine secondary organic aerosol from biogenic amines[J].Environmental science and technology,2008,42(24):9116-9121.
[14] MLLER C,IINUMA Y,KARSTENSEN J,et al.Seasonal variation of aliphatic amines in marine sub-micrometer particles at the Cape Verde islands[J].Atmospheric chemistry and physics,2009,9(24):9587-9597.
[15] HU Q J,YU P R,ZHU Y J,et al.Concentration,size distribution and formation of trimethylaminium and dimethylaminium ions in atmospheric particles over marginal seas of China[J].Journal of the atmospheric sciences,2015,72(9):3487-3498.
[16] ZHANG Y,ZHANG H H,YANG G P,et al.Chemical characteristics and source analysis of aerosol composition over the Bohai Sea and the Yellow Sea in spring and autumn[J].Journal of the atmospheric sciences,2015,72(9):3563-3573.
[17] DICKSON D M J,KIRST G O.Osmotic adjustment in marine eukaryotic algae:The role of inorganic ions,quaternary ammonium,tertiary sulphonium and carbohydrate solutes.I.Diatoms and a rhodophyte[J].New phytologist,1987,106(4):645-655.
[18] SWAPNIL P,SINGH M,SINGH S,et al.Recombinant glycinebetaine improves metabolic activities,ionic balance and salt tolerance in diazotrophic freshwater cyanobacteria[J].Algal research,2015,11:194-203.
[19] SCHOLZ B,LIEBEZEIT G.Compatible solutes and fatty acid composition of five marine intertidal microphytobenthic Wadden Sea diatoms exposed to different temperature regimes[J].Diatom research,2013,28(4):337-358.
[20] MAO Y X,CHEN N C,CAO M,et al.Functional characterization and evolutionary analysis of glycine-betaine biosynthesis pathway in red seaweed Pyropia yezoensis[J].Marine drugs,2019,17(1):70-81.
[21] PRASAD K V S K,SARADHI P P.Enhanced tolerance to photoinhibition in transgenic plants through targeting of glycinebetaine biosynthesis into the chloroplasts[J].Plant science,2004,166(5):1197-1212.
[22] BOYD C,GRADMANN D.Impact of osmolytes on buoyancy of marine phytoplankton[J].Marine biology,200 141(4):605-618.
[23] HASSINE A B,GHANEM M E,BOUZID S,et al.An inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus L.differ in their ability to accumulate proline and glycinebetaine in response to salinity and water stress[J].Journal of experimental botany,2008,59(6):1315-1326.
[24] WHAPHAM C A,BLUNDEN G,JENKINS T,et al.Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract[J].Journal of applied phycology,1993,5(2):231-234.
[25] DICKSON D M,JONES R G,DAVENPORT J.Steady state osmotic adaptation in Ulva lactuca[J].Planta,1980,150(2):158-165.
[26] ASHRAF M,FOOLAD M R.Roles of glycine betaine and proline in improving plant abiotic stress resistance[J].Environmental and experimental botany,2007,59(2):206-216.
[27] BEALE R,AIRS R.Quantification of glycine betaine,choline and trimethylamine N-oxide in seawater particulates:Minimisation of seawater associated ion suppression[J].Analytica chimica acta,2016,938:114-122.
[28] 張英杰,廖子亚,赵百锁.嗜盐菌中甘氨酸甜菜碱的合成途径及其生物学功能[J].微生物学报,2020,60(6):1074-1089.
[29] WADITEE R,TANAKA Y,AOKI K,et al.Isolation and functional characterization of N-methyltransferases that catalyze betaine synthesis from glycine in a halotolerant photosynthetic organism Aphanothece halophytica[J].The journal of biological chemistry,2003,278(7):4932-4942.
[30] KAGEYAMA H,TANAKA Y,TAKABE T.Biosynthetic pathways of glycinebetaine in Thalassiosira pseudonana;functional characterization of enzyme catalyzing three-step methylation of glycine[J].Plant physiology and biochemistry,2018,127:248-255.
[31] MOORE D J,REED R H,STEWART W D P.A glycine betaine transport system in Aphanothece halophytica and other glycine betaine-synthesising cyanobacteria[J].Archives of microbiology,1987,147(4):399-405.
[32] SUN J,MAUSZ M A,CHEN Y,et al.Microbial trimethylamine metabolism in marine environments[J].Environmental microbiology,2019,21(2):513-520.
[33] SUN J,STEINDLER L,THRASH J C,et al.One carbon metabolism in SAR11 pelagic marine bacteria[J].PLoS One,201 6(8):1-12.
[34] KIENE R P.Uptake of choline and its conversion to glycine betaine by bacteria in estuarine waters[J].Applied and environmental microbiology,1998,64(3):1045-1051.
[35] BERNARD T,POCARD J A,PERROUND B,et al.Variations in the response of salt-stressed Rhizobium strains to betaines[J].Archives of microbiology,1986,143(4):359-364.
[36] CHEN Y.Comparative genomics of methylated amine utilization by marine Roseobacter clade bacteria and development of functional gene markers (tmm,gmaS)[J].Environmental microbiology,201 14(9):2308-2322.
[37] KING G M.Metabolism of trimethylamine,choline,and glycine betaine by sulfate-reducing and methanogenic bacteria in marine sediments[J].Applied and environmental microbiology,1984,48(4):719-725.
[38] OREN A.Formation and breakdown of glycine betaine and trimethylamine in hypersaline environments[J].Antonie van leeuwenhoek,1990,58(4):291-298.
[39] CHEN Y,PATEL N A,CROMBIE A,et al.Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase[J].Proceedings of the national academy of sciences,201 108(43):17791-17796.
[40] MLLER E,FAHLBUSCH K,WALTHER R,et al.Formation of N,N-dimethylglycine,acetic acid,and butyric acid from betaine by Eubacterium limosum[J].Applied and environmental microbiology,198 42(3):439-445.
[41] DIAZ M R,VISSCHER P T,TAYLOR B F.Metabolism of dimethylsulfoniopropionate and glycine betaine by a marine bacterium[J].FEMS Microbiology Letters,199 96(1):61-65.
[42] DONG H P,HONG Y G,LU S H,et al.Metaproteomics reveals the major microbial players and their biogeochemical functions in a productive coastal system in the northern South China Sea[J].Environmental microbiology reports,2014,6(6):683-695.
[43] HEIJTHUIJSEN J H F G,HANSEN T A.Betaine fermentation and oxidation by marine Desulfuromonas strains[J].Applied and environmental microbiology,1989,55(4):965-969.
[44] MOHAMMAD F A A,REED R H,STEWART W D P.The halophilic cyanobacterium Synechocystis DUN52 and its osmotic responses[J].FEMS Microbiology Letters,1983,16(2/3):287-290.
[45] BLUNDEN G,SMITH B E,IRONS M W,et al.Betaines and tertiary sulphonium compounds from 62 species of marine algae[J].Biochemical systematics and ecology,199 20(4):373-388.
[46] AL-AMOUDI O A,ALI A Y.Some practical aspects of measurements of betaines and their sulphur analogues by the use of HPLC[J].Journal of microbiological methods,1989,10(4):289-296.
[47] 韓丽君,范晓,周永航.海藻中甜菜碱的研究[J].海洋科学集刊,1999,41(00):86-91.
[48] 赵鹏,徐继林,刘雪梅,等.海带等4种大型海藻中甜菜碱液质分析研究[J].中国药学杂志,201 46(24):1886-1889.
[49] CHUDEK J A,FOSTER R,MOORE D J,et al.Identification and quantification of Methylated Osmolytes in algae using Proton Nuclear Magnetic Resonance Spectroscopy[J].British phycological journal,1987,22(2):169-173.
[50] VAN ALSTYNE K L.The distribution of DMSP in green macroalgae from northern New Zealand,eastern Australia and southern Tasmania[J].Journal of the marine biological association of the UK,2008,88(4):799-805.
[51] SPIELMEYER A,GEBSER B,POHNERT G.Dimethylsulfide sources from microalgae:Improvement and application of a derivatization-based method for the determination of dimethylsulfoniopropionate and other zwitterionic osmolytes in phytoplankton[J].Marine chemistry,201 124(1/2/3/4):48-56.
[52] BLUNDEN G,EL BAROUNI M M,GORDON S M,et al.Extraction,purification and characterisation of Dragendorff-positive compounds from some British marine algae[J].Botanica marina,198 24(8):451-456.
[53] VALVERDE J,HAYES M,MCLOUGHLIN P,et al.Cardioprotective potential of irish macroalgae:Generation of glycine betaine and dimethylsulfoniopropionate containing extracts by accelerated solvent extraction[J].Planta medica,2015,81(8):679-684.
[54] HORI K,YAMAMOTO T,MIYAZAWA K,et al.Distribution of quaternary ammonium bases in seven species of marine algae[J].Journal of the faculty of applied biological science hiroshima nuiversity,1979,18(1):65-73.
[55] REED R H.Osmotic adjustment and organic solute accumulation in Chaetomorpha capillaris[J].British phycological journal,1989,24(1):21-37.
[56] BLUNDEN G,GORDON S M,MCLEAN W F H,et al.The distribution and possible taxonomic significance of quaternary ammonium and other Dragendorff-positive compounds in some genera of marine algae[J].Botanica marina,198 25(12):563-568.
[57] KELLER M D,KIENE R P,MATRAI P A,et al.Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton.I.Batch cultures[J].Marine biology,1999,135(2):237-248.
[58] DICKSON D M J,KIRST G O.The role of β-dimethylsulphoniopropionate,glycine betaine and homarine in the osmoacclimation of Platymonas subcordiformis[J].Planta,1986,167(4):536-543.
[59] ZHANG X H,LIU J,LIU J L,et al.Biogenic production of DMSP and its degradation to DMS-their roles in the global sulfur cycle[J].Science China life sciences,2019,62(10):1296-1319.
[60] KELLER M D,BELLOWS W K,GUILLARD R R L.Dimethyl sulfide production in marine phytoplankton[M]//ACS symposium series.Washington,DC:American Chemical Society,1989:167-182.
[61] SCIUTO S,CHILLEMI R,PIATTELLI M.Onium compounds from the red alga Pterocladia capillacea[J].Journal of natural products,1988,51(2):322-325.
[62] PATTI A,MORRONE R,CHILLEMI R,et al.Thetines and betaines of the red alga Digenea simplex[J].Journal of natural products,1993,56(3):432-435.
[63] MACKINNON S L,HILTZ D,UGARTE R,et al.Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts[J].Journal of applied phycology,2010,22(4):489-494.
[64] BLUNDEN G,CRIPPS A L,GORDON S M,et al.The characterisation and quantitative estimation of betaines in commercial seaweed extracts[J].Botanica marina,1986,29(2):155-160.
[65] LYON B R,LEE P A,BENNETT J M,et al.Proteomic analysis of a sea-ice diatom:Salinity acclimation provides new insight into the dimethylsulfoniopropionate production pathway[J].Plant physiology,201 157(4):1926-1941.
[66] CURSON A R J,WILLIAMS B T,PINCHBECK B J,et al.DSYB catalyses the key step of dimethylsulfoniopropionate biosynthesis in many phytoplankton[J].Nature microbiology,2018,3(4):430-439.
[67] KETTLES N L,KOPRIVA S,MALIN G.Insights into the regulation of DMSP synthesis in the diatom Thalassiosira pseudonana through APR activity,proteomics and gene expression analyses on cells acclimating to changes in salinity,light and nitrogen[J].PLoS One,2014,9(4):1-11.
[68] VAIRAVAMURTHY A,ANDREAE M O,IVERSON R L.Biosynthesis of dimethylsulfide and dimethylpropiothetin by Hymenomonas carterae in relation to sulfur source and salinity variations[J].Limnology and oceanography,1985,30(1):59-70.
[69] STEFELS J,VAN BOEKEL W H M.Production of DMS from dissolved DMSP in axenic cultures of the marine phytoplankton species Phaeocystis sp.[J].Marine ecology progress series,1993,97(1):11-18.
[70] DACEY J W H,WAKEHAM S G.Oceanic dimethylsulfide:Production during zooplankton grazing on phytoplankton[J].Science,1986,233(4770):1314-1316.
[71] REED R H,CHUDEK J A,FOSTER R,et al.Osmotic adjustment in cyanobacteria from hypersaline environments[J].Archives of microbiology,1984,138(4):333-337.
[72] INCHAROENSAKDI A,WUTIPRADITKUL N.Accumulation of glycinebetaine and its synthesis from radioactive precursors under salt-stress in the cyanobacterium Aphanothece halophytica[J].Journal of applied phycology,1999,11(6):515-523.
[73] OREN A.Diversity of organic osmotic compounds and osmotic adaptation in cyanobacteria and algae[M]//SECKBACH J.Algae and cyanobacteria in extreme environments.Dordrecht:Springer,2007:639-655.
[74] REED R H,STEWART W D P.Osmotic adjustment and organic solute accumulation in unicellular cyanobacteria from freshwater and marine habitats[J].Marine biology,1985,88(1):1-9.
[75] HU Q J,QU K M,GAO H W,et al.Large increases in primary trimethylaminium and secondary dimethylaminium in atmospheric particles associated with cyclonic eddies in the northwest Pacific Ocean[J].Journal of geophysical research:Atmospheres,2018,123(21):12133-12146.
[76] ZHANG S,LENG X Y,FENG Y Y,et al.Ecological provinces of spring phytoplankton in the Yellow Sea:Species composition[J].Acta oceanologica sinica,2016,35(8):114-125.
[77] MULHOLLAND M M,OTTE M L.The effects of nitrogen supply and salinity on DMSP,glycine betaine and proline concentrations in leaves of Spartina anglica[J].Aquatic botany,200 72(2):193-200.
[78] ZEHR J P,KUDELA R M.Nitrogen cycle of the open ocean:From genes to ecosystems[J].Annual review of marine science,201 3(1):197-225.
[79] ANDREAE M O.The ocean as a source of atmospheric sulfur compounds[M]//BUAT-MENARD P.The role of air-sea exchange in geochemical cycling.Dordrecht,USA:D.Reidel Publishing Company,1986:331-362.
[80] LISS P S,HATTON A D,MALIN G,et al.Marine sulphur emissions[J].Philosophical transactions of the royal society B biological sciences,1997,352(1350):159-169.
[81] KELLER M D,KIENE R P,MATRAI P A,et al.Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton.II.N-limited chemostat cultures[J].Marine biology,1999,135(2):249-257.
[82] YANG G P,LI C X,SUN J.Influence of salinity and nitrogen content on production of dimethylsulfoniopropionate(DMSP) and dimethylsulfide (DMS) by Skeletonema costatum[J].Chinese journal of oceanology and limnology,2011(2):378-386.
[83] GIBB S W,MANTOURA R F C,LISS P S,et al.Distributions and biogeochemistries of methylamines and ammonium in the Arabian Sea[J].Deep-sea research part II,1999,46(3/4):593-615.
[84] LI C X,WANG B D,YANG G P,et al.Occurrence and turnover of biogenic sulfur in the Bering Sea during summer[J].Journal of geophysical research:Oceans,2017,122(11):8567-8592.
[85] JIAO N Z,LIU C Z,HONG H S,et al.Dynamics of dimethylsulfide and dimethylsulfoniopropionate produced by phytoplankton in the Chinese seas-distribution patterns and affecting factors[J].Acta botanica sinica,2003,45(7):774-786.
[86] VAN ALSTYNE K L,KOELLERMEIER L,NELSON T A.Spatial variation in dimethylsulfoniopropionate (DMSP) production in Ulva lactuca (Chlorophyta) from the Northeast Pacific[J].Marine biology,2007,150(6):1127-1135.
[87] KELLER M D,MATRAI P A,KIENE R P,et al.Responses of coastal phytoplankton populations to nitrogen additions:Dynamics of cell-associated dimethylsulfoniopropionate (DMSP),glycine betaine (GBT),and homarine[J].Canadian journal of fisheries and aquatic sciences,2004,61(5):685-699.
[88] DALLOSTO M,OVADNEVAITE J,PAGLIONE M,et al.Antarctic sea ice region as a source of biogenic organic nitrogen in aerosols[J].Scientific reports,2017,7(1):6047-6056.
